Abstract
Objective
Mitral annular structure and dynamics after mitral ring annuloplasty using transesophageal echocardiography during the operation have been reported. We evaluated mitral annular structure and dynamics of three different rings in the mid-term period postoperatively.
Methods
Thirty-one patients underwent mitral valve repair for degenerative mitral insufficiency. The MEMO 3D ring (semi-flexible), Carpentier–Edwards Physio II ring (semi-rigid), and St. Jude Medical Rigid Saddle Ring (rigid) were implanted in 15, 12, and eight patients, respectively, from September 2009 to February 2015. Electrocardiogram-gated three-dimensional computed tomography was performed in the mid-term period postoperatively.
Results
The postoperative antero-posterior rate of reduction in diameter from end-diastole to end-systole was slightly larger in the MEMO3D (0.57 ± 0.69%) than in the Physio II (0.08 ± 0.60%) and Rigid Saddle Ring (0.11 ± 0.59%). There was no significant difference in the commissure-to-commissure rate of reduction in diameter among the groups. The postoperative end-systolic annular height to commissure width ratio was significantly larger in the Physio II (20.4 ± 1.7%) and Rigid Saddle Ring (21.3 ± 1.7%) than in the MEMO3D (10.8 ± 3.1%, both p < 0.0001). The rate of increase in the postoperative annular height to commissure width ratio from end-diastole to end-systole was significantly larger in the MEMO3D (2.1 ± 1.7%) than in the Physio II (0.1 ± 0.4%) and Rigid Saddle Ring (0.1 ± 0.6%).
Conclusions
The Physio II and Rigid Saddle Ring can restore the physiological and three-dimensional annular shape, and the MEMO3D can preserve physiological annular dynamics in mid-term period postoperatively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The mitral valve leaflet is concave toward the left ventricle with a mitral annular three-dimensional saddle shape during the systolic phase [1]. This structural feature contributes to reducing stress of the leaflet and stabilizing blood flow at left ventricular outflow during systole [2, 3]. The range of optimal annular height to commissure width ratio (AHCWR) was reported as 15–20% in an experimental study [3]. Mitral annular dynamics also play a pivotal role in function of left ventricular inflow and outflow in the cardiac cycle [3, 4].
Mitral valve repair with an annuloplasty ring is the gold standard technique for degenerative mitral valve insufficiency. Some types of physiological annular rings are available based on mitral annular physiology. A saddle-shaped ring can be useful for making a flattened mitral annulus for recovering the physiological shape to maintain long-term valve function [5,6,7]. However, a semi-flexible ring can preserve folding dynamics, which is one of the physiological annular dynamics, even though it cannot restore the physiological, three-dimensional, annular shape [8].
Trans-esophageal real-time three-dimensional echocardiography (RT3DE) can precisely evaluate the mitral annulus, but it is a burden for patients. Therefore, most studies on RT3DE have only been performed during the operation, and it is difficult to assess it in detail at the mid-term period after the operation. Many animal studies have been designed as acute experiments by implanting radiopaque markers [3, 9, 10], sonomicrometry equipment [5, 11] or using an in vitro stimulator [6].
In this study, we investigated mitral annular dynamics after implantation of three different annuloplasty rings. These rings included a rigid saddle-shaped ring [Rigid Saddle Ring (RSR)], a semi-rigid saddle-shaped ring (Physio II), and a semi-flexible ring [MEMO 3D ring (MEMO3D)] at the postoperative mid-term period by electrocardiogram (ECG)-gated three-dimensional computed tomography (3D-CT) instead of RT3DE.
Patients and methods
Ethics statement
This study was approved by the Institutional Review Board of Hyogo College of Medicine.
Patient population
From September 2009 and February 2015, patients who underwent elective mitral valve (MV) repair with the Carpentier–Edwards Physio II (Physio II) ring (Edwards Lifesciences LLC., Irvine, CA), St. Jude Medical RSR (St Jude Medical, St. Paul, MN), or MEMO3D (Sorin S.p.A., Milano, Italy) for severe mitral regurgitation (Carpentier’s classification type II) due to myxomatous valve disease were included. Patients who required other concomitant valvular surgery were excluded. The choice of annuloplasty ring was based on the surgeon’s preference. Patients who could not undergo postoperative 3D-CT were excluded. Using these criteria, 15 patients with the MEMO3D, 12 with the Physio II, and eight with the RSR were enrolled. The mean period for performing postoperative 3D-CT was 3.6 ± 1.6 years after the operation.
Surgical procedure
Eighteen patients underwent surgery through sternotomy and 17 through right mini-thoracotomy. All of the patients underwent MV repair under cardioplegic arrest with moderate hypothermic cardiopulmonary bypass. Prolapsed leaflets were repaired with implantation of artificial chordae, or triangular or quadrangular resection of the leaflet and suture technique. A suitable size of ring annuloplasty was then decided.
