Abstract
Background
We assessed the mitral annular motion after mitral valve repair with the Sorin Memo 3D® (Sorin Group Italia S.r.L., Saluggia, Italy), which is a unique complete semirigid annuloplasty ring intended to restore the systolic profile of the mitral annulus while adapting to the physiologic dynamism of the annulus, using transesophageal real-time three-dimensional echocardiography.
Methods
17 patients (12 male; mean age 60.4 ± 14.9 years) who underwent mitral annuloplasty using the Memo 3D ring were investigated. Mitral annular motion was assessed using QLAB®version8 allowing for a full evaluation of the mitral annulus dynamics. The mitral annular dimensions were measured throughout the cardiac cycle using 4D MV assessment2® while saddle shape was assessed through sequential measurements by RealView®.
Results
Saddle shape configuration of the mitral annulus and posterior and anterior leaflet motion could be observed during systole and diastole. The mitral annular area changed during the cardiac cycle by 5.7 ± 1.8%.The circumference length and diameter also changed throughout the cardiac cycle. The annular height was significantly higher in mid-systole than in mid-diastole (p < 0.05).
Conclusions
The Memo 3D ring maintained a physiological saddle-shape configuration throughout the cardiac cycle. Real-time three-dimensional echocardiography analysis confirmed the motion and flexibility of the Memo 3D ring upon implantation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mitral valve reconstruction has become the gold standard in the surgical treatment of mitral regurgitation both in ischemic and degenerative mitral valve disease showing excellent long-term results [1]. Mitral valve repair preserving the leaflets and the subvalvular apparatus is often complemented by annuloplasty with a prosthetic ring to correct annular dilatation and allow remodeling [2]. However, a rigid ring stabilization results in a stiff, planar annular area preventing the native mitral annulus to maintain its typical saddle-shaped configuration and natural dynamism during the cardiac cycle. Therefore, flexible rings have been used resulting in less than required annular stabilization, leading to a round and less physiologic mitral orifice over time with increased leaflet stress and thus impaired long-term results of mitral repair [3].
To overcome this dilemma, semirigid mitral annuloplasty rings as the Sorin Memo 3D Annulopasty Ring® (Sorin Group Italia S.r.L., Saluggia, Italy) have been introduced into clinics [4], the partial rigidity restores the physiologic annular ratio, while the flexibility allows for better adaptation to the complex dynamism of the mitral valve during the cardiac cycle. Unlike other semirigid rings, the Memo3D, while planar at implantation, allows for a physiologic saddle-shape configuration during systole and diastole with full flexibility of the annulus during the cardiac cycle. Although the Memo 3D is a complete semirigid annuloplasty ring intended to adapt the physiologic dynamism of the annulus and to accommodate to the three-dimensional motion of the annulus, there is little information about these annular dynamics throughout the cardiac cycle. In this study, we assessed the mitral annular dynamics after mitral valve repair with the Memo 3D ring using transesophageal full-volume real-time three-dimensional echocardiography and various novel analysis software programs.
Patients and methods
We evaluated 17 patients with mitral valve reconstruction in the study (Table 1). Written informed consent was obtained by all patients for both implantation of the approved ring and intraoperative transesophageal echocardiographic evaluation. The transesophageal echocardiographic evaluation is a routine technique performed in our department to verify the proper valve function in every mitral valve reconstruction. Approval by the local ethics committee was obtained.
Surgical technique
All patients were operated under general anesthesia and the heart was accessed either via full sternotomy or right mini-thoracotomy according to surgical indications. Cardiopulmonary bypass was initiated in usual fashion, and cardioplegic arrest was induced. After opening the left atrium in the typical position, a detailed analysis of the valve pathology was performed. Mitral valve reconstruction was achieved by resecting the prolapsing valve leaflet portions and suturing with polypropylene 5–0 sutures, if excessive leaflet materials was identified as main cause for regurgitation, or by chordae replacement techniques using loop technique, respectively. After sizing the mitral valve annulus and the anterior leaflet of the mitral valve according to the instructions for use of the manufacturer, with special respect to the antero-posterior (AP) and anterolateral–posteromedial (AL–PM) diameter, proper size ring was implanted in each patient using interrupted stitches. Competence of the mitral valve was confirmed by water testing prior to closure of the left atrium. After cross-clamp release, proper reperfusion and deairing extracorporeal circulation was terminated without inotropic support in all patients. Transesophageal echocardiography after chest closure confirmed proper valve function in all patients and further investigations were done before transferring the patient to the intensive care for further uneventful treatment.
