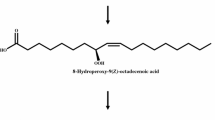
Abstract
Whole cells of recombinant Escherichia coli expressing diol synthase from Aspergillus nidulans produced 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid from α-linolenic acid via 8-hydroperoxy-9,12,15(Z,Z,Z)-octadecatrienoic acid as an intermediate. The optimal conditions for 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid production using whole recombinant cells were exhibited at pH 7.0, 40 °C, and 250 rpm with 40 g/L cells, 12 g/L, α-linolenic acid, and 5 % (v/v) dimethyl sulfoxide in a 250-mL baffled flask containing 50 mL reaction solution. Under these conditions, whole recombinant cells produced 9.1 g/L 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid for 100 min, with a conversion yield of 75 % (w/w), a volumetric productivity of 5.5 g/L/h, and specific productivity of 137 mg/g-cells/h. As an intermediate, 8-hydroperoxy-9,12,15(Z,Z,Z)-octadecatrienoic acid was observed at approximately 1.4 g/L after 100 min. With regard to dihydroxy fatty acid production, this is the highest reported volumetric and specific productivities thus far. This is the first report on the biotechnological production of 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydroxy fatty acids comprise of one or more hydroxyl groups and a carbon chain with a carboxyl group at the α-carbon position. Hydroxy fatty acids show a higher reactivity, solvent miscibility, hydrophilicity, and viscosity than non-hydroxylated fatty acids [1, 2]. Therefore, they are widely used as starting materials for the synthesis of resins, nylons, polyurethanes, plastics, and polymers, as additives for the manufacture of lubricants, surfactants, and stabilizers [3, 4], and as precursors for the synthesis of lactones [5]. Hydroxy fatty acids have been shown to function as signaling compounds in host-pathogen interactions in fungi [6], and have pharmaceutical activities, including anti-bacterial, anti-fungal, and anti-diabetic properties [2, 4, 7, 8]. Among hydroxy fatty acids, dihydroxy fatty acids are used in the manufacturing polyurethane rigid foams and skin care products [9], and they are known to be better surfactants than monohydroxy fatty acids [10]. Some dihydroxy fatty acids, called precocious sexual inducer (psi) factors, regulate the sexual and asexual life cycles in filamentous fungi [11].
α-Linolenic acid (18:3Δ9,12,15), polyunsaturated fatty acid, and omega-3 fatty acid, are an essential dietary nutrient for vertebrates [12]. Dihydroxy fatty acid derived from α-linolenic acid such as 9,16-dihydroxyoctadecatrienoic acid exhibits anti-aggregatory and anti-inflammatory properties [13]. Thus, the production of dihydroxy fatty acid from α-linolenic acid via biotransformation is important. Several microorganisms, including Agaricus bisporus [14], Clavibacter sp. ALA2 [15], Flavobacterium sp. DS5 [16], Fusarium oxysporum [17], Gaeumannomyces graminis [18], Lysinibacillus fusiformis [19], Magnaporthe grisea [20], Nocardia cholesterolicum [21], and Stenotrophomonas maltophilia [22], are capable of converting α-linolenic acid into several kinds of hydroxy fatty acids. Specially, Agaricus bisporus [14], Clavibacter sp. ALA2 [15], Fusarium oxysporum [17], and several strains of Pseudomonas aeruginosa such as PR3, 42A2, and 32T3 [23–29], have been shown to qualitatively convert fatty acids into dihydroxy fatty acids. The quantitative production of diverse dihydroxy fatty acids from different fatty acids, including palmitoleic acid, oleic acid, eicosenoic acid, ricinoleic acid, and linoleic acid, has been reported in P. aeruginosa PR3 and 42A2 containing diol synthase [30–36] and E. coli expressing A. nidulans diol synthase [37]. However, the quantitative production of dihydroxy fatty acid from α-linolenic acid by diol synthase or cells containing diol synthase has not yet been attempted.
In the present study, the biotechnological production of 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid from α-linolenic acid was achieved using whole recombinant cells expressing diol synthase from A. nidulans (Fig. 1). To increase the production, the reaction conditions such as pH, temperature, solvent, agitation speed, and concentrations of cell and substrate were optimized. Under the optimized conditions, an increase in the production of 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid from α-linolenic acid was achieved.
