Abstract
The present study reports the olive oil quality properties, namely free fatty acid, peroxides index, purity properties [fatty acid and triacylglycerol (TAG) composition], antioxidant compounds (total phenol, carotenoid and chlorophyll content) and oxidative stability. These analyses were performed for three rare cultivars grown in southern Tunisia (region of Tataouine). All the samples have presented good quality according to the international olive council norms. Nevertheless, there was a difference in many of the evaluated parameters as a function of ripening stages and cultivars. The oleic acid and linoleic acid amounts of olive oils increased with the ripening progress, with the highest rates were found in Fakhari cultivar. The most abundant triacylglycerols of olive oils were OOO, POO, LOO + PLnP, PLO + SLL and POP. Moreover, a general decrease (p < 0.05) of the total phenolic content, pigments content and oxidative stability were observed during maturation. The principal component analysis was used to classify nine olive oils samples depending on cultivars and maturity degree.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Olive oil is the main component of the Mediterranean diet. As a dietary fat, it has several therapeutic benefits, in particular to regulate plasma levels of cholesterols and prevent cancers, cardiovascular illnesses, and inflammatory diseases [1, 2]. Furthermore, virgin olive oil does not only have a high resistance to oxidative deterioration for its high content of monounsaturated-to-polyunsaturated fatty acid ratio, but also contains a pool of minor compounds of powerful antioxidant activities, among which polyphenols stand out. These compounds contribute to the satisfactory organoleptic characteristics of oil like aroma and flavor of the olive oil complex, they are largely responsible for its shelf life [3, 4]. Moreover, pigments and squalene was reported to play a role in preventing olive oil oxidation [5, 6]. Tunisia is one of the most important olive oil-producing countries in the world after Spain, Italy, and Greece with an average of production equal to 180,000 tons in 2012 [7]. Almost all studies on Tunisian virgin olive oil have focused on the characterization of two varieties: Chétoui in the northern regions and Chemlali in the central and southern parts [8]. Consequently, there is a lack of information on the chemical characteristics of several minor varieties that are sustained in restricted areas in different parts of Tunisia. A good example is the case of the region of southern Tunisia (Tataouine). Because of the severe pedoclimatic conditions (shallow soils, high temperature, and low rainfall), the traditional diet in the region is rather simple, and it is based on some specific plant foods, like cereals or palm fruits, with olive oil being the main fat source [9]. The varieties of that region are still commercially unexplored, and their VOO qualities and composition have not been fully characterized. For oil-mill purposes, the better harvesting time should be when the olives achieve their highest oil content and the oil quality is the best. Olive growers usually use the changes in fruit color as a guideline to begin the harvest period. However, this parameter is not always valid because fruit maturation and oil accumulation rate may change with the cultivar and environmental conditions. In fact, several authors have studied the influence of genetic, environmental and agronomic factors on olive oil yield and quality to establish the optimum harvesting period [6, 10,11,12,13,14].
However, little is known about how geographic and climate conditions may affect the properties of the olive oil that is being produced in Tataouine. Therefore, the aim of this study is to describe the differences found between the fatty profiles and oxidative state of three olive cultivars (Jemri, Touffehi and Fakhari), as well as their evolution throughout the ripening process.
Materials and methods
Chemicals
All chemicals and reagents were of analytical grade. Squalene ≥ 98 was purchased from Sigma–Aldrich (Steinheim, Germany). Methanol, hexane, acetone, acetic acid, and cyclohexane HPLC-grade solvents were purchased from Riedel–deHaen (Switzerland). The solvents were of appropriate purity. Double distilled water was used in the HPLC mobile phase. Folin–Ciocalteu reagent and gallic acid were obtained from Fluka (Switzerland).
Sampling and oil extraction
The analysis was applied to samples of VOOs from the South of Tunisia (Zone of Tataouine) (See Fig. 1). The climate of the production area is characterized by warm temperate climate with dry seasons and hot summer. The olive samples chosen for this study were from selected olive varieties: Jemri, Fakhari and Touffehi. To control the changes in the olive oil composition during the maturity progress, samples were selected at three different harvesting times, beginning from October 12 to December 23, according to their color. All the samples were obtained under a two-phase extraction system, corresponding to the same crop harvest (2015–2016). The annual rainfall and temperature averages during the studied year 2015/2016 were 140 mm and 20–45 °C, respectively. Oil extraction were carried out using an Abencor laboratory oil-mill (Abengoa, Spain) equipped with a crusher, a vertical malaxator and a paste centrifuge from handpicked fresh olives (2.5 kg). The extractor is equipped with a metal crusher, a mixer whose internal partition is in rustproof steel, having a vertical or horizontal axis, and a basket centrifuge. Fruits were cleaned from leaves and milled in the hammer crusher; the olive paste was then mixed for 30 min at 28 °C. Afterwards, the oil was separated by a vertical centrifuge (3500 rpm over 3 min). The produced oils were filled in dark bottles and stored at 3 °C until analysis.
