Abstract
This study presents a combined approach of establishing cultivar differences between Aegean olive oils, obtained from economically important olive oil producing cultivars (cv. Ayvalik and Memecik), based on chemometric evaluation of their content and in particular composition of the minor compounds. Evaluation of minor compounds with principal component analysis and linear discriminant analysis (LDA) indicated differentiation according to the cultivars. LDA produced a 100% correct group classification. Moreover, stigmasterol, apparent β-sitosterol and total sterols were found to have the highest discriminating power. Memecik oils were characterized by the highest content of antioxidant compounds (α-tocopherol, phenolic compounds and total phenolic compounds). On the other hand, Ayvalik oil had the highest level of total sterols. The data were analyzed statistically to evaluate the differences according to variety and crop season. The minor compounds of Ayvalik and Memecik oils presented statistically significant differences (p < 0.01) according to variety, except for the hydroxytyrosol and clerosterol content. The amount of α-tocopherol, total phenolic compounds, apparent β-sitosterol and total sterols varied with respect to crop season. A good correlation was observed between the amount of α-tocopherol, total phenolic compounds, apparent β-sitosterol and total sterols and some climatic variables.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The consumption of extra virgin olive oil, the most characteristic component of the Mediterranean diet, is currently increasing because of its nutritional and health-promoting effects, which have been attributed to its high content of monounsaturated fatty acids and antioxidants [1, 2].
Turkey is the world’s fifth largest producer of olive oil. Turkey, which exports a great majority of its production accounts for 10% of total world exports [3]. The Aegean coast, Turkey’s leading olive growing region, accounts for 75–80% of the total national production, and has mainly Ayvalik and Memecik as economically important cultivars [4]. Olive oil is a traditional product from the Aegean region and can be labeled as a product with a geographical indication. North Aegean olive oils obtained from Ayvalik cultivar and South Aegean olive oils obtained from Memecik cultivar have received the registered geographical signs [5].
The chemical characterization of olive oil is important to accomplish authentication studies concerning geographical origin and cultivar composition. Such characterization of monovarietal olive oils has been studied for Spanish and Italian cultivars [6–8]. However, not much information on the chemical composition of Turkish olive oil is available in the literature [4, 9–11]. Therefore, this study aims at providing data about the chemical characteristics of the two major Turkish cultivars.
The chemical characterization of monovarietal olive oil depends on olive variety, geographical, agronomic, climatic and technological factors. Temperature and rainfall may affect olive oil composition. Some studies have been carried out to evaluate the effects of climatic conditions on olive oil composition [12]. However, data are limited about the relationship between climatic variables and the composition of olive oil, especially for the Aegean coast.
To our knowledge, few references were found in the literature regarding characterization of Aegean olive oils with respect to their minor compounds [4, 10]. The first aim of this study was to characterize and classify Aegean extra virgin olive oils according to their minor compounds. Secondly, the influence of the crop season and climatic conditions on the minor compounds was studied as well.
Materials and Methods
Samples
Commercial monovarietal extra virgin olive oils were collected from TARIS (Olive and Olive Oil Agricultural Sales Cooperatives Union, Izmir, Turkey) during the 2006/2007 and 2007/2008 harvest seasons. North Aegean olive oils obtained from the Ayvalik cultivar (n:8) and South Aegean olive oils obtained from the Memecik cultivar (n:8) were studied. Olives were harvested during the period October-December and immediately processed to olive oil in the TARIS plant. Thirteen oils were extracted using a triple-phase decanter and three oils (Ayvalik oils) with the dual-phase decanter. All samples were kept at 4 °C and were protected from light prior to analysis.
Chemicals
All reagents used were analytical or HPLC grade (Merck, Riedel–de–Haen, Acros and Biosolve).
Analytical Methods
Determination of the free fatty acids was carried out following the AOCS official method Ca 5a-40. Isopropanol was neutralized with 0.1 N sodium hydroxide solution (1–2 drops). Five grams of oil was weighed in an Erlenmeyer flask and the neutralized solvent was added to the sample. The mixture was titrated with 0.1 N sodium hydroxide with phenolphthalein as the indicator. UV absorption characteristics (K232 and K270) were determined following the analytical methods described in the Regulation EC/1989/2003 [13]. In a 10-ml flask, 0.1 g oil was weighed and dissolved in cyclohexane. Absorbance of the solution at 232 and 270 nm was measured in 10-mm quartz cuvettes using a Varian Carry 50 probe UV–visible spectrophotometer, with pure solvent (cyclohexane) as the blank.