Production of 5S,8R-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid from α-linolenic acid via 8R-hydroperoxy-9,12,15(Z,Z,Z)-octadecatrienoic acid intermediate by whole recombinant cells expressing diol synthase from A. nidulans. The stereo-specificity of these compounds produced by diol synthase from A. nidulans has been reported previously [11]
Materials and Methods
Preparation of Dihydroxy Fatty Acid Standards
A double-site (H1004A-C1006S) variant of A. nidulans 5,8-diol synthase produced only 8-hydroperoxy-9,12,15(Z,Z,Z)-octadecatrienoic acid (not yet published). Recombinant cells expressing the wild-type and double-site variant of A. nidulans diol synthase were used for the preparation of 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid and 8-hydroperoxy-9,12,15(Z,Z,Z)-octadecatrienoic acid, respectively. The reactions were performed as described previously [37]. After the reactions, the fractions containing the reaction products were collected by solvent fractional crystallization at low temperature [38]. The partially purified products were extracted with two volumes of ethyl acetate. The solvent was removed from the extracts using a rotary evaporator. After evaporation, methanol was added to the solvent-free solutions, which were then applied to an HPLC system (Agilent 1260, Santa Clara, CA, USA) coupled with a UV detector and analyzed at a detection wavelength of 202 nm using a Nucleosil C18 semi-prep column (10.0 × 250 mm; Phenomenex, Torrance, CA, USA) and a separate fraction collector. The column was eluted at 40 °C, with a flow rate of 0.25 mL/min. The product fractions were collected and the solvent using a rotary evaporator was removed, leaving solid extracts. 5,8-Dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid and 8-hydroperoxy-9,12,15(Z,Z,Z)-octadecatrienoic acid with high purity (>99 %) were obtained and used as standard compounds in subsequent analyses.
Microorganisms, Plasmids, Gene Cloning, and Culture Conditions
A. nidulans ATCC 10074, E. coli ER2566 (New England Biolabs, Hertfordshire, UK), and pET-21a(+) plasmid (Novagen, Madison, WI, USA) were used as the sources of DNA template for the diol synthase gene, host cells, and expression vector, respectively. P. aeruginosa PR3 was kindly provided by Professor Hak-Ryul Kim at the Kyungpook National University (Daegu, South Korea). Although the production of dihydroxy fatty acids by P. aeruginosa PR3 has been published [23, 30–32, 39], the cell mass of P. aeruginosa PR3 in the production was not reported. P. aeruginosa PR3 was cultivated as described previously [39], and the strain was used for determining the cell mass of P. aeruginosa PR3 using a linear calibration curve relating optical density at 610 nm versus dry cell weight. The cell mass of P. aeruginosa PR3 was used for the calculation of volumetric and specific productivities for dihydroxy fatty acids. The diol synthase gene (GenBank accession number AY502073) from A. nidulans was obtained from a previous construction in E. coli [37]. The recombinant E. coli cells expressing diol synthase from A. nidulans were cultivated in a 2-L flask containing 500 mL Luria–Bertani (LB) medium and 50 μg/mL ampicillin at 37 °C with shaking at 200 rpm. When the optical density of the culture reached 0.6 at 600 nm, isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 0.1 mM. The culture was incubated at 16 °C with shaking at 150 rpm for 16 h to express the enzyme. The whole cells obtained were used for 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid production.
Effects of pH, Temperature and Thermostability
Unless otherwise stated, the reactions were performed in 50 mM HEPES (pH 7.0) containing 2 g/L cells and 0.14 g/L α-linolenic acid, which was purchased from Sigma-Aldrich (St. Louis, MO, USA), at 40 °C for 5 min with agitation at 250 rpm in a 250-mL baffled flask containing 50 mL reaction solution. Agitation was carried out in a shaking incubator (Vision Scientific, Bucheon, Korea). The effects of pH and temperature on the production of 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid from α-linolenic acid were investigated by varying the pH from 6.0 to 8.0 using 50 mM HEPES buffer at a constant temperature of 40 °C, and by varying the temperature from 30 to 60 °C at a constant pH of 7.0. The thermal stability of recombinant cells expressing diol synthase from A. nidulans for the production of 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid from α-linolenic acid was determined after incubating the cells at four different temperatures (35, 40, 45, and 50 °C). Samples were withdrawn at regular time intervals, and the activity was assessed by measuring 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid production under the standard reaction conditions described above. The half-life of recombinant cells expressing diol synthase from A. nidulans was calculated using the Sigma Plot 9.0 software (Systat Software, San Jose, CA, USA).
Effect of Solvent
The effect of solvent on 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid production was examined using ethanol, methanol, ethyl acetate, cyclohexane, diethyl ether, 1-propanol, dimethyl sulfoxide, 1-butanol, hexane, and isopropanol at the concentrations of 5 and 10 % (v/v) in 50 mM HEPES buffer (pH 7.0) with 2 g/L cells and 0.14 g/L α-linolenic acid at 40 °C for 5 min. To determine the optimum concentration of 1-propanol or dimethyl sulfoxide for the production of 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid from α-linolenic acid at the high concentrations of cells and substrate, the reactions were performed in 50 mM HEPES buffer (pH 7.0) with 20 g/L cells and 3 g/L α-linolenic acid at 40 °C for 10 min with agitation at 250 rpm by varying the concentration from 0 to 13 % (v/v) in a 250-mL baffled flask containing 50 mL reaction solution.
