Abstract
Enzymatic epoxidation of vegetable oils in the presence of free fatty acids has been well studied in recent years, by mainly using long chain fatty acids (e.g., stearic acid) as the active oxygen carrier. However, for the previous enzymatic processes, the acid value (AV) of final epoxidized oils using long chain fatty acids is high, and the free fatty acid is not easily removed in the post treatment with water. Aiming at developing a more sustainable process, enzymatic epoxidation of sunflower oil was revisited using different free fatty acids catalyzed by Novozym 435 (lipase B from Candida antarctica, provided by Novozymes, Bagsvaerd, Denmark). When long chain stearic acid was introduced into the epoxidation in toluene solvent, the epoxy oxygen group content (EOC) of 6.41 ± 0.19 % was obtained. Due to the poor water solubility of stearic acid, the AV of the final epoxidized oil product was very high (53.40 ± 1.34) after it was washed with water. Alternatively, current study shows that the epoxidation process using short chain butyric acid produced the final epoxidized oil with lower AV of 2.57 ± 0.11. When the enzymatic epoxidation of sunflower oil was optimized in the presence of butyric acid and Novozym 435, EOC of 6.84 ± 0.21 % was obtained, reaching an oxriane conversion of 96.4 ± 3.0 %. Therefore, introducing short chain butyric acid as an active oxygen carrier will provide an alternative to the present enzymatic epoxidation process and produce the desired epoxidized oil products with much lower AV only after simple water-treatments, which will make the enzymatic epoxidation more attractive.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
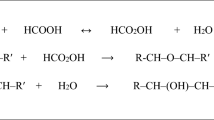
Epoxidized vegetable oils can be used as environmentally friendly lubricants, plasticizers, rubber-like materials and polymer stabilizers [1–5]. Up to now, various plant oils (e.g., from soybean, sunflower, canola, and rapeseed) have been investigated as epoxidation materials. One of the main methods to produce epoxidized vegetable oil is the Prileshajev-epoxidation of unsaturated plant oils by peracetic acid [6], which involves the use of strong mineral acids (e.g., H2SO4) (Scheme 1). However, the use of strong mineral acids to catalyze peroxy acid formation often results in undesired oxirane ring-opening reactions and corrosion problems [7, 8].
Compared with the classical chemical protocol, enzymatic epoxidation of vegetable oils has attracted more and more attention in recent years [9–16]. Formation of stable peracids directly from free fatty acids (Scheme 1), significant suppression of side reactions, high conversion and mild reaction conditions make it superior to chemical protocols [17]. Meanwhile, enzymatic epoxidation of vegetable oils obtains relatively high epoxy oxygen group content (EOC) using long chain fatty acids (mainly stearic acid) as an active oxygen carrier [14–16]. However, for the enzymatic process, the acid value (AV) of final epoxidized oils using stearic acid is high and the free fatty acid is not easily removed in the post treatment with water, and alkali treatment is not preferred because generated soaps can hinder epoxidized oil separation (Fig. 1). Therefore, a suitable fatty acid, which is easily removed from epoxidized oils after reaction workup, is very much required to increase the efficiency of this well-established enzymatic epoxidation method.
To the best of our knowledge, short chain (C1-4) fatty acids, such as formic and acetic acids, have been mainly used in strong mineral acid-catalyzed epoxidation of plant oils but not for enzymatic epoxidation. Sunflower oil is rich in polyunsaturated fatty acids (~85 %), which makes it useful as a potential raw material for production of epoxy products. In this work, butyric acid has been successfully used as an active oxygen carrier in the presence of immobilized lipase Novozym 435 for epoxidation of sunflower oil using hydrogen peroxide (H2O2) as an oxygen donor to convert free fatty acid into peroxy acid for the first time. Solvents were found to have a significant impact on the enzymatic epoxidation reaction. Notably, employing butyric acid as an active oxygen carrier, the final epoxidized oil was obtained with high EOC and very low AV.