Computed tomography
All computed tomography (CT) scans were performed using a 128-slice CT scanner (Siemens AG, Munich, Germany). Studies were performed with retrospective ECG gating using a helical technique at 120 kVp, with tube current adjusted in the range of 600–1000 mAs based on the patient’s weight. Manual image analysis was performed using Osirix Lite ver.8.0.2 (Pixmeo SARL, Bernex, Switzerland) by a single cardiac surgeon.
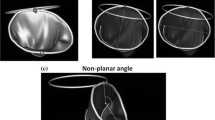
Digital imaging and communication in medicine data at two time points, end-systole and end-diastole, were obtained based on the ECG. The annuloplasty ring was reconstructed by adjusting the window level in the 3D volume-rendering viewer. The annuloplasty ring was described in the lateral view and the frontal view. In the lateral view, the annuloplasty ring was set at the plane where the highest points of the anterior and posterior annulus were placed in parallel rows. Annular height, which was measured between the highest and lowest points on the ring, and the antero-posterior (AP) diameter were then measured at end-systolic and end-diastolic phases (Fig. 1a). In the frontal view, the ring was set at a symmetrical appearance where the AP distance was similar to the AP distance measured in the lateral view. The commissure-to-commissure (CC) diameter was measured at two time points (Fig. 1b). The annular height to commissure width ratio (AHCWR) was calculated as an index to express three-dimensional annular construction. The rate of reduction in the AP and CC diameters and the rate of increase in the AHCWR from end-diastole to end-systole were evaluated.
Annular modeling. The ring was reconstructed by adjusting the window level in the three-dimensional volume-rendering viewer. a Lateral view of the ring. The highest points of the anterior and posterior annulus were placed on parallel rows. The dashed line shows the antero-posterior diameter and the continuous line shows the annular height. b Frontal view of the ring. The dashed line shows the anterior–posterior diameter and the continuous line shows the commissure-to-commissure diameter
Statistical analysis
All of the data are reported as mean ± SD. Only patients with complete data sets were included and analyzed in this study. Comparison of the data was performed by analysis of variance between groups. Values of p less than 0.05 were considered to indicate statistical significance. The software JMP 9.0.2 for Mac (SAS, Cary, NC, USA) was used for analysis.
Results
The mean age of the patients at the operation, preoperative values of echocardiography, and the size of the annular ring were not different among the annular ring groups (Table 1). In patients with only anterior leaflet or bileaflet prolapse, only implantation of artificial chordae was performed in the Physio II and RSR groups. Either implantation of artificial chordae or triangular resection was performed in the MEMO3D group (Table 2). In patients with only posterior leaflet prolapse, implantation of artificial chordae, or triangular or quadrangular resection of the leaflet was carried out (Table 3). There was no significant difference in the implanted annuloplasty ring size among the groups (Table 4).
The echocardiography in postoperative mid-term period showed trivial or mild mitral regurgitation in all patients except one patient with moderate mitral regurgitation in MEMO3D group. The rates of reduction in the AP diameter from end-diastole to end-systole at the postoperative mid-term period were 0.57 ± 0.69, 0.08 ± 0.60, and 0.11 ± 0.59% in the MEMO3D, Physio II, and RSR groups, respectively (Fig. 2). There was no significant difference in this rate among the groups, although the value in the MEMO3D group was slightly higher than that in the other groups.
Rate of reduction of the postoperative anterior–posterior (AP) diameter throughout the cardiac cycle. The rate of reduction of the postoperative AP diameter from end-diastole to end-systole was slightly higher with the MEMO3D compared with the other rings. Data are mean ± SD. AP antero-posterior, RSR rigid saddle ring
The rates of reduction in the CC diameter from end-diastole to end-systole at the postoperative mid-term period were 0.72 ± 1.89, 0.93 ± 1.31, and 0.08 ± 0.82% in the MEMO3D, Physio II, and RSR groups, respectively (Fig. 3). There was no significant difference in this rate among the groups.
At the mid-term postoperative period, the Physio II and RSR maintained the AHCWR values (20.4 ± 1.7 and 21.3 ± 1.7%, respectively) which were equivalent to normal values. However, the MEMO3D had a significantly smaller AHCWR (10.8 ± 3.1%) compared with the other rings (Fig. 4).