Optimization of Reaction Conditions
The effect of agitation speed on the production of 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid from α-linolenic acid by whole recombinant cells expressing diol synthase from A. nidulans was examined in 50 mM HEPES buffer (pH 7.0) containing 20 g/L cells, 3 g/L α-linolenic acid, and 5 % (v/v) dimethyl sulfoxide by varying the agitation speed from 0 to 270 rpm in a 250-mL baffled flask containing 50 mL reaction solution at 40 °C for 10 min.
To determine the optimal cell concentration required for the maximum production of 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid from α-linolenic acid, the reactions were performed in 50 mM HEPES buffer (pH 7.0) containing cells with a constant substrate concentration of 9 g/L α-linolenic acid and 5 % (v/v) dimethyl sulfoxide at 40 °C for 60 min with agitation at 250 rpm by varying the cell concentration from 10 to 50 g/L in a 250-mL baffled flask containing 50 mL reaction solution. To determine the optimal substrate concentration, the reactions were performed at 40 °C in 50 mM HEPES buffer (pH 7.0) containing with a constant cell concentration of 40 g/L cells and 5 % (v/v) dimethyl sulfoxide with shaking at 250 rpm for 60 min by varying the substrate concentration from 3 to 18 g/L in a 250-mL baffled flask containing 50 mL reaction solution.
Time-course of Production of 5,8-Dihydroxy-9,12,15(Z,Z,Z)-Octadecatrienoic Acid from α-Linolenic Acid
The time-course reactions of the production of 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid from α-linolenic acid by whole recombinant cells expressing diol synthase from A. nidulans via 8-hydroperoxy-9,12,15(Z,Z,Z)-octadecatrienoic acid as an intermediate were conducted under optimized conditions at 40 °C in 50 mM HEPES buffer (pH 7.0) containing 40 g/L cells, 12 g/L α-linolenic acid, and 5 % (v/v) dimethyl sulfoxide with shaking at 250 rpm in a 250-mL baffled flask containing 50 mL reaction solution for 100 min. A sample of 0.5 mL reaction solution was withdrawn at time interval and was analyzed with the HPLC system described below.
Analytical Methods
The cell mass was determined using a linear calibration curve relating optical density at 600 nm of recombinant E. coli expressing diol synthase from A. nidulans versus dry cell weight. The reaction solution was extracted with two volumes of ethyl acetate, and then the solvent was removed from the extract. The ethyl acetate fraction was then evaporated to dryness using a rotary evaporator, and methanol was added. 5,8-Dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid and α-linolenic acid were quantitatively analyzed using an HPLC system (Agilent 1260, Santa Clara, CA, USA) equipped with a UV detector at a detection wavelength of 202 nm and a reverse phase Nucleosil C18 column (3.2 × 150 mm; Phenomenex). The column was eluted at 40 °C using a gradient of solvent A (acetonitrile/water/acetic acid, 50/50/0.1, v/v/v) and solvent B (acetonitrile/acetic acid, 100/0.1, v/v) as follows: 100 % solvent A at a flow rate of 0.25 mL/min for 0–5 min; solvent B for 5–21 min at 0.25 mL/min; for 21–22 min at 0.4 mL/min; 100 % solvent B at 0.4 mL/min for 22–27 min; solvent A at 0.4 mL/min for 27–32 min; and 100 % solvent A at 0.25 mL/min for 32–35 min.
Liquid chromatography-mass spectrometry/mass spectrometry (LC–MS/MS) analysis of dihydroxy fatty acids was performed using an electrospray ionization (ESI) interface with a Thermo-Finnigan LCQ Deca XP plus ion trap mass spectrometer (Thermo Scientific, Pittsburgh, PA, USA) at the National Instrumentation Center for Environmental Management (NICEM) facility (Seoul National University, Seoul, South Korea). The instrument consisted of an LC pump, an auto sampler, and a photodiode array detector. The operation parameters were as follows: 275 °C capillary temperature, 5 kV ion source voltage, 30 psi nebulizer gas, 46 V capillary voltage in positive mode and 15 V in negative ionization mode, 0.01 min average scan time, 0.02 min average time to change polarity, and collision energy of approximately 35 % abundance of the precursor ion.
Results and Discussion
Identification of the Reaction Product and Intermediate Obtained from the Conversion of α-Linolenic Acid by Whole Recombinant Cells
The reaction product and intermediate obtained from the conversion of α-linolenic acid using whole recombinant cells expressing the wild-type and double-site variant (H1004A-C1006S) diol synthase of A. nidulans, respectively, were analyzed by LC–MS/MS, and their mass spectra were obtained. The total molecular mass of the product was represented by a peak at m/z 309 in LC/MS (Supplementary data, Fig. S1A). The peaks have been known to be formed from the cleavage of the hydroxylated carbon at α-carbon of hydroxy fatty acid [40]. The major peak at m/z 291 was formed by the loss of H2O from the total molecular mass. The peaks at m/z 115 and 173 resulted from the cleavage of the hydroxyl groups at the C5 and C8 positions, respectively. The product was identified as 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid on the basis of these fragment peaks. The reaction intermediate obtained from the conversion of α-linolenic acid using whole recombinant cells expressing the double-site variant diol synthase gave the mass spectrum of LC–MS/MS as shown in Supplementary data, Fig. S1B. The total molecular mass of the product was represented by a peak at m/z 309, and the peak at m/z 173 resulted from the cleavage of hydroperoxy group at the C8 position. These peaks indicate that the product was 8-hydroperoxy-9,12,15(Z,Z,Z)-octadecatrienoic acid.