Total sterol content was determined according to a modified DGF official method [14]. First, 1.5 g oil was weighed in a flask and 2 ml of internal standard (0.1% cholesterol in chloroform) was added to the flask. The sample was saponified with 6 ml aqueous 10 M KOH in 10 ml of ethanol. Saponification took at least 90 min. The unsaponifiable fraction was extracted twice with diethyl ether. The combined fractions were washed twice with 0.5 M KOH (7 ml) and two to four times saturated salt solution (7 ml) until the washing water was neutral, and then the organic phase was dried with anhydrous sodium sulfate. The residue obtained after evaporation was redissolved in 0.5 ml pyridine and 1 ml N,O-bis-(trimethylsilyl)-trifluoroacetamide containing 1% trimethyl-chlorosilane solution, and derivatized for 30 min at 80 °C. The samples were injected into an Agilent 6890N gas chromatograph equipped with a capillary column Alltech EC5 (0.25mm × 0.25μm × 30 m). Detection was by a flame ionization detector (360 °C). The initial oven temperature for the first 35 min was isothermal at 285 °C and, followed by an increase at 10 °C/min to 310 °C, which was held for 10 min. The carrier gas was helium at a flow rate of 0.5 ml/min. The identification of sterols was performed according to the relative retention time and mass spectra. The internal standard (cholesterol) method was used for the quantification.
α-Tocopherol analysis was carried out following the AOCS Official Method Ce-8-89. Two grams of oil were weighed into a 25-ml flask and dissolved in hexane. After filtration through a 0.45-μm PTFE membrane filter. Twenty microliters of the sample solution was injected into an Agilent 1100 Series HPLC system. A normal phase, Purospher Star Silica column (250 × 4.6 mm id, 5 μm particle size) was used. The mobile phase was hexane/iso-propanol (99.5:0.5) at a flow rate of 1.5 ml/min. Diode array detection was accomplished at 292 nm. The total phenolic compounds content of the oils was determined colorimetrically using the Folin Ciocalteu reagent according to the method of Gutfinger [15]. The phenolic fraction of the oil was obtained by solid-phase extraction (SPE) using the diol-bonded SPE cartridges (GracePure SPE Diol 500 mg/3 ml) according to the procedure of Mateos et al. [16] The phenolic extract was redissolved in 1 ml methanol. Folin-Ciocalteu reagent (diluted 10×) and 20% sodium carbonate solution were added to the phenolic extract. The solution was left in a dark place to protect from light and kept for 2 h before measurement. The absorbance of each sample was determined at 760 nm in a 1-cm cuvette against the blank sample (prepared as described before but with 1 ml water instead of polar extract solution). The measurement was done on a Varian Cary 50 Probe UV–Visible spectrophotometer. The calculation of phenolic compounds was based on an external standard calibration curve. Calibration curves were constructed using caffeic acid and gallic acid within the range 0–400 mg/ml. The total phenol content was expressed as mg caffeic acid equivalents (CAE) per kg of oil and mg gallic acid equivalents (GAE) per kg of oil.
Phenolic compounds were analyzed by reversed-phase high performance liquid chromatography (HPLC) and detected by both Diode Array Detector and Mass Spectroscopy.
Sample Preparation
The phenolic fraction of the oil was extracted by solid-phase extraction (SPE) according to the method mentioned in total phenol content analysis. The residue was dissolved in 0.5 ml methanol. Samples were filtered through a 0.45-μm PTFE Membrane filter into the vial.
LC–MS Injection
Analyses were performed on an Agilent 1100 LC–MSD System equipped with a quaternary pump, Phenomex-Luna C18 column (4.6 mm i.d. × 250 mm, 5 μm particle size), vacuum degasser, autosampler, 1100 6-port autoinjector valve, Diode Array Detector. The device was controlled by Agilent software v A.09.03. The determination of phenolic compounds was carried out at 280 nm and 320 nm. A gradient of three solvents was used to achieve the separation of analyzed compounds [4]. The flow of the mobile phase was maintained at the level of 1 ml/min and injection amount was 20 μl. The mobile phase consisted of 0.2% acetic acid in water (A), methanol (B) and acetonitrile(C). B and C solvents were mixed in 50:50 (v/v).