Materials and Methods
Materials
Sunflower oil with an iodine value (IV) of 122 g I2/100 g was purchased from a local supermarket (Zhengzhou, China) (fatty acids composition (wt%): C16:0, 6.4 %; C18:0, 6.1 %; C18:1, 30.0 %; C18:2, 57.5 %; AV = 0.15mgKOH/g). Hydrogen peroxide was purchased from Luoyang Chemical Reagent Co., Ltd (Luoyang, China) as 30 % w/w solution. Acetic acid (purity >99 %), butyric acid (purity >99 %), caproic acid (purity >98 %), capric acid (purity >99 %) and lauric acid (purity >99 %) were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Caprylic acid (purity >99 %), myristic acid (purity >99 %), palmitic acid (purity >99 %) and stearic acid (purity >99 %) were purchased from Sigma-Aldrich Co. (St. Louis, MO). Immobilized lipase (Novozym 435, Candida antarctica lipase immobilized on polyacrylic resin, 10,000 PLU/g) was purchased from Novozymes A/S (Bagsvaerd, Denmark). All other reagents were analytical grade and used without further purification.
Reaction Procedure
The epoxidation reaction was carried out in a 150-mL three-neck round-bottom flask and placed in a water bath with reflux condensation. Firstly, 5.0 g of sunflower oil, calculated amounts of free fatty acid and lipase, and 30 mL of nonpolar solvent (e.g., toluene or benzene) were added to the reactor in the order as listed. Then the reaction mixture was heated to 50 °C and homogenized at 800 rpm with an IKA magnetic stirrer (IKA, Guangzhou, China). Secondly, 10 mL of 30 %(w/w) hydrogen peroxide was added dropwise in 10 min to the reaction mixture through a funnel. After complete addition of hydrogen peroxide, the epoxidation reaction was continued for the desired time (Note: because benzene is a carcinogen, it was handled with extreme caution).
General Procedure for Purification of the Epoxidation Product
After completion of the epoxidation reaction, Novozym 435 was removed by filtration. The filtrate was washed five times using distilled hot water (70 °C), and then dried with anhydrous sodium sulfate (Na2SO4) for 30 min at room temperature. Solids were removed by the filtration procedure as described above. Finally, the solvent and the remaining trace amount of water were removed by a rotary evaporator at vacuum below 1 mmHg, and the epoxidized sunflower oil was obtained.
Analytical Techniques
Epoxy oxygen group content (EOC) was determined by the titration method with hydrobromic acid solution in acetic acid [18]. From the oxirane content, the relative percentage conversion to oxirane was determined by the following formula:
where OOex is the content of oxirane oxygen experimentally determined, and OOth is the theoretical maximum oxirane oxygen content determined by the following equation:
where A i(126.9) and A o(16.0) are the atomic weights of iodine and oxygen respectively, and IV0 is the initial iodine value of the sunflower oil. The theoretical maximum oxirane oxygen content in 100 g of sunflower oil (OOth) is 7.1 %.
Results and Discussion
Based on the previous reports on enzymatic epoxidation of vegetable oils [14–17], the enzymatic epoxidation of sunflower oil was investigated in the presence of commonly used long chain stearic acid (C18:0) and immobilized lipase Novozym 435. With the increase of reaction time (Fig. 2a), the EOC of epoxidized sunflower oil increased rapidly and reached the maximum (6.41 ± 0.19 %) after 6 h. Further reaction time (8–10 h) led to decreased EOC values (5.45 ± 0.15 % ~ 5.75 ± 0.22 %,) due to hydrolysis of the epoxy group, which had been also observed in previous reports [16, 17].