Effects of pH and Temperature on the Production of 5,8-Dihydroxy-9,12,15(Z,Z,Z)-Octadecatrienoic Acid from α-Linolenic Acid by Whole Recombinant Cells
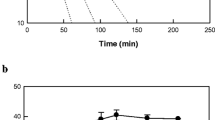
The maximum conversion rate of α-linolenic acid to 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid by whole cells of recombinant E. coli expressing diol synthase from A. nidulans was observed at pH 7.0 and 40 °C (Fig. 2a, b). The maximum conversion rate of linoleic acid to 5,8-dihydroxy-9,12(Z,Z)-octadecadienoic acid by recombinant cells expressing diol synthase from A. nidulans was observed previously at pH 7.5 and 35 °C [37]. These different optimum pH and temperature values may be due to different substrate solubility. The stability of recombinant cells expressing diol synthase from A. nidulans was measured, and the half-lives of the cells at 35, 40, 45, and 50 °C were 1,133, 232, 77, and 28 min, respectively (Fig. 2c).
Effects of pH and temperature on the production of 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid from α-linolenic acid by whole recombinant cells expressing diol synthase from A. nidulans. a Effect of pH. The reactions were performed in 50 mM HEPES buffer with 2 g/L cells and 0.14 g/L α-linolenic acid at 40 °C for 5 min by varying the pH from 6.0 to 8.0. b Effect of temperature. The reactions were performed in 50 mM HEPES (pH 7.0) containing 2 g/L cells and 0.14 g/L α-linolenic acid for 5 min by varying the temperature from 30 to 60 °C. c Effect of temperature on enzyme stability. Recombinant cells were incubated at 35 (unfilled squares), 40 (filled square), 45 (unfilled circles), and 50 °C (filled circles) in 50 mM HEPES buffer (pH 7.0) for various periods of time. A sample was withdrawn at each time point and assayed in 50 mM HEPES (pH 7.0) containing 2 g/L cells and 0.14 g/L α-linolenic acid at 40 °C for 5 min. Data present the means of three experiments and error bars represent standard deviations
Effect of Solvent on the Production of 5,8-Dihydroxy-9,12,15(Z,Z,Z)-Octadecatrienoic Acid from α-Linolenic Acid by Whole Recombinant Cells
The effect of organic solvent on the hydroxylation activity of recombinant cells expressing diol synthase from A. nidulans was examined at the concentrations of 5 and 10 % (v/v) with 2 g/L cells and 0.14 g/L α-linolenic acid. Among the solvents tested, the activity was the highest in the presence of 5 % (v/v) 1-propanol, followed by 5 % (v/v) dimethyl sulfoxide, and their activities were 1.4 and 1.3-fold higher than the hydroxylation activity in the absence of an organic solvent, respectively (Fig. 3a). The effects of solvent concentrations of the two solvents for the production of 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid were investigated with the high concentrations of cells (20 g/L) and substrate (3 g/L). The optimal concentrations of 1-propanol and dimethyl sulfoxide for 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid production were 1 and 5 % (v/v), respectively (Fig. 3b, c). At these concentrations, the activities were 1.2- and 1.4-fold higher than that in the absence of solvent, respectively. The higher production of 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid using dimethyl sulfoxide was resulted from the higher substrate solubility at high concentrations in dimethyl sulfoxide than those in 1-propanol. Thus, 5 % (v/v) dimethyl sulfoxide was used for 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid production by whole recombinant cells expressing diol synthase from A. nidulans.
Effect of solvent on the production of 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid from α-linolenic acid by whole recombinant cells expressing diol synthase gene from A. nidulans. a Effect of solvent type. The reactions were performed in 50 mM HEPES buffer (pH 7.0) with 2 g/L cells, 0.14 g/L α-linolenic acid, and 5 % (v/v) (filled square) or 10 % (v/v) (unfilled square) solvent at 40 °C for 5 min. b Effect of 1-propanol concentration. The reactions were performed in 50 mM HEPES buffer (pH 7.0) containing 20 g/L cells, 3 g/L α-linolenic acid, and 1-propanol at 40 °C for 10 min. c Effect of dimethyl sulfoxide concentration. The reactions were performed in 50 mM HEPES buffer (pH 7.0) containing 20 g/L cells, 3 g/L α-linolenic acid, and dimethyl sulfoxide at 40 °C for 10 min. Data present the means of three experiments and error bars represent standard deviations
Organic solvents are widely used to dissolve free fatty acids in aqueous solution. As dimethyl sulfoxide was used to increase the concentration of fatty acid as a substrate [41] in the production of 13-hydroxy-9,11-octadecadienoic acid with immobilized soybean lipoxygenase, the conversion yield was 1.4-fold higher than that in the absence of any treatment [42]. These results indicate that the increased production of 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid by the addition of dimethyl sulfoxide was due to an increase in substrate solubility.