The Agilent 11000 series mass detector (LC-MSD) was an Agilent G1946D (SL) quadrupole mass spectrophotometer equipped with electrospray ionization (ESI) System. Nitrogen was nebulizing gas (pressure 50 psi, flow 13 l/min). The heated capillary temperature and voltage were maintained at 350 °C and 4.1 kV, respectively, and the fragmentor voltage was 70 eV. In the mass spectrum full scan was performed in the range: m/z 100–1,000. Mass spectrometry data were acquired in the negative ionization mode.
Identification of Compounds
The phenolic compounds were identified on the basis of their retention times, their UV and mass spectra and their molecular masses compared with those of standard compounds. When standards were not available, literature data was used for the identification.
Quantification
Quantification was performed using phenolic compound standard calibration curves. Since hydroxytyrosol acetate and oleuropein aglycon were not commercially available, the quantification of such compounds was based on hydroxytyrosol and oleuropein. Calibration curves were in the range of 10–50 mg/kg. The content of each phenolic compound present in the polar fraction of the oil samples was expressed in mg/kg of olive oil.
All analyses were carried out in triplicate. ANOVA was performed to evaluate significant differences on the studied parameters. PCA (principal component analysis) and linear discriminant analysis (LDA) were used to characterize and to classify the studied olive oils according to the minor compounds. Statistica 6.0 software (StatSoft Inc., Tulsa, OK, USA) was used for the statistical data processing.
Results and Discussion
Chemical Composition of Aegean Olive Oils
The quantity of free fatty acids and UV spectrophotometric measurements are used for quality assessment. The values of acidity fell within the ranges established for the quality category “extra virgin” olive oil (FFA < 0.8%). The values of specific UV absorbances (K232 and K270) were below the limits (K232 < 2.50 and K270 < 0.25), except one Ayvalik sample and one Memecik sample which had a value of K232 above the upper limit of the 2.5 established by Regulation EC/1989/2003, which may be ascribed to the low quality of the olive fruits. It was reported that UV characteristics of olive oil were mainly affected by the fruit quality. Improper harvest, transport and storage cause damage to the olive fruits [17].
Tocopherols are the main lipid-soluble antioxidants found in the olive oil. In this study, only α-tocopherol could be detected in Aegean olive oils. The content of α-tocopherol varied from 155.2 to 224.4 mg/kg for Ayvalik oils (Table 1). The level of α-tocopherol in Ayvalik oils was similar to some Spanish olive oils (Cornicabra and Picual) [18]. However, it was lower than in Arbequina olive oil (Spain) and Koroneiki olive oil (Greece) [18, 19]. The content of α-tocopherol ranged from 198.7 to 326.8 mg/kg for Memecik oils (Table 2). The amount of α-tocopherol in Memecik oils was higher than in some Spanish olive oils such as Cornicabra, Arbequina and Picual [18]. However, it was similar to Koroneiki olive oil (Greece) [19].
Olive oil includes phenolic compounds, which act as natural antioxidants, and also contribute to the sensory properties of olive oil. The bitter and the pungent taste of extra virgin olive oils is related to phenolic compounds. Representative LC chromatograms of Aegean olive oils are presented in Fig. 1. The following compounds were identified by LC–MS: hydroxytyrosol, hydroxytyrosol acetate, tyrosol, p-coumaric acid, ferulic acid, luteolin, apigenin and oleuropein aglycon. From Table 1, it can be seen that the phenolic alcohols content of Ayvalik samples shows great variations. The content of hydroxytyrosol ranged from 1.6 to 21.4 mg/kg and tyrosol varied from 2.2 to 9.1 mg/kg. The content of phenolic acids was less than 1 mg/kg. The level of luteolin, the major flavone detected, ranged from 0.8 to 2.8 mg/kg while apigenin, another major flavone detected, constituted less than 1 mg/kg. The amount of phenolic compounds of Memecik oils is presented in Table 2. While hydroxytyrosol is considered as the major phenolic alcohol in olive oil, Memecik cultivar was richer in tyrosol, ranging from 7.4 to 20.1 mg/kg Tyrosol content of Memecik oils seemed to be higher than Ayvalik oils and Spanish oils such as Arbequina and Hojiblanca [20]. Hydroxytyrosol was the second phenolic alcohol and varied from 0.5 to 14.2 mg/kg. The content of phenolic acids was lower than 1 mg/kg, except in two samples. The content of flavones ranged from 1 to 3.7 mg/kg and 0.8 to 1.4 mg/kg, for luteolin and apigenin, respectively.