Effect of reaction time (a), reaction temperature (b), amount of hydrogen peroxide (c), enzyme load (d), and amount of stearic acid (e) on the epoxy oxygen group content (EOC) of epoxidized sunflower oil in toluene. (a enzyme load 3 % (related to oil), hydrogen peroxide (10 mL, molar ratio of H2O2/C=C-bonds = 3.7:1), stearic acid (1.0 mol/1000 g), and 50 °C; b enzyme load 3 % (related to oil), hydrogen peroxide (10 mL), stearic acid (1.0 mol/1000 g), and 6 h; c enzyme load 3 % (related to oil), stearic acid (1.0 mol/1000 g), 50 °C, and 6 h; d hydrogen peroxide (10 mL), stearic acid (1.0 mol/1000 g), 50 °C, and 6 h; e enzyme load 3 % (related to oil), hydrogen peroxide (10 mL), 50 °C, and 6 h)
The effect of reaction temperature on the enzymatic epoxidation of sunflower oil is shown in Fig. 2b. With the increase of reaction temperature (30–70 °C), the EOC of epoxidized sunflower oil first increased up to 6.41 ± 0.19 % (50 °C) and then decreased with higher temperature. Higher reaction temperature may lead to fast reaction rates, but to undesired side reactions, such as hydrolysis of the epoxy groups or ester groups.
The effect of hydrogen peroxide (H2O2) amounts was also studied, and the results are presented in Fig. 2c. A bell-shaped relationship exists for hydrogen peroxide content vs EOC. So 10 mL of hydrogen peroxide (molar ratio of H2O2/C=C-bonds of 3.7:1) was the optimal amount in this work. By adding H2O2, H2O is also added. Excess amounts of H2O may lead to hydrolysis of the epoxy groups or ester groups.
The amount of immobilized lipase Novozym 435 was also investigated in the range of 1 ~ 5 % (wt%, related to oil) (Fig. 2d). The results showed that an increase in the amount of immobilized lipase Novozym 435 (1 ~ 3 %) was accompanied by an increase in the EOC of epoxidized sunflower oil from 5.72 ± 0.23 % to 6.41 ± 0.19 %. By further increasing the amount of immobilized lipase Novozym 435 (4 ~ 5 %), EOC of epoxidized oil showed a slightly decrease.
The effect of stearic acid concentration was investigated for concentrations ranging from 0.4 to 1.6 mol/1000 g (related to oil) (Fig. 2e). The results showed that the maximum EOC (6.41 ± 0.19 %) of epoxidized sunflower oil (6 h) was obtained when 1.0 mol/1000 g (related to oil) of stearic acid was added. Further increasing the amount of stearic acid (1.2 mol/1000 g or 1.6 mol/1000 g) decreased the EOC of the final epoxidized sunflower oil. Also, a high content of free fatty acid remaining in the final product is undesirable. Though free fatty acid can be removed from the reaction mixture by alkali treatment, this procedure requires an extra production step, which makes the epoxidation less economical [13].
Though long chain free fatty acids (e.g., stearic acid) were favored for improving the efficiency of enzymatic epoxidation of plant oils in most cases [14–16], using long chain free fatty acids still had some drawbacks, such as the high acid value (AV) that existed in the final epoxidized products. The long chain free fatty acids cannot be removed easily from the epoxidized oil through the simple water-washing workup (Fig. 1), which would undoubtedly affect the properties of the final epoxidized products. Aiming at developing a more sustainable process for enzymatic epoxidation of vegetable oils, a set of saturated free fatty acids with different carbon chain lengths were investigated as oxygen donors based on the optimized reaction conditions obtained for stearic acid (Fig. 2), noting the solubility of free fatty acid in water increases as the carbon chain of free fatty acid decreases [19]. With increasing chain length of free fatty acids up to 18 carbons, the EOC of epoxidized sunflower oil increased to the maximum (6.41 ± 0.19 %) in the presence of toluene solvent (Table 1). However, adding stearic acid (C18:0) to the reaction system as oxygen carrier led to high AV of 53.40 ± 1.34 (mgKOH/g) (Table 1). Using the same amount of palmitic acid (C16:0) only slightly decreased EOC (6.16 ± 0.29 %) and achieved a similar AV (52.59 ± 2.40). The use of medium chain free fatty acids (C8:0–C14:0) produced moderately high EOC (5.09 ± 0.21 % ~ 5.57 ± 0.12 %) and similarly high AV (54.86 ± 0.99 ~ 55.98 ± 2.04). When the chain length of the free fatty acids decreased to six carbons, the epoxidized oil possessed a moderate EOC of 5.51 ± 0.17 % and a greatly lower AV of 10.44 ± 0.29. The use of butyric acid (C4:0) yielded epoxidized sunflower oil with a moderate EOC of 4.26 ± 0.16 % and relatively low AV of 4.55 ± 0.16. However, further decreasing the carbon chain length to 2 (acetic acid) did not efficiently promote enzymatic epoxidation (EOC of 0.45 ± 0.02 %) though lower AV (2.29 ± 0.11) was obtained.