Determination of agronomic indices
The maturity progress was controlled by calculating the maturity index of olives before each extraction. The maturity index (MI) of olives was established by visual appreciation of the color samples of 100 fruits according to a color scale varying from green-intense skin to a black skin and an entirely violet pulp. The maturity index values range from 0 to 7 as described by Hermoso et al. [15]. For the oil content, it was determined in the subsamples using a nuclear magnetic resonance fat analyzer according to the method of Lazzez et al. [16]. Subsequently, three subsamples of approximately 100 fresh fruits were arbitrarily chosen to evaluate the fruit fresh weight. Next, they were dried in a forced-air oven at 105 °C during 42 h until dehydration. Dried samples were weighed to estimate fruit dry weight.
Quality indices determination
Free acidity, conventionally expressed in oleic acid (g/100 g) and peroxide value (PV) (meqO2/kg) were determined according to the International Olive Council standard (IOC) [7].
Pigment content
Carotenoids and chlorophylls (mg/kg of oil) were determined at 470 and 670 nm, respectively, in cyclohexane using the specific extinction values according to the method of Minguez Mosquera et al. [17]
-
chlorophyll (mg/kg) = (A670 × 106)/(613 × 100 × d)
-
carotenoid (mg/kg) = (A470 × 106)/(2.000 × 100 × d)
where A is the absorbance and d is the spectrophotometer cell thickness (1 cm). The chlorophyll and carotenoid concentrations are expressed as mg of pheophytin and lutein, respectively, per kg of oil.
Fatty acid and squalene determinations
About 0.120 g of olive oil (accurately weighed, ± 0.001 g) in 2,2,4-Trimethylpentane (isooctane, 2 mL) was trans-methylated with a cold solution of KOH (2 M) (200 µL) according to the European Standard NF EN ISO 12966-2 [10]. Fatty acid methyl esters (FAME) were analyzed according to the European Standard NF EN ISO 5508 [11]. Analyses were performed on an Agilent Technology gas chromatograph 7890A (GC) equipped with a split/split-less injector (T = 250 °C) and flame ionization detector (FID) (T = 250 °C). A silica capillary column (60 m × 0.25 mm i.d., 0.25 µm film thickness) coated with polyethylene glycol (Supelcowax, Supelco, France) was used. The carrier gas was hydrogen (column flow 1 mL/min), and the split ratio was 1:60. The oven temperature was programmed as follows: 20 min at 210 °C, from 210 to 245 °C at 6 °C/min, 20 min at 245 °C.
The identification of FAs was performed by the comparison of retention times with those of olive oil FAs, whose composition is known [12]. FA percentages were determined by internal standardization without taking into account the mass response factors. The coefficients of variation in FA percentages were lower than 5% for the most important FAMEs and lower than 10% for some minor ones. Squalene was analyzed by gas chromatography, at the same time as the fatty acid methyl esters. The quantification method is easy to implement and requires no additional sample preparation. Squalene was well separated from lignoceric acid (24:0) (retention times 38.48 and 37.55 min, respectively). It was determined along with the FAs using an external standard calibration curve (Eq. 1), where A is the peak area of squalene and C is the concentration expressed in mg/mL (linearity 0.030–0.60 mg/mL, R2 = 0.999).
The obtained results, calculated based on the analyzed oil weight, were expressed in mg/kg with a coefficient of variation equal to 1.5% according to the experimental error. Nomenclature Fatty acids: 16:0, palmitic acid (hexadecanoic acid); 16:1ω9, hypogenic acid (7-hexadecenoic acid); 16:1ω7, palmitoleic acid (9-hexadecenoic acid); 17:0, margaric acid (heptadecanoic acid); 17:1ω8, margaroleic acid, 18:0, stearic acid, (9-heptadecenoic acid); 18:1ω9, oleic acid (9-octadecenoic acid); 18:1ω7, z-vaccenic acid (11-octadecenoic acid); 18:2ω6, linoleic acid (9,12-octadecadienoic acid); 18:3ω3, linolenic acid (9,12,15-octadecatrienoic acid); 20:0, arachidic acid (eicosanoic acid); 20:1ω9, gondoic acid (11-eicosenoic acid); 22:0, behenic acid (docosanoic acid); 24:0, lignoceric acid (tetracosanoic acid).