Postoperative annular height to commissure width ratio (AHCWR) in end-systole. The AHCWR of the Physio II and RSR reached approximately 20%, which is equivalent to normal values. This finding suggested that the Physio II and RSR restored or preserved the mitral annulus in the three-dimensional saddle shape. However, the AHCWR of the MEMO3D was significantly smaller than that of the other rings. Therefore, the MEMO3D could not restore the annulus. Data are mean ± SD (*p < 0.001). AHCWR annular height to commissure width ratio, RSR rigid saddle ring
The increase in rates of the AHCWR from end-diastole to end-systole at the postoperative mid-term period were 2.1 ± 1.7, 0.1 ± 0.4, and 0.1 ± 0.6% in the MEMO3D, Physio II, and RSR groups, respectively. The MEMO3D had a significantly larger increase in the rate of the AHCWR compared with that in the other groups (Fig. 5).
Increase in rate of the postoperative annular height commissure to width ratio (AHCWR) from end-diastole (ED) to end-systole (ES). The increase in rate of the postoperative AHCWR from ED to ES was significantly larger with the MEMO3D than with the Physio II and RSR. Data are mean ± SD (*p < 0.001). AHCWR annular height to commissure width ratio, ED end-diastole, ES end-systole, RSR rigid saddle ring
Discussion
Generally, evaluating mitral annular dynamics at the mid-term period is difficult because trans-esophageal echocardiography instead of trans-thoracic echocardiography is required to obtain precise data. In this study, we evaluated mitral annular dynamics after ring implantation with ECG-gated 3D-CT, which is thought to be a less invasive modality than trans-esophageal echocardiography. In the mid-term period postoperatively, the MEMO3D ring (semi-flexible ring) was able to preserve one of the mitral annular dynamics, folding dynamics. However, no annular dynamics were observed with the Physio II and RSR. The physiological saddle shape of the mitral annulus was maintained in the Physio II and RSR, but the MEMO3D could not preserve the saddle shape.
The mitral annulus has a saddle-shaped 3D structure. Levine and colleagues evaluated the mitral annulus in 20 adults with two-dimensional echocardiography [1]. They showed that mitral leaflets were concave toward the left ventricle with the non-planar shape of the mitral annulus using the metal ring model. Another study reported that leaflet stress was significantly reduced, with an AHCWR between 15 and 20% [2], which is equivalent to normal values. Therefore, the saddle-shaped ring is designed to contribute to durability of the leaflet after mitral valve repair. Some reports have shown that a saddle-shape ring has lower leaflet stress compared with a flat annular ring [6, 7, 11,12,13]. In this study, Physio II and RSR preserved the physiological annular structure in the mid-term postoperatively. Therefore, these annuloplasty rings might be effective for durability.
A physiological feature of the mitral annulus is annular dynamics. The normal mitral annulus shows some dynamics that correspond with left ventricular contraction throughout the cardiac cycle. In an sheep model, mitral annular contraction was confirmed in the septal–lateral and commissure–commissure directions [4]. The folding dynamics was also introduced in this report. The authors mentioned that the anterior annulus is displaced away from the least-squares annular plane fitted to the posterior annulus toward the left atrium. This is effective in preventing left ventricular out-flow obstruction in the end-systolic phase. We have previously reported that folding dynamics were preserved immediately after mitral annuloplasty with the MEMO3D [8].
Whether mitral annular dynamics are preserved in the mid-term period after mitral annuloplasty is unclear. Some studies on this issue have been published using an acute animal model [3, 10] and there are intraoperative data from trans-esophageal echocardiography [8, 14,15,16]. Precisely evaluating the mitral annulus with trans-thoracic echocardiography can be difficult, although trans-thoracic echocardiography can be performed less invasively compared with trans-esophageal echocardiography. This is the reason why we applied CT scans to evaluate the dynamics of the mitral annulus where the annuloplasty ring was implanted. CT is a less invasive method than trans-esophageal echocardiography and is easily performed at the outpatient clinic. Furthermore, the precise 3D structure can be obtained with ECG-gated scans at any time point during the cardiac cycle. To the best of our knowledge, no reports have evaluated mitral annular dynamics with ECG-gated 3D-CT.
The MEMO3D is a semi-flexible ring that accommodates the annulus to three-dimensional motion. Folding dynamics can be preserved immediately after an operation with the MEMO3D [8, 15]. However, the MEMO3D cannot restore a flattened annulus to the saddle shape, which is in contrast to the Physio II or RSR. Folding dynamics are preserved with the Tailor band to the same degree as controls in an acute animal model [4]. A previous study showed that anterior leaflet strain after ring annuloplasty was higher in the group using a three-dimensional annular ring than that with a flexible annular band, which can preserve annular dynamics [10]. The authors speculated that this strain affects the durability of long-term mitral valve repair [10]. In our study, folding annular dynamics was preserved at the mid-term period postoperatively, as well as during the operation, which we previously reported [8]. The MEMO3D might be able to maintain durability of repair after the operation. However, long-term, clinical trials on this issue are required.