Considering the outcome of enzymatic epoxidation (EOC) and AV in the final epoxidized oils, butyric acid (C4:0) was chosen as the active oxygen carrier and different common nonpolar organic solvents were compared in enzymatic epoxidation of sunflower oil. When toluene was replaced with benzene as the solvent, epoxidized sunflower oil was obtained with lowest AV of 2.57 ± 0.11 and highest EOC of 6.85 ± 0.24 % (Table 2), which reached the highest conversion of 96.5 ± 3.4 %. Other commonly used nonpolar solvents, such as hexane, cyclohexane, ethyl acetate and chloroform appeared to provide lower efficiency (EOC = 0.16 ± 0.01 % ~ 3.76 ± 0.17 %), though a lower AV (0.45 ± 0.06 ~ 6.09 ± 0.16) was observed (Table 2). In addition, a control experiment confirmed that no epoxidation conversion was observed in the absence of immobilized lipase Novozym 435.
The enzymatic epoxidation of sunflower oil was further optimized in the presence of butyric acid (C4:0), benzene as solvent and immobilized lipase Novozym 435 (Fig. 3). With the increase of reaction time from 2 to 6 h (Fig. 3a), the EOC of the epoxidized oil increased rapidly from 4.18 ± 0.19 % to 6.85 ± 0.24 %, and similar EOC (6.84 ± 0.26 %) was observed after 5 h. Further prolonging the reaction time to 8–10 h led to the decreased EOC values (6.28 ± 0.19 % to 6.64 ± 0.17 %) due to partial hydrolysis of the epoxide bonds. In addition, the AV increased in general when the reaction time ranged from 2 to 10 h, and medium AV (2.52 ± 0.15) was observed after 5 h (Fig. 4a). Therefore, 5 h was chosen as the most favorable reaction time.
Effect of reaction time, temperature, amount of hydrogen peroxide, enzyme load, and amount of butyric acid on the enzymatic epoxidation of sunflower oil in benzene. (a enzyme load 3 % (related to oil), hydrogen peroxide (10 mL, molar ratio of H2O2/C=C-bonds = 3.7:1), butyric acid (1.0 mol/1000 g), and 50 °C; b enzyme load 3 %, hydrogen peroxide (10 mL), butyric acid (1.0 mol/1000 g), and 5 h; c enzyme load 3 %, butyric acid (1.0 mol/1000 g), 50 °C, and 5 h; d hydrogen peroxide (10 mL), butyric acid (1.0 mol/1000 g), 50 °C, and 5 h; e enzyme load 3 %, hydrogen peroxide (10 mL), 50 °C, and 5 h)
Effect of reaction time, temperature, amount of hydrogen peroxide, enzyme load, and amount of butyric acid on the enzymatic epoxidation in benzene. (a enzyme load 3 %, hydrogen peroxide (10 mL), butyric acid (1.0 mol/1000 g), and 50 °C; b enzyme load 3 %, hydrogen peroxide (10 mL), butyric acid (1.0 mol/1000 g), and 5 h; c enzyme load 3 %, butyric acid (1.0 mol/1000 g), 50 °C, and 5 h; d hydrogen peroxide (10 mL), butyric acid (1.0 mol/1000 g), 50 °C, and 5 h; e enzyme load 3 %, hydrogen peroxide (10 mL), 50 °C, and 5 h)
The effect of reaction temperature on the enzymatic epoxidation of sunflower oil was studied. With the increase of reaction temperature (30–50 °C), the EOC of epoxidized sunflower oil increased significantly (Fig. 3b). The maximum EOC (6.84 ± 0.26 %) of epoxidized sunflower oil was obtained at 50 °C. Higher reaction temperatures (60–70 °C) provided epoxidized sunflower oil with lower EOC values (4.35 ± 0.22 % to 6.55 ± 0.16 %). The AV of epoxidized sunflower oil changed slightly (1.98 ± 0.18–2.87 ± 0.16) (Fig. 4b), which was not affected by the reaction temperature obviously. Therefore, 50 °C was chosen as the most suitable reaction temperature.