Image acquisition and intraoperative transesophageal echocardiography
A Phillips iE-33® (Phillips Medical Systems, Bothell, Wash, USA) X7-2t transesophageal echocardiography probe was used for all patients in the usual technique. After performing the routine echocardiographic investigation of the mitral valve competence, hemodynamic performance, ventricular function, and the completeness of deairing at the end of extracorporeal circulation, additional real-time three-dimensional echocardiography was performed to obtain full volume data in hemodynamically stable situations without any inotropic support and under general anesthesia using QLAB®version8 (Phillips Medical Systems, Bothell, Wash, USA). Electrocardiographically gated full-volume images were acquired over four cardiac cycles at a frame rate of 17–30 frames/s. After gain optimization, images were recorded and stored on digital versatile discs for further offline analysis to transfer to a Windows-based workstation for analysis by the TomTec Image Arena Browser (TomTec Imaging Systems, GmbH, Munich, Germany) and RealView® (YD, Nara, Japan). The MV geometric analysis was performed using the TomTec 4D MV Assessment 2.0 program (TomTec Imaging Systems GmBH Munich, Germany). The methodology for intraoperative dynamic geometric analysis has been carried out as previously described [5].
-
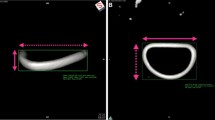
mitral annular motion was assessed using QLAB®version8 (Phillips Medical Systems, Bothell, Wash, USA) (Fig. 1a)
Example of a 65-year-old patient with severe mitral regurgitation due to P2 prolapse after surgical reconstruction using artificial chordae with loop technique and mitral annuloplasty using a 30 mm MEMO 3D ring; a three-dimentional picture was displayed using QLAB. Upper figures shows the mitral valve after implantation of Memo-3D at mid-systolic phase from two different direction (right and left). Lower figures revealed those at mid-diastolic phase. b Graphical illustration of leaflet and annular structures motion was demonstrated in 3D by 4D MV assessment 2. Left figures show mitral annulus and mitral valve leaflets at mid-systolic phase (upper) and mid-diastolic phase (lower). Blue dot indicated the center of anterior leaflet annulus, and red dot indicated the center of posterior leaflet. Right figures focused on the motion of mitral annulus at mid-systolic phase (upper) and mid-diastolic phase (lower). Yellow line indicated mitral annulus,and white circle indicated the position of aortic valve
-
mitral annular dimensions, especially circumference area and length, annular antero-posterior (AP) and anterolateral–posteromedial (AL–PM) diameter were assessed throughout the cardiac cycle using 4D MV assessment2 (Fig. 1b)
-
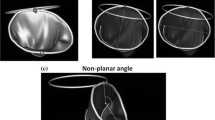
for analysis of annular height, we checked sequential measurements in 6 phases of the cardiac cycle by RealView (Fig. 2). Annular height was defined as the maximum difference in mm between the highest and the lowest point of mitral annulus in early, mid- and late systole, and in early, mid- and late diastole.
Statistical analysis
Data were analyzed using the Statview 5.0 program (SAS Institute Inc., Cary, NC). Results are expressed as the mean ± standard deviation. For descriptive analysis, all data are expressed as mean and standard deviation. For comparison of mitral annulus dimensions during cardiac cycle, a Mann–Whitney U test was used, defining a p value < 0.05 to be significant.
Results
Patient characteristics (Table 1)
The mean age of all mitral valve repair groups was 60 ± 15 years and 71% were male. Left ventricular ejection fraction was similar among all groups. Loop technique was utilized in 7 patients (41%). Concomitant procedures included coronary artery bypass grafting in one case, maze procedures in two cases of permanent atrial fibrillation and tricuspid annuloplasty in another case. The mean ring size was 28.9 ± 2.1 mm. All patients had degenerative mitral regurgitation.
Annular motion after MV repair
QLAB analysis retained full data sets in all cases and qualitative analysis overall showed the motion of mitral annulus throughout the cardiac cycle on both anterior and posterior portions (Fig. 1a). The results of 4D MV assessment in this study group showed overall the motion of mitral annulus throughout the cardiac cycle on both anterior and posterior leaflet side. The mitral annulus had a pronounced saddle-shape configuration in systolic phase as well as in diastolic phase. Also, both flexible anterior and posterior annular motion was observed (Fig. 1b).