Analysis of triacylglycerol composition
Triacylglycerols were analyzed by an HPLC system composed of a Merck liquid chromatography model LaChrom equipped with a Merck RP-18 Supersphere 100 column (250 mm × 4 mm, particle size 4 µm, temperature 28 °C) coupled with a Merck refractometric L-7490 detector. The mobile phase was propionitrile with a flow rate linear gradient (0.5–1 mL/min) for 47 min. A volume of 10 µl of each sample prepared at a concentration of 5% (w / v) in propionitrile was injected into an HPLC apparatus. Triacylglycerols in virgin olive oils were separated according to equivalent carbon number (ECN), defined as CN-2n, where CN is the total acyl carbon number and n is the number of double bonds of fatty acids.
Total phenol content determination
The total phenol content of the olive oil samples was determined using the Folin Ciocalteu method [18]. Briefly, a 50 mL aliquot of the extracts was assayed with 250 mL Folin reagent and 500 Ml sodium carbonate (200 g/L). The mixture was vortexed and diluted with water to a final volume of 5 mL. After incubation for 30 min at room temperature, the absorbance was read at 765 nm; total phenols were expressed as gallic acid equivalents (GAE) using a calibration curve of a freshly prepared gallic acid solution (y = 0.0012x + 0.0345, R2 = 0.9997).
Determination of oxidative stability by Rancimat apparatus
The oil samples (5.0 g) were heated in Rancimat equipment at 120 °C (Metrohm Ltd., Herisau, Switzerland) with a continuous airflow of 20 L/h passing through the samples. The conductivity cells were filled with 60 mL of deionized water. The time needed for the appearance of a sudden water conductivity rise, caused by the adsorption of volatiles derived from oil oxidation, was registered in hours as the induction time [19].
Chemometric analysis
Principal component analysis (PCA) was performed on all the chemical values: FAs, TAG, squalene, total phenols, and oxidative stability regrouped for the three cultivars depending on the ripening stage. All the chemometric analyses were performed using XLSTAT software for Windows (v.2013.2.03, Addinsoft, New York, USA).
Results and discussion
Maturity index
The maturity indices (MI) for each cultivar during harvest time olives are shown in Table 1. The MI values of the samples were determined for each variety. Concerning the MI of Jemri olive samples, they were classified into three groups; green (MI = 3.5); purple (MI = 4.2); black (MI = 5.46). As for those of Touffehi olives, they were found as green (MI = 1.52); purple (MI = 2.78); black (MI = 3.83). For Fakhari oil, MI classification is as follows: green (MI = 1.72); purple (MI = 3.4); black (MI = 4.11). There is an increase in MI values from October to December for each cultivar, with the highest MI value recorded for Jemri cultivar.
Oil content
During fruit ripening period, oil content reached its maximum at the last harvest sample for all studied samples. Subsequently, it increased significantly as the ripening progressed. This tendency is in agreement with that found in the findings of different authors [20, 21]. The cultivars under investigation showed values of oil content between 17.02% and 18.57% in fresh fruit and between 40.57% and 54.32% in dry fruit as shown in Table 1. The oil content in fresh fruit is profoundly affected by moisture levels. Therefore, the use of oil content in dry fruit is very accurate to compare between varieties [10]. In fact, Jemri olive fruits contain the high oil content (54.32%) followed by Touffehi and Fakhari cultivars. The behavior of this parameter is closely related to the activity of the triglyceride-forming biosynthesis pathway until the fruit reaches full maturation [22].
Free acidity and peroxide indices
As shown in Table 1, the free acidity of three olive oils ranged from 0.19 to 0.36%. Meanwhile, the peroxide values of the studied olive oils ranged from 3.21 to 10.19 meq O2/kg. Both the free acidity and peroxide values were within the limit of the values established by IOC [7]. All olive oil samples were categorized as “extra virgin olive oil”. Indeed, free fatty acidity values increased slightly during ripening. In contrast, peroxide indices significantly decreased in the same harvest time. This result is in accordance with other studies [6, 23, 24].