Optimization of Reaction Conditions for the Production of 5,8-Dihydroxy-9,12,15(Z,Z,Z)-Octadecatrienoic Acid from α-linolenic Acid by Whole Recombinant Cells
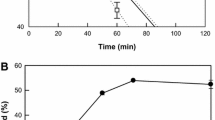
The production of 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid from α-linolenic acid by whole recombinant cells increased with an increase in the agitation speed (Fig. 4a). However, above 250 rpm, the increase in 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid production with increasing the agitation speed was not critical. Thus, the agitation speed was determined as 250 rpm. The optimal cell concentration was evaluated at agitation speed of 250 rpm with 9 g/L α-linolenic acid by varying the cell concentration from 10 to 50 g/L after 60 min. When the concentration was less than 40 g/L cells, the production increased with an increase in the cell concentration. However, above 40 g/L concentration, it reached a plateau (Fig. 4b), indicating that the optimum concentration of recombinant cells was 40 g/L. The production of 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid from α-linolenic acid was assessed over a 60-min period by varying the substrate concentration from 3 to 18 g/L while keeping the cell concentration constant at 40 g/L cells. Below 12 g/L α-linolenic acid, the production increased with an increase in the concentration of α-linolenic acid (Fig. 4c). However, above 12 g/L α-linolenic acid, the production of 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid decreased as the concentration of α-linolenic acid increased. Thus, the optimum substrate concentration for 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid production was 12 g/L α-linolenic acid, and the conversion yield at this concentration was approximately 72 % (w/w).
Effects of agitation speed and concentrations of cells and substrate for the production of 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid from α-linolenic acid by whole recombinant cells expressing diol synthase from A. nidulans. a Effect of agitation speed. The reactions were performed in 50 mM HEPES buffer (pH 7.0) with 20 g/L cells, 3 g/L α-linolenic acid, and 5 % (v/v) dimethyl sulfoxide at 40 °C for 10 min. b Effect of cell concentration. The reactions were performed in 50 mM HEPES buffer (pH 7.0) containing cells, 9 g/L α-linolenic acid, and 5 % (v/v) dimethyl sulfoxide at 40 °C for 60 min with agitation at 250 rpm. c Effect of substrate concentration on the production (filled circles) and conversion yield (filled squares) of 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid. The reactions were performed in 50 mM HEPES buffer (pH 7.0) containing 40 g/L cells, α-linolenic acid, and 5 % (v/v) dimethyl sulfoxide at 40 °C for 60 min with agitation at 250 rpm. Data present the means of three experiments and error bars represent standard deviations
Production of 5,8-Dihydroxy-9,12,15(Z,Z,Z)-Octadecatrienoic Acid from α-Linolenic Acid by Whole Recombinant Cells Under the Optimized Conditions
The production of 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid by whole recombinant cells expressing diol synthase from A. nidulans was optimal at pH 7.0, 40 °C, 12 g/L α-linolenic acid, and 40 g/L cells in the presence of 5 % (v/v) dimethyl sulfoxide in a 250-mL baffled flask containing 50 mL reaction mixture with shaking at 250 rpm. Under these optimized conditions, time-course reactions for the production of 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid by whole recombinant cells were performed for 100 min (Fig. 5). The cells were stable at 40 °C for 100 min because the half-life of the cells at this temperature for 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid production was 232 min (Fig. 4). Whole recombinant cells produced 9.1 g/L 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid from 12 g/L α-linolenic acid after 100 min, with a conversion yield of 75 % (w/w), a volumetric productivity of 5.5 g/L/h, and specific productivity of 137 mg/g-cell/h. Whole recombinant cells also produced 1.0 g/L 8-hydroperoxy-9,12,15(Z,Z,Z)-octadecatrienoic acid as an intermediate at 20 min. After the time, the concentration of the monohydroxy fatty acid was maintained at 1.0–1.4 g/L during the time-course reactions.