Representative phenolic compounds chromatograms of Aegean olive oils at 280 nm (a) and 320 nm (b) hydroxytyrosol (1), tyrosol (2), o-coumaric acid (IS 1 ), p-coumaric acid (3), ferulic acid (4), hydroxytyrosol acetate (5), p-hydroxyphenylacetic acid (IS 2 ), luteolin (6), oleuropein aglycon (7), apigenin (8)
The total phenolic compounds (as measured by the Folin-Ciocalteu method) of Ayvalik oils ranged from 76.1 to 159.5 mg caffeic acid equivalents/kg (Table 1). The total phenolic compounds of Ayvalik oils was studied by Andjelkoviç et al. [4] and Ocakoglu et al. [10] in the 2006/2007 harvest season. Their findings were similar to our results. The total phenolic compounds of Memecik oils ranged from 107 to 226.3 mg caffeic acid equivalents/kg (Table 2). Similar values were reported by Ocakoglu et al. [10] for Memecik oils from the 2006/2007 harvest season. Aegean oils had a lower content of total phenolic compounds than Spanish cultivars such as Cornicabra, Picual and Arbequina and Italian cultivar (Coratina) [18, 21]. However, the content of total phenolic compounds of Aegean oils was higher than reported for Tunisian cultivars (Chemlai and Chetoui) [22].
Sterols are major constituents of the unsaponifiable fraction. The sterol composition is very important in the evaluation of the nutritional value, but also in the quality control of olive oils, since it can be used to detect adulteration. A representative chromatogram of the unsaponifiable fraction of Aegean olive oils is presented in Fig. 2. Squalene, cycloartenol, 24-methylene cycloartenol and citrostadienol were identified as well. However for the quantification the desmethylsterols were selected as those are the sterols included in olive oil legislation. In this study, the major detected desmethylsterols were β-sitosterol, ∆5-avenasterol, campesterol, stigmasterol and clerosterol. The sterol composition of Ayvalik oil is presented in Table 1. The percentage of β-sitosterol ranged from 74 to 78.2%. The second major sterol was ∆5-avenasterol, which ranged from 17.3 to 22.2%. The total content of desmethylsterols was higher than 1,000 mg/kg which is the legal minimum value. Moreover, the contents of apparent β-sitosterol (sum of percentages of clerosterol, β-sitosterol, and ∆5-avenasterol) were above the legal minimum value of 93%. The content of campesterol was lower than the legal maximum value of 4%. The results obtained in this work indicated that Ayvalik oils had a higher content of total sterols (2135–2673 mg/kg) than many European cultivars, such as Arbequina, Cornicabra and Picual, Coratina and Koroneiki [18, 21]. The sterol composition of Memecik oils is presented in Table 2. The percentage of β-sitosterol varied from 80.8 to 83%. The Δ5-avenasterol content ranged from 11.3 to 12.8%. The level of campesterol was remarkably high, with a range from 4 to 5%. The content of total sterols was higher than the legal minimum value and the content of apparent β-sitosterol was above the legal minimum value. However, the content of campesterol exceeded the legal maximum value of 4%, a similar observation was previously made for Cornicabra olive oil [23]. The results obtained in this work indicated that the content of total sterols of Memecik oils (1157–1676 mg/kg) was similar to some Spanish cultivars (Arbequina, Cornicabra and Picual), an Italian cultivar (Coratina) and Greek cultivar. (Koroneiki) [18, 21].