The optimization of the amount of hydrogen peroxide is important mainly for lowering the cost by reducing the amount of unreacted peroxide at the end of the reaction. With increasing the amount of hydrogen peroxide reagent from 3 mL (molar ratio of H2O2/C=C-bonds = 1.1:1) to 10 mL (molar ratio of H2O2/C=C-bonds = 3.7:1), the EOC of epoxidized sunflower oil increased from 3.52 ± 0.14 % to 6.84 ± 0.26 % (Fig. 3c). A further increase of hydrogen peroxide reagent caused a slight decline of conversion. Moreover, the AV of epoxidized sunflower oil increased slightly (1.87 ± 0.12–2.89 ± 0.17) (Fig. 4c), which indicated that the higher amount of water that accompanies the hydrogen peroxide reagent may lead to hydrolysis of the triglycerides’ ester bonds. So 10 mL (molar ratio of H2O2/C=C-bonds = 3.7:1) of hydrogen peroxide was the optimum amount in this work.
Figure 3d showed that the formation of epoxide groups was strongly affected by the catalyst concentration, if the amount of lipase was lower than 3.0 wt% of oil. The results showed that the EOC of epoxidized sunflower oil offered 6.84 ± 0.26 % in the presence of 3.0 % immobilized lipase Novozym 435, below which the efficiency of epoxidation rapidly decreased. Further increasing the amount of immobilized lipase Novozym 435 (4–5 %) led to lower EOC values (6.56 ± 0.22 % to 6.65 ± 0.19 %) due to the hydrolysis of the triglycerides. The AV of epoxidized sunflower oil increased (1.35 ± 0.11–5.42 ± 0.35), when the amount of lipase was increased from 1 to 5 % (Fig. 4d), which was related to lipolysis of the triglycerides.
In the final step, butyric acid was used in concentrations ranging from 0.4 to 1.4 mol/1000 g (related to oil) to study the influence of FFA concentration on conversion (Fig. 3e). The results showed that the maximum EOC (6.82 ± 0.21 %–6.84 ± 0.26 %) of epoxidized sunflower oil was obtained when 0.8–1.0 mol/1000 g (related to oil) of butyric acid was used. Further increasing the amount of butyric acid (1.2 mol/1000 g or 1.4 mol/1000 g) could not increase the EOC of the final epoxidized sunflower oil. Importantly, a high content of free fatty acid remaining in the final product was undesirable. The AV of final epoxidized oil decreased from 3.22 ± 0.18 to 2.52 ± 0.15, when the concentration of butyric acid was increased from 0.4 to 1.0 mol/1000 g, and did not change from 1.0 to 1.4 mol/1000 g butyric acid (Fig. 4e).
Conclusions
The present study indicated that enzymatic epoxidation of sunflower oil was successfully achieved under mild conditions using immobilized lipase Novozym 435 as catalyst. Using saturated long carbon chain free fatty acid (e.g., stearic acid) led to good oxriane conversion of 90.3 ± 2.7 % and EOC of 6.41 ± 0.19 % of the epoxidized sunflower oil with high AV of 53.40 ± 1.34. While using the same molar amount of short chain free fatty acid butyric acid as a peracid precursor in benzene could produce the desired epoxidized sunflower oil product with much lower AV of 2.57 ± 0.11. Moreover, the enzymatic epoxidation of sunflower oil was optimized in the presence of butyric acid (C4:0) and immobilized lipase Novozym 435 and excellent EOC of 6.84 ± 0.21 % was obtained, which could reach to the highest oxriane conversion of 96.4 ± 3.0 %. Therefore, introducing short carbon chain butyric acid as an active oxygen carrier will provide an alternative to the present enzymatic epoxidation process and produce the desired epoxidized oil products with much lower AV only after simple water treatments, which will make the enzymatic epoxidation more attractive. A future goal is to replace benzene with a more environmentally friendly and lower toxicity solvent.