Time-course reactions for the production of 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid from α-linolenic acid by whole recombinant cells expressing diol synthase from A. nidulans under the optimized conditions. 5,8-Dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid (filled circles), α-linolenic acid (unfilled circles), and 8-hydroperoxy-9,12,15(Z,Z,Z)-octadecatrienoic acid (filled triangles). The reactions were performed in 50 mM HEPES buffer (pH 7.0) containing 40 g/L cells, 12 g/L α-linolenic acid, and 5 % (v/v) dimethyl sulfoxide at 40 °C for 100 min with agitation at 250 rpm. Data present the means of three experiments and error bars represent standard deviations
Whole recombinant cells expressing hydratase from S. maltophilia produced 14.3 g/L 10-hydroxy-12,15(Z,Z)-octadecadienoic acid from 17.5 g/L α-linolenic acid with volumetric and specific productivities of 0.79 g/L/h and 16 mg/g-cell/h, respectively [22]. This is the highest reported concentration, yield, and volumetric and specific productivities for the production of hydroxy fatty acid from α-linolenic acid. The volumetric and specific productivities of recombinant cells in the present study were 7.0- and 8.6-fold higher than those obtained by whole recombinant cells expressing oleate hydratase from S. maltophilia, respectively. However, the concentration and yield in the present study were 1.6-fold and 7 % lower, respectively.
The quantitative production of dihydroxy fatty acids from fatty acids has been reported in only two microorganisms, namely P. aeruginosa PR3 and recombinant E. coli expressing diol synthase from A. nidulans (Table 1). P. aeruginosa PR3 produced 7,10-dihydroxy-8(E)-octadecenoic acid from oleic acid [23], 7,10-dihydroxy-8(E)-octadecenoic acid from triolein [31], 7,10-dihydroxy-8(E)-hexadecenoic acid from palmitoleic acid [32], 9,12-dihydroxy-10(E)-eicosenoic acid from eicosenoic acid [30], and 7,10-dihydroxy-8(E)-octadecenoic acid from olive oil [39]. The specific productivity of P. aeruginosa PR3 was determined by calibrating 1.0 optical density at 610 nm to 0.54 g/L cell mass. Among the production of these dihydroxy fatty acids, the production of 7,10-dihydroxy-8(E)-octadecenoic acid from oleic acid exhibited the highest concentration, conversion yield, and volumetric productivity, which were 9.1 g/L, 89 % (w/w), and 0.19 g/L/h, respectively. Moreover, the production of 7,10-dihydroxy-8(E)-octadecenoic acid from olive oil showed the highest specific productivity of 47 mg/g-cell/h. The volumetric and specific productivities of recombinant cells in the present study were 29- and 3-fold higher than those obtained using whole P. aeruginosa PR3, respectively. However, the concentration and conversion yield in the present study was same and 1.2-fold lower, respectively. Whole recombinant cells expressing diol synthase from A. nidulans produced 4.98 g/L 5,8-dihydroxy-9,12(Z,Z)-octadecadienoic acid from 5.0 g/L linoleic acid for 150 min with a volumetric productivity of 2.5 g/L/h and a specific productivity of 85 mg/g-cell/h [37]. The volumetric and specific productivities for the production of 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid from α-linolenic acid by whole recombinant cells expressing diol synthase from A. nidulans were 2.2- and 1.6-fold higher than those for the production of 5,8-dihydroxy-9,12(Z,Z)-octadecadienoic acid from linoleic acid.
Conclusions
To increase the production of 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid from α-linolenic acid by whole recombinant cells expressing diol synthase from A. nidulans, the reaction conditions such as pH, temperature, thermostability, solvent, agitation speed, and cell and substrate concentrations were optimized. Under the optimized conditions, whole recombinant cells produced 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid with the highest specific and volumetric productivities as compared to dihydroxy fatty acid production described previously. To the best of our knowledge, this is the first report on the biotechnological production of 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid. These results will contribute to the improved industrial production of 5,8-dihydroxy-9,12,15(Z,Z,Z)-octadecatrienoic acid via a biological process.