Statistical Evaluation of Differences Between both Cultivars
Two-way Anova (cultivar and crop season: independent variables) was applied to evaluate the differences between the composition of Aegean olive oils. All chemical parameters of Ayvalik and Memecik olive oils showed statistically significant differences, except hydroxytyrosol and clerosterol (p < 0.01). Memecik oils had the highest level of antioxidant compounds such as α-tocopherol, and phenolic compounds. The oxidative stability of olive oil was attributed to its fatty acid composition and inherent antioxidant compounds. It was reported that the contribution of these factors to oil stability was 30, 27, and 11% for phenolic compounds, fatty acids and α-tocopherol, respectively. The oleic acid is very important for the oxidative stability of olive oil since the rate of oxidation increases with the number of the double bonds [24]. Memecik oils were found to have a higher percentage of oleic acid than Ayvalik oils [11]. In this study, Memecik oils were reported to have a higher content of total phenolic compounds and α-tocopherol compared to Ayvalik oils. Thus, it may follow that the oxidative stability of Memecik oils was higher than that of Ayvalik oils. On the other hand, Ayvalik oils had the highest content of total sterols and the highest percentage of Δ5 avenasterol and apparent β-sitosterol. Sterols are considered as functional food ingredient due to their protective effects on some diseases. They facilitate the reduction of total plasma cholesterol and LDL-cholesterol by opposing its absorption in the intestinal tract. They also prevent various types of cancer [25]. The total sterols content of Ayvalik oil was remarkably higher than that of Memecik oils. Therefore, it is possible to conclude that analyzed Ayvalik oil samples were of superior quality to those of Memecik oil samples with regard to their sterol content, but Memecik was of higher quality regarding the content of oleic acid, α-tocopherol and phenolic compounds.
Variability in the minor compounds of Aegean olive oil samples led us to perform multivariate statistical techniques to describe the characteristics of olive oil samples from Ayvalik and Memecik cultivars. A set of 15 variables was selected, which presented statistical differences with respect to the cultivars.
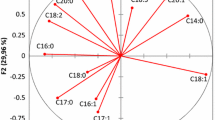
Principal component analysis (PCA) was used to find the variables that have more weight for characterizing samples from Aegean olive oils. The first two PCs explained 74.3% of the total variance. The first PC accounted for 65% of the total variance and was highly correlated with, p-coumaric acid, oleuropein aglycon, campesterol, stigmasterol, β-sitosterol, Δ5-avenasterol, apparent β-sitosterol and total sterols, where all of them had a higher loading than 0.8 (absolute value, Table 3). Five variables ( p-coumaric acid, oleuropein aglycon, campesterol, stigmasterol and β-sitosterol) had negative loading values on PC1 whereas three variables (Δ5-avenasterol, apparent β-sitosterol and total sterols) had positive loading values. PCA allows us to observe groupings of samples, which define the structure of the data set. The interpretation of the results of PCA is usually carried out by visualization of the component scores in a biplot. In Fig. 3, it can be observed that the PC1-PC2 plot permits an excellent differentiation of two groups of oils. Ayvalik oils have positive scores for the first component while Memecik oils have negative scores. In this way, the two cultivars constitute two distinctive groups. Therefore, it seems that the first component is related to the variety. An examination of factor loadings and scores showed that Ayvalik oils were characterized by a relatively higher content of Δ5-avenasterol, apparent β-sitosterol and total sterols. However, Memecik oils were correlated with p-coumaric acid, oleuropein aglycon, campesterol, stigmasterol and β-sitosterol. These minor compounds contain useful information to distinguish the two cultivars.
The LDA was used to classify Aegean olive oils according to the minor compounds. LDA was applied to a reduced data matrix. The eight variables that had a higher loading than 0.8 were selected. Stepwise procedure of discriminant analysis led us to select three variables. Stigmasterol, apparent β-sitosterol and total sterols were the significant variables to classify olive oils according to cultivar. The percentage of grouped samples correctly classified was 100%.
Summarizing the PCA and LDA results, the chemometric studies revealed that the variables which had the most distinctive power were stigmasterol, apparent β-sitosterol and total sterols. These parameters can be used as biomarker to classify Ayvalik and Memecik olive oils with respect to cultivar.