Annular changes after MV repair
Mitral annular area changed during the cardiac cycle (increased in diastole; reduced in systole; percent reduction 5.7 ± 1.8%). Circumference length, AP diameter, and AL–PM diameter also changed throughout the cardiac cycle (Fig. 3).
Annular height in mid-systole was significantly higher than in mid-diastole (p < 0.05). Annular height increased markedly from early-systole to mid-systole, and decreased rapidly from early-diastole to mid-diastole (Fig. 4).
Discussion
In the present study, we show the structure of the mitral annulus, and its dynamics between mid-diastole and mid-systole after MV repair using the Memo 3D ring. Intraoperative transesophageal RT3DE was performed to calculate these values in vivo and allowed the acquisition of different parameters of the mitral annulus with commercially available software. It represents one of the first studies to fully assess changes of the mitral annulus before and after MV repair using a certain mitral ring. This imaging modality has proven to be suitable to evaluate not only the MR degree but also the annular dynamics and changes before and after surgery. Furthermore, it allows to acquire various measurements at closed heart conditions that confirm those findings obtained under direct inspection during cardiac surgery. Our findings provide several novel insights in MV repair using prosthetic rings providing valuable information to clarify the effects of a certain prosthetic ring on the mitral annular dynamics and remodeling in patients undergoing MV repair.
In case of severe mitral regurgitation, either due to ischemic cardiomyopathy or degenerative diseases, mitral reconstruction using various techniques of leaflet reconstruction combined with mitral annuloplasty has become the treatment of choice [1]. Traditional annuloplasty rings are rigid and planar, fixing the mitral valve annulus into unphysiologic configuration with negative effects on leaflet motion as well as fixation of the mitral orifice area during cardiac cycle [6]. In long-term study, rigid rings have shown worse outcomes in terms of improvement of ejection fraction and preservation of mitral valve areas during cardiac cycle without additional effects on overall survival, mortality, reoperation, recurrence of regurgitation, and left ventricular performance [3].
In contrast, fully flexible rings and repair with autologous pericardium result in favorable mitral annulus dynamics and preserved left ventricular function during stress conditions [7]. Unfortunately the use of flexible rings results in a significant lower level of annulus stabilization over the time, leading to a loss of the natural 2:3 antero-posterior to anterolateral–posteromedial diameter, and eventually resulting in a more or less round and unphysiologic mitral orifice [3]. Flexible mitral annuloplasty rings have, therefore, failed to further improve symptoms and survival as compared to semi-rigid rings in short term studies [8], since the natural annular saddle shape acts to optimize the leaflet curvature and thus to reduce peak leaflet stress especially at the anterior leaflets [9].
The concept of the Memo 3D bases on restoring the natural anterior/posterior to lateral/lateral relationship (annular remodeling) while allowing the natural three-dimensional motion of the mitral annulus during cardiac cycle. In limited, selective prospected single center trials with short- and medium-term follow-up, the Memo 3D showed excellent hemodynamic results preserving both mitral annular flexibility and functionality through standard two-dimensional transthoracic echocardiography [4, 10].
Recent studies have demonstrated the suitability of modern real-time three-dimensional echocardiography in terms of the shape of the annuloplasty ring and its height during cardiac cycle. So far confirmation of the preservation of mitral annulus flexibility after implantation of a Memo 3D up to 5 years has been performed by means of conventional transthoracic echocardiography [10], thus with the limitation of planar analysis only. We selected this prosthetic ring to assess the usefulness of real-time three-dimensional echocardiography evaluation because of its unique feature. In this study, the use of real-time three-dimensional echocardiography demonstrated that the use of this annuloplasty ring allows for a saddle-shaped configuration whilst stabilizing the mitral annulus. Furthermore, it was showed that the annular dynamics throughout the cardiac cycle closely mimic the normal mitral valve motion by Topilsky et al. [11]. They described that annular height increased markedly in early-systole, and decreased in late diastole in normal patients, Although they did not show the significant difference of inter commissure length between systolic and diastolic phase, annular area, AP diameter, and circumference length changed significantly between systolic and diastolic phase like our results. However, the degree of change between two phases was higher in normal subjects compared to our results. Further studies need to investigate the long-term effect of the preservation of this natural saddle-shaped configuration after mitral annuloplasty both in terms of valvular function over time and preservation or even restoration of ventricular function [12].