References
Metzger JO, Bornscheuer U (2006) Lipids as renewable resources: current state of chemical and biotechnological conversion and diversification. Appl Microbiol Biotechnol 71:13–22
Hou CT (2009) Biotechnology for fats and oils: new oxygenated fatty acids. N Biotechnol 26:2–10
Ogunniyi D (2006) Castor oil: a vital industrial raw material. Bioresour Technol 97:1086–1091
Mutlu H, Meier M (2010) Castor oil as a renewable resource for the chemical industry. Eur J Lipid Sci Technol 112:10–30
Romero-Guido C, Belo I, Ta TM, Cao-Hoang L, Alchihab M, Gomes N, Thonart P, Teixeira JA, Destain J, Wache Y (2011) Biochemistry of lactone formation in yeast and fungi and its utilisation for the production of flavour and fragrance compounds. Appl Microbiol Biotechnol 89:535–547
Tsitsigiannis DI, Keller NP (2007) Oxylipins as developmental and host-fungal communication signals. Trends Microbiol 15:109–118
Paul S, Hou CT, Kang SC (2010) alpha-Glucosidase inhibitory activities of 10-hydroxy-8(E)-octadecenoic acid: an intermediate of bioconversion of oleic acid to 7,10-dihydroxy-8(E)-octadecenoic acid. N Biotechnol 27:419–423
Martin-Arjol I, Bassas-Galia M, Bermudo E, Garcia F, Manresa A (2010) Identification of oxylipins with antifungal activity by LC-MS/MS from the supernatant of Pseudomonas 42A2. Chem Phys Lipids 163:341–346
Hou CT (2008) New bioactive fatty acids. Asia Pac J Clin Nutr 17(Suppl 1):192–195
Seo MH, Kim KR, Oh DK (2013) Production of a novel compound, 10,12-dihydroxystearic acid from ricinoleic acid by an oleate hydratase from Lysinibacillus fusiformis. Appl Microbiol Biotechnol 97:8987–8995
Brodhun F, Gobel C, Hornung E, Feussner I (2009) Identification of PpoA from Aspergillus nidulans as a fusion protein of a fatty acid heme dioxygenase/peroxidase and a cytochrome P450. J Biol Chem 284:11792–11805
Alhazzaa R, Sinclair AJ, Turchini GM (2013) Bioconversion of alpha-linolenic acid into n-3 long-chain polyunsaturated fatty acid in hepatocytes and ad hoc cell culture optimisation. PLoS One 8:e73719
Liu M, Chen P, Vericel E, Lelli M, Beguin L, Lagarde M, Guichardant M (2013) Characterization and biological effects of di-hydroxylated compounds deriving from the lipoxygenation of ALA. J Lipid Res 54:2083–2094
Wadman MW, van Zadelhoff G, Hamberg M, Visser T, Veldink GA, Vliegenthart JF (2005) Conversion of linoleic acid into novel oxylipins by the mushroom Agaricus bisporus. Lipids 40:1163–1170
Hosokawa M, Hou CT, Weisleder D (2003) Production of novel tetrahydroxyfuranyl fatty acids from alpha-linolenic acid by Clavibacter sp. strain ALA2. Appl Environ Microbiol 69:3868–3873
Hou CT (1995) Is strain DS5 hydratase a C-10 positional specific enzyme? Identification of bioconversion products from alpha and gamma linolenic acids by Flavobacterium sp. DS5. J Ind Microbiol 14:31–34
Brodhun F, Cristobal-Sarramian A, Zabel S, Newie J, Hamberg M, Feussner I (2013) An iron 13S-lipoxygenase with an alpha-linolenic acid specific hydroperoxidase activity from Fusarium oxysporum. PLoS One 8:e64919
Brodowsky ID, Hamberg M, Oliw EH (1992) A linoleic acid (8R)-dioxygenase and hydroperoxide isomerase of the fungus Gaeumannomyces graminis. Biosynthesis of (8R)-hydroxylinoleic acid and (7S,8S)-dihydroxylinoleic acid from (8R)-hydroperoxylinoleic acid. J Biol Chem 267:14738–14745
Kim BN, Joo YC, Kim YS, Kim KR, Oh DK (2011) Production of 10-hydroxystearic acid from oleic acid and olive oil hydrolyzate by an oleate hydratase from Lysinibacillus fusiformis. Appl Microbiol Biotechnol 95:929–937
Cristea M, Osbourn AE, Oliw EH (2003) Linoleate diol synthase of the rice blast fungus Magnaporthe grisea. Lipids 38:1275–1280
Koritala S, Bagby MO (1992) Microbial conversion of linoleic and linolenic acids to unsaturated hydroxy fatty acids. J Am Oil Chem Soc 69:575–578
Oh HJ, Shin KC, Oh DK (2013) Production of 10-hydroxy-12,15(Z, Z)-octadecadienoic acid from alpha-linolenic acid by permeabilized cells of recombinant Escherichia coli expressing the oleate hydratase gene of Stenotrophomonas maltophilia. Biotechnol Lett 35:1487–1493
Kuo TM, Manthey LK, Hou CT (1998) Fatty acid bioconversions by Pseudomonas aeruginosa PR3. J Am Oil Chem Soc 75:875–879
Estupinan M, Diaz P, Manresa A (2014) Unveiling the genes responsible for the unique Pseudomonas aeruginosa oleate-diol synthase activity. Biochim Biophys Acta 1842:1360–1371