References
Biermann U, Friedt W, Lang S (2000) New syntheses with oils and fats as renewable raw materials for the chemical industry. Angew Chem Int Ed 39:2206–2224
Wu X, Zhang X, Yang S (2000) The study of epoxidized rapeseed oil used as a potential biodegradable lubricant. J Am Oil Chem Soc 77:561–563
Hwang HS, Erhan SZ (2001) Modification of epoxidized soybean oil for lubricant formulations with improved oxidative stability and low pour point. J Am Oil Chem Soc 78:1179–1184
Sharma BK, Adhvaryu A, Liu Z (2006) Chemical modification of vegetable oils for lubricant applications. J Am Oil Chem Soc 83:129–136
Campanella A, Rustoy E, Baldessari A (2010) Lubricants from chemically modified vegetable oils. Bioresour Technol 101:245–254
Formo MW (1982) Miscellaneous oil and fat products. In: Swern D (ed) Bailey’s industrial oil and fat products, 4th edn. Wiley, New York, pp 366–371
Sonnet PE, Lankin ME, McNeill GP (1995) Reactions of dioxiranes with selected oleochemicals. J Am Oil Chem Soc 72:199–204
Rüsch GKM, Warwel S (1999) Complete and partial epoxidation of plant oils by lipase-catalyzed perhydrolysis. Ind Crops Prod 9:125–132
Gen Klaas MR, Warwel S (1996) Chemoenzymatic epoxidation of unsaturated fatty acid esters and plant oils. J Am Oil Chem Soc 73:1453–1457
Hilker I, Bothe D, Prüss J (2001) Chemo-enzymatic epoxidation of unsaturated plant oils. J Chem Eng Sci 56:427–432
Piazza GJ, Foglia TA, Nuñez A (2001) Optimizing reaction parameters for the enzymatic synthesis of epoxidized oleic acid with oat seed peroxygenase. J Am Oil Chem Soc 78:589–592
Orellana-Coca C, Törnvall U, Adlercreutz D (2005) Chemo-enzymatic epoxidation of oleic acid and methyl oleate in solvent-free medium. Biocatal Biotransfor 23:431–437
Vlček T, Petrović ZS (2006) Optimization of the chemoenzymatic epoxidation of soybean oil. J Am Oil Chem Soc 83:247–252
Sun SD, Yang GL, Bi YL (2011) Enzymatic epoxidation of corn oil by perstearic acid. J Am Oil Chem Soc 88:1567–1571
Lu H, Sun S, Bi YL (2010) Enzymatic epoxidation of soybean oil methyl esters in the presence of free fatty acids. Eur J Lipid Sci Tech 112:1101–1105
Sun SD, Ke X, Cui LL (2011) Enzymatic epoxidation of Sapindus mukorossi seed oil by perstearic acid optimized using response surface methodology. Ind Crops Prod 33:676–682
Daniel M-S, Nicolás RL, Vicente G, Vicente G-F (2014) Chemoenzymatic epoxidation of alkenes based on peracid formation by a Rhizomucor miehei lipase-catalyzed perhydrolysis reaction. Tetrahedron 70:1144–1148
Paquot C, Hautfenne A (1987) Standard methods for the analysis of oils, fats and derivatives, 7th edn. Blackwell Scientific Publications, Oxford, pp 118–119
John LM, McBain JW (1948) The hydrolysis of soap solutions. II. The solubilities of higher fatty acids. J Am Oil Chem Soc 25:40–41
Acknowledgments
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (No. 21102036), the Young-aged Backbone Teacher Funds of Henan Province of China (No. 2014GGJS-058) and Basic Research Funds in Henan Universities of Henan University of Technology (No. 2015RCJH01).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Liu, W., Chen, J., Liu, R. et al. Revisiting the Enzymatic Epoxidation of Vegetable Oils by Perfatty Acid: Perbutyric Acid Effect on the Oil with Low Acid Value. J Am Oil Chem Soc 93, 1479–1486 (2016). https://doi.org/10.1007/s11746-016-2897-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-016-2897-3