Study limitations
There are several potential limitations with this study. First, the relatively small number of patients in this study represents limitation. Another limitation is the single time of investigation at the end of the operation. As it was difficult to obtain the data of whole mitral annulus during cardiac cycle before operation due to difficult motion capture, we were unable to investigate the comparison before and after operation. Also, follow-up RT3DE will be necessary to reveal the long-term flexibility as demonstrated here.
Conclusion
In this study, the Memo 3D ring demonstrates a saddle-shape configuration throughout the cardiac cycle despite its planar shape before implantation, which showed its shape to the physiologic dynamism of the mitral annulus. Real-time three-dimensional echocardiography analysis using various analysis software programs was useful to show detailed motion and flexibility of the mitral prosthetic ring upon implantation.
References
American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 2006;114:e84–e231.
Nardi P, Pellegrino A, Olevano C, Scafuri A, Lio A, Polisca P, et al. Mitral valve repair for the treatment of degenerative mitral valve disease with or without prosthetic ring annuloplasty: long-term outcomes. J Cardiovasc Surg (Torino) 2013;54:305–12.
Hu X, Zhao Q. Systematic evaluation of the flexible and rigid annuloplasty ring after mitral valve repair for mitral regurgitation. Eur J Cardiothorac Surg. 2011;40:480–7.
Bruno PG, Leva C, Santambrogio L, Lazzarini I, Musazzi G, Del Rosso G, et al. Early clinical experience and echocardiographic results with a new semirigid mitral annuloplasty ring: the Sorin Memo 3D. Ann Thorac Surg. 2009;88:1492–8.
Sugeng L, Coon P, Weinert L, Jolly N, Lammertin G, Bednarz JE, et al. Use of real-time 3-dimensional transthoracic echocardiography in the evalu ation of mitral valve disease. J Am Soc Echocardiogr. 2006;19:413 – 21.
Dall’Agata A, Taams MA, Fioretti PM, Roelandt JR, Van Herwerden LA. Cosgrove-Edwards mitral ring dynamics measured with transesophageal three-dimensional echocardiography. Ann Thorac Surg. 1998;65:485–90.
Borghetti V, Campana M, Scotti C, Domenighini D, Totaro P, Coletti G, et al. Biological versus prosthetic ring in mitral-valve repair: enhancement of mitral annulus dynamics and left-vent, ricular function with pericardial annuloplasty at long-term. Eur J Cardiothorac Surg. 2000;17:431–9.
Chee T, Haston R, Togo A, Domenighini D, Totaro P, Coletti G, et al. Is a flexible mitral annuloplasty ring superior to semi-rigid or rigid ring in terms of improvement in symptoms and survival? Interact Cardiovasc Thorac Surg. 2008;7:477–84.
Jimenez JH, Liou SW, Padala M, He Z, Sacks M, Gorman RC, et al. A saddle-shaped annulus reduces systolic strain on the central region of the mitral valve anterior leaflet. J Thorac Cardiovasc Surg. 2007;134:1562–8.
Santarpino G, Pfeiffer S, Fischlein T. First-in-man implantation of a Sorin Memo 3D ring: mitral annular flexibility is still preserved at 5 years of follow-up! Int J Cardiol. 2012;23;159:e23–e4.
Topilsky Y, Vaturi O, Watanabe N, Bichara V, Nkomo VT, Michelena H, et al. Real-time 3-dimensional dynamics of functional mitral regurgitation: a prospective quantitative and mechanistic study. J Am Heart Assoc. 2013;2:e000039.
Vergnat M, Levack MM, Jassar AS, Jackson BM, Acker MA, Woo YJ, et al. The influence of saddle-shaped annuloplasty on leaflet curvature in patients with ischaemic mitral regurgitation. Eur J Cardiothorac Surg. 2012;42:493–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Rights and permissions
About this article
Cite this article
Nishi, H., Toda, K., Miyagawa, S. et al. Annular dynamics of memo3D annuloplasty ring evaluated by 3D transesophageal echocardiography. Gen Thorac Cardiovasc Surg 66, 214–219 (2018). https://doi.org/10.1007/s11748-018-0886-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-018-0886-1