Statistical Evaluation of Differences Between both Crop Seasons
α-Tocopherol, total phenolic compounds, apparent β-sitosterol and total sterol content of Ayvalik and Memecik oils presented significant differences with respect to the crop seasons (p < 0.01). Samples from the 2007/2008 crop season contained more α-tocopherol, total phenolic compounds and total sterols than those from 2006/2007. These differences may be related to the variations in climatic conditions such as temperature and rainfall. The climatic data collected from the Turkish State Meteorological Service are presented in Fig. 4. and 5. Total phenolic compounds and α-tocopherol content of olive oils were reported to reach the highest level in the most dry years [26]. In our study, the content of antioxidant compounds of olive oils was the highest level in the 2007/2008 crop season, which seemed to have the lowest total rainfall of the January–September period. The changes in chemical composition of olive fruits are related to biochemical reactions taking place during olive development and ripening [27]. The biochemical reactions may be affected by climatic conditions. Multiple linear regression analysis was performed to analyze the relationship between minor compounds and climatic data. The linear regression model selected four parameters as independent variables: annual rainfall (x 1), total rainfall of the January–September period (x 2; corresponding to the rain before harvest season), average temperature of July–September period (x 3; corresponding to the development of the olive fruits) and average temperature of September–October period (x 4; corresponding to the ripening of the olive fruits). The minor compounds, tocopherol, total phenols, apparent β-sitosterol and total sterols, measured for the two crop seasons, were selected as dependent variables. A good correlation was observed between minor compounds and climatic variables, with a r-squared of 0.989; 0.998; 0.938; 0.996, for tocopherol, total phenols, apparent β-sitosterol and total sterols, respectively. The following regression equations were obtained:
The correlations between climatic variables and the minor compounds of olive oil may be explained by the effect of climatic variables on biochemical reactions, especially enzymatic reactions. The activity of enzymes is affected by environmental factors such as temperature and water availability. The studies of irrigation effects on olive composition revealed that the activity of l-phenylalanine ammonia lyase, which is responsible for biosynthesis of phenolic compounds, increases with water stress [28]. In our study, total polyphenol content of samples of the 2007/2008 crop season was found to be higher than those of the 2006/2007 crop season, which was registered as having the highest annual rainfall.
The results of our study confirm that the crop season may affect the chemical composition of olive oil, especially the antioxidants but the total sterol content as well. The effect of crop season can be attributed to climatic conditions, especially, the rainfall and average temperature during the growth and ripening of the olive fruits. Therefore, it is important to include different cultivation years for each cultivar in a characterization study. In this study, samples from two harvest years of Ayvalik and Memecik cultivars were used to set up chemometric analysis which is capable of discriminating between both cultivars, irrespective of the intra-cultivar variability over the cultivation years.
References
Del Caro A, Vacca V, Poiana M, Fenu P, Piga A (2006) Influence of technology, storage and exposure on components of extra virgin olive oil (Bosana cv) from whole and de-stoned fruits. Food Chem 98:311–316
Gimeno E, Castellote AI, Lamuela-Raventos RM, De Torre, la Torre MC, Lopez-Sabater MC (2002) The effects of harvest and extraction methods on the antioxidant content (phenolics, α-tocopherol and β-carotene) in virgin olive oil. Food Chem 78:207–211
International Olive Oil Council (2009). http://www.internationaloliveoil.org/downloads/exportiations1-ang.PDF
Andjelkoviç M, Acun S, Van Hoed V, Verhe R, Van Camp J (2009) Chemical composition of Turkish olive oil-Ayvalik. J Am Oil Chem Soc 86:135–140
Turkish Patent Institute (2009). http://www.tpe.org.tr
Marini F, Magri AL, Bucci R, Balestrieri F, Marini D (2006) Class-modeling techniques in the authentication of Italian oils from Sicily with a protected denomination of origin (PDO). Chemometr Intell Lab Sys 80:140–149