Conclusion
The Physio II and RSR can restore the physiological, three-dimensional, annular shape at the mid-term postoperative period, although the physiological annular dynamics was completely disappeared. The MEMO3D preserves annular dynamics and folding dynamics in the mid-term period postoperatively. However, the MEMO3D cannot restore the annulus to the saddle shape. Therefore, it might be possible to select a suitable annular ring depending on the preoperative three-dimensional annular shape.
References
Levine A, Triulzi O, Harrigan P, Weyman E. The relationship of mitral annular shape to the diagnosis of mitral valve prolapse. Circulation. 1987;75:756–67.
Salgo IS, Gorman JH, Gorman RC, Jackson BM, Bowen FW, Plappert T, et al. Effect of annular shape on leaflet curvature in reducing mitral leaflet stress. Circulation. 2002;106:711–7.
Kvitting JP, Bothe W, Göktepe S, Rausch MK, Swanson JC, Kuhl E, et al. Anterior mitral leaflet curvature during the cardiac cycle in the normal ovine heart. Circulation. 2010;122:1683–9.
Dagum P, Timek T, Green GR, Daughters GT, Liang D, Ingels NB, et al. Three-dimensional geometric comparison of partial and complete flexible mitral annuloplasty rings. J Thorac Cardiovasc Surg. 2001;122:665–73.
Jensen MO, Jensen H, Levine RA, Yoganathan AP, Andersen NT, Nygaard H, et al. Saddle-shaped mitral valve annuloplasty rings improve leaflet coaptation geometry. J Thorac Cardiovasc Surg. 2011;142:697–703.
Padala M, Hutchison RA, Croft LR, Jimenez JH, Gorman RC, Gorman JH, et al. Saddle shape of the mitral annulus reduces systolic strains on the P2 segment of the posterior mitral leaflet. Ann Thorac Surg. 2009;88:1499–504.
Ryan LP, Jackson BM, Hamamoto H, Eperjesi TJ, Plappert TJ, St John-Sutton M, et al. The influence of annuloplasty ring geometry on mitral leaflet curvature. Ann Thorac Surg. 2008;86:749–60 (discussion 749–760).
Ryomoto M, Mitsuno M, Yamamura M, Tanaka H, Fukui S, Tsujiya N, et al. Is physiologic annular dynamics preserved after mitral valve repair with rigid or semirigid ring? Ann Thorac Surg. 2014;97:492–7.
Bothe W, Kvitting JP, Swanson JC, Hartnett S, Ingels NB, Miller DC. Effects of different annuloplasty rings on anterior mitral leaflet dimensions. J Thorac Cardiovasc Surg. 2010;139:1114–22.
Bothe W, Kuhl E, Kvitting JP, Rausch MK, Göktepe S, Swanson JC, et al. Rigid, complete annuloplasty rings increase anterior mitral leaflet strains in the normal beating ovine heart. Circulation. 2011;124:S81–96.
Jensen MO, Jensen H, Smerup M, Levine RA, Yoganathan AP, Nygaard H, et al. Saddle-shaped mitral valve annuloplasty rings experience lower forces compared with flat rings. Circulation. 2008;118:S250–5.
Vergnat M, Jackson BM, Cheung AT, Weiss SJ, Ratcliffe SJ, Gillespie MJ, et al. Saddle-shape annuloplasty increases mitral leaflet coaptation after repair for flail posterior leaflet. Ann Thorac Surg. 2011;92:797–803.
Mahmood F, Gorman JH, Subramaniam B, Gorman RC, Panzica PJ, Hagberg RC, et al. Changes in mitral valve annular geometry after repair: saddle-shaped versus flat annuloplasty rings. Ann Thorac Surg. 2010;90:1212–20.
Nishi H, Toda K, Miyagawa S, Yoshikawa Y, Fukushima S, Kawamura M, et al. Annular dynamics after mitral valve repair with different prosthetic rings: a real-time three-dimensional transesophageal echocardiography study. Surg Today. 2016;46:1083–90.
Tsuneto A, Eishi K, Miura T, Tanigawa K, Matsukuma S, Minami T, et al. Comparison of saddle-shape flexibility and elliptical-shape stability between Cosgrove–Edwards and Memo-3D annuloplasty rings using three-dimensional analysis software. Gen Thorac Cardiovasc Surg. 2016;64:325–32.
Grewal J, Suri R, Mankad S, Tanaka A, Mahoney DW, Schaff HV, et al. Mitral annular dynamics in myxomatous valve disease: new insights with real-time three-dimensional echocardiography. Circulation. 2010;121:1423–31.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ryomoto, M., Mitsuno, M., Yamamura, M. et al. Physiological mitral annular dynamics preserved after ring annuloplasty in mid-term period. Gen Thorac Cardiovasc Surg 65, 627–632 (2017). https://doi.org/10.1007/s11748-017-0805-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-017-0805-x