Martinez E, Hamberg M, Busquets M, Diaz P, Manresa A, Oliw EH (2010) Biochemical characterization of the oxygenation of unsaturated fatty acids by the dioxygenase and hydroperoxide isomerase of Pseudomonas aeruginosa 42A2. J Biol Chem 285:9339–9345
Pelaez M, Orellana C, Marques A, Busquets M, Guerrero A, Manresa A (2003) Natural estolides produced by Pseudomonas sp. 42A2 grown on oleic acid: production and characterization. J Am Oil Chem Soc 80:859–866
Nilsson T, Martinez E, Manresa A, Oliw EH (2010) Liquid chromatography/tandem mass spectrometric analysis of 7,10-dihydroxyoctadecenoic acid, its isotopomers, and other 7,10-dihydroxy fatty acids formed by Pseudomonas aeruginosa 42A2. Rapid Commun Mass Spectrom 24:777–783
Busquets M, Deroncele V, Vidal-Mas J, Rodriguez E, Guerrero A, Manresa A (2004) Isolation and characterization of a lipoxygenase from Pseudomonas 42A2 responsible for the biotransformation of oleic acid into (S)-(E)-10-hydroxy-8-octadecenoic acid. Antonie Van Leeuwenhoek 85:129–139
Rodriguez E, Espuny M, Manresa A, Guerrero A (2001) Identification of (E)-11-hydroxy-9-octadecenoic acid and (E)-9-hydroxy-10-octadecenoic acid by biotransformation of oleic acid by Pseudomonas sp. 32T3. J Am Oil Chem Soc 78:593–597
Back KY, Sohn HR, Hou CT, Kim HR (2011) Production of a novel 9,12-dihydroxy-10(E)-eicosenoic acid from eicosenoic acid by Pseudomonas aeruginosa PR3. J Agric Food Chem 59:9652–9657
Chang IA, Bae JH, Suh MJ, Kim IH, Hou CT, Kim HR (2008) Environmental optimization for bioconversion of triolein into 7,10-dihydroxy-8(E)-octadecenoic acid by Pseudomonas aeruginosa PR3. Appl Microbiol Biotechnol 78:581–586
Bae JH, Suh MJ, Kim BS, Hou CT, Lee IJ, Kim IH, Kim HR (2010) Optimal production of 7,10-dihydroxy-8(E)-hexadecenoic acid from palmitoleic acid by Pseudomonas aeruginosa PR3. N Biotechnol 27:352–357
Kim H, Kuo TM, Hou CT (2000) Production of 10,12-dihydroxy-8(E)-octadecenoic acid, an intermediate in the conversion of ricinoleic acid to 7,10,12-trihydroxy-8(E)-octadecenoic acid by Pseudomonas aeruginosa PR3. J Ind Microbiol Biotechnol 24:167–172
Cullere J, Durany O, Busquets M, Manresa A (2001) Biotransformation of oleic acid into (E)-10-hydroxy-8-octadecenoic acid and (E)-7,10-dihydroxy-8-octadecenoic acid by Pseudomonas sp. 42A2 in an immobilized system. Biotechnol Lett 23:215–219
Guerrero A, Casals I, Busquets M, Leon Y, Manresa A (1997) Oxidation of oleic acid to (E)-10-hydroperoxy-8-octadecenoic and (E)-10-hydroxy-8-octadecenoic acids by Pseudomonas sp. 42A2. Biochim Biophys Acta 1347:75–81
De Andres C, Mercade E, Guinea J, Manresa A (1994) 7,10-Dihydroxy-8 (E)-octadecenoic acid produced by Pseudomonas 42A2: evaluation of different cultural parameters of the fermentation. World J Microbiol Biotechnol 10:106–109
Seo MJ, Shin KC, Oh DK (2014) Production of 5,8-dihydroxy-9,12(Z, Z)-octadecadienoic acid from linoleic acid by whole recombinant Escherichia coli cells expressing diol synthase from Aspergillus nidulans. Appl Microbiol Biotechnol 98:7447–7456
Chen TC, Ju YH (2001) An improved fractional crystallization method for the enrichment of gamma-linolenic acid in borage oil fatty acid. Ind Eng Chem Res 40:3781–3784
Suh MJ, Baek KY, Kim BS, Hou CT, Kim HR (2011) Production of 7,10-dihydroxy-8(E)-octadecenoic acid from olive oil by Pseudomonas aeruginosa PR3. Appl Microbiol Biotechnol 89:1721–1727
Oliw EH, Su C, Skogstrom T, Benthin G (1998) Analysis of novel hydroperoxides and other metabolites of oleic, linoleic, and linolenic acids by liquid chromatography-mass spectrometry with ion trap MSn. Lipids 33:843–852
Emken EM, Dutton HJ (1971) Lipoxygenase-oxidized soap stock as source of hydroxy conjugated octadecadienoic acids. J Am Oil Chem Soc 48:324–329
Omar MN, Moynihan H, Hamilton R (2003) Scaling-up the production of 13S-hydroxy-9Z,11E-octadecadienoic acid (13S-HODE) through chemoenzymatic technique. Bull Korean Chem Soc 24:397–399
Acknowledgments
This study was supported by the Korean Healthcare Technology R&D Project, Ministry for Health & Welfare, Republic of Korea (No. 2012-009) and the Marine Biomaterials Research Center grant from the Marine Biotechnology Program funded by the Ministry of Oceans and Fisheries, Korea.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Seo, MJ., Shin, KC., Jeong, YJ. et al. 5,8-Dihydroxy-9,12,15(Z,Z,Z)-Octadecatrienoic Acid Production by Recombinant Cells Expressing Aspergillus nidulans Diol Synthase. J Am Oil Chem Soc 92, 193–202 (2015). https://doi.org/10.1007/s11746-014-2581-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-014-2581-4