Alves MR, Cunha SC, Amaral JS, Pereira JA, Olieveria MB (2005) Classification of PDO olive oils on the basis of their sterol composition by multivariate analysis. Anal Chim Acta 549:166–178
Aparicio R, Luna G (2002) Characterisation of monovarietal virgin olive oils. Eur J Lipid Sci Technol 104:614–627
Gürdeniz G, Ozen, Tokatlı F (2008) Classification of Turkish olive oil with respect to cultivar, geographic origin, and harvest year, using fatty acid profile and mid-IR spectroscopy. Eur Food Res Technol 227:1275–1281
Ocakoglu D, Tokatli F, Ozen B, Korel F (2009) Distribution of simple phenols, phenolic acids and flavonoids in Turkish monovarietal extra virgin olive oils for two harvest years. Food Chem 113:401–410
Dıraman H, Dibeklioğlu H (2009) Characterization of Turkish virgin olive oils produced from early harvest olives. J Am Oil Chem Soc 86:663–674
Romero MP, Tovar MJ, Ramo T, Motilva MJ (2003) Effect of crop season on the composition of virgin olive oil with protected designation of origin “Les Garrigues”. J Am Oil Chem Soc 80:423–431
European Union Commission. Regulation EEC (1989/2003) on the characteristic of olive oil and olive pomace and their analytical methods. Official Journal of the European Commission L295
Verleyen T, Forcades M, Verhe R, Dewettinck K, Huyghebaert A, De Greyt W (2002) Analysis of free and esterified sterols in vegetable oils. J Am Oil Chem Soc 79:117–122
Gutfinger T (1981) Polyphenols in olive oil. J Am Oil Chem Soc 58:966–968
Mateos R, Espartero JL, Trujillo M, Rios JJ, Leon-Camacho M, Alcudia F, Cert A (2001) Determination of phenols, flavones and lignans in virgin olive oils by solid phase extraction and high performance liquid chromatography with diode array ultraviolet detection. J Agric Food Chem 49:2185–2192
Ben Temime S, Wael T, Bechir B, Leila A, Douja D, Mokthar Z (2006) Changes in olive oil quality of Chetoui variety according to origin of plantation. J Food Lipids 13:88–99
Pardo JE, Cuesta MA, Alvarruiz E (2007) Evaluation of potential and real quality of virgin olive from the designation of origin “Aceite Campo de Montiel” (Ciudad Real, Spain). Food Chem 100:977–984
Psomiadou E, Tsimidou M, Boskou D (2000) α-Tocopherol content of Greek virgin olive oils. J Agric Food Chem 48:1770–1775
Garcia A, Brenes M, Garcia P, Romero C, Garrido A (2003) Phenolic content of commercial olive oils. Eur Food Res Technol 216:520–525
Stefanoudaki E, Kotsifaki F, Koutsaftakis A (2000) Sensory and chemical profiles of three European olive varieties (Olea europea L.); an approach for the characterization and authentication of the extracted oils. J Sci Food and Agric 80:381–389
Dhifi W, Hamrouni S, Ayachi S, Chahed T, Saidani M, Marzouk B (2004) Biochemical characterization of some Tunisian olive oils. J Food Lipids 11:287–296
Del Alamo RMR, Fregapane G, Aranda F, Gomez-Alanso S, Salvador MD (2004) Sterol and alcohol composition of Cornicabra virgin olive oil: the campesterol content exceeds the upper limit of 4% established by EU regulations. Food Chem 84:533–537
Morello JR, Motilva MJ, Tovar MJ, Romero MP (2004) Changes in commercial virgin olive oil (cv Arbequina) during storage, with special emphasis on the phenolic fraction. Food Chem 85:357–364
Ben Temime S, Manai H, Methenni K, Baccouri B, Abaza L, Daoud D, Casas JS, Bueno EO, Zarrouk M (2008) Sterolic composition of Cheutoi virgin olive oil: influence of geographical origin. Food Chem 110:368–374
Beltran G, Aguilera MP, Del Rio C, Sanchez S, Martinez L (2005) Influence of fruit ripening process on the natural antioxidant content of Hojiblanca virgin olive oils. Food Chem 89:207–215
Conde C, Delrot S, Geros H (2008) Physiological, biochemical and molecular changes occurring during olive development and ripening. J Plant Phys 165:1545–1562
Gomez-Rico A, Salvador MD, Moriana A, Perez D, Olmedilla N, Ribas F, Fregapene G (2007) Influence of difference irrigation strategies in a traditional Cornicabra cv olive orchard on virgin olive oil composition and quality. Food Chem 100:568–578
Acknowledgment
The authors would like to express their thanks to the Administrative Board of TARIS (Olive and Olive Oil Agricultural Sales Cooperatives Union); Cahit Çetin, Head of Administrative Board and the technical personnel: Mükerrem Keskiner, Veli Özdemir, Özlem Yalçın, and Meltem Zengin for supplying the olive oil samples and for their assistance.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ilyasoglu, H., Ozcelik, B., Van Hoed, V. et al. Characterization of Aegean Olive Oils by Their Minor Compounds. J Am Oil Chem Soc 87, 627–636 (2010). https://doi.org/10.1007/s11746-009-1538-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-009-1538-5