Chlorophyll and carotenoid content
Due to their antioxidant nature in the dark and pro-oxidant in the light, the pigments play an important role in the oxidative stability of the oil during its storage. Furthermore, they are mainly responsible for the color of olive oil, which varies from yellow–green to greenish gold [25]. The values of the analyzed olive oils, chlorophylls and carotenes are fluctuating between 0.21 and 3.33 mg/kg and from 1.53 to 6.52 mg/kg, respectively. Touffehi oils showed on average higher pigment content than Fakhari and Jemri oils. The evolution of the pigment content during olive maturation (Table 1) shows a progressive decrease of these pigments. It is found that the oils obtained from olives harvested at the first stage of maturation contain significant amounts of chlorophylls and carotenes. However, the levels decrease considerably during ripening until reaching very low values in fully ripe olives. Taking the example of the Touffehi variety, the amount of chlorophyll decreased from 2.52 in October to 0.53 mg/kg in December. This behavior is also observed in the studies developed by Mraicha et al. [26] It can be explained by the chlorophylls transformation into anthocyanins during maturation, from green fruit to the purple coloration. In the same context, other studies have shown that the concentration of chlorophylls may exceed 80 mg/kg for oils obtained from olives in the early stage of maturity. These values may decrease to about 2 mg/kg when the fruit is fully ripe [24, 27]. Therefore, as maturation progresses, photosynthetic activity decline and the level of the two pigments: chlorophylls and carotenoids gradually decreases [28]. The values of chlorophylls found in the present study are lower than those described in Tunisia (cv, Arbequina) [29] and Turkish (cv, Halhali) EVOO [30], whereas the levels of carotenoids are similar to those shown in olive oils from Tunisia and Brazil [5, 21]. However, the concentration of pigments can vary significantly depending not only on harvesting time and olive cultivars, but also on geographic and climate characteristics which seem to affect the pigment content of olive oils [5, 31].
Fatty acids composition
The fatty acids composition is presented in Table 2. Fourteen fatty acids were identified and quantified. The results showed that the fatty acid composition varied considerably depending on the variety and the maturity stage. As expected, the major fatty acids found in all samples is oleic acid (C18:1) ranging from 54.26 to 73.01%. Fakhari oil is the richest one in oleic acid followed by Toffehi and Jemri. According to the IOC [1], the EVOO is defined with a percentage of oleic acid between 55 and 83℅. This is the case of the two varieties of Fakhari and Toffehi during the 3 harvest periods. These percentages are close to those of the Tunisian varieties Zalmati and Chetoui, cultivated in the Tunisian center, which are 62.15% and 65.66%, respectively [32]. However, for the Jemri variety, oleic acid amount is slightly lower than the commercial standard (54.26%) in October. Similar results were found by Laroussi et al [33] for other Tunisian varieties. They have reported that a percentage of oleic acid less than 55% can be observed under very specific conditions (low maturity index < 2 and excessive heat ≥ 41 C). In fact, high temperatures delay the biosynthesis of fatty acids. Indeed, maturation progress favors the increase of oleic acid levels for all the studied varieties. Indeed, the oleic acid amount increased as the skin color of the olives changed from green to black. For example, this rate varied from 65 to 68.35% between October and December for Touffehi oils. The same behavior was recorded for the percentages of linoleic acid that marks an improvement during ripening for all varieties. On the contrary, the percentages of palmitic acid were high at the beginning of fruit ripening and then decreased gradually. These results are consistent with those found by Chtourou et al. [21], who observed an increase in linoleic acid levels during maturation for the Oueslati variety cultivated in the center of Tunisia.
The sum of saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), and polyunsaturated fatty acids (PUFAs), as well as the oleic acid/linoleic acid ratio were also determined during the maturation in all samples. The olive oils of the three studied varieties had high concentrations of MUFA in the Fakhari variety followed by Touffehi and Jemri oils. These values strongly correlate with oleic acid levels, the major acid in VOO. The values of the sum of UFA mainly take into account the oleic and linoleic acids, which explains the similar variation as the MUFAs. Fakhari and Touffehi olive oils have higher levels of unsaturated fatty acids than Jemri oils. The ratio between (C18:1) and (C18:2) shows that the Fakhari variety is the most stable compared to the other varieties with a rate of 7.59℅. The PUFA level reflects the richness of the oils in linoleic and linolenic acids. The highest average value of PUFA was obtained for the Jemri variety. In fact, the level of linoleic acid increased gradually from 13.64 to 19.18% during fruit ripening. This variation is due to the enzymatic activity of the oleate desaturase enzyme which converts oleic acid to linoleic acid [33].
Finally, the sum of SFA evaluates the level of saturated fatty acids of the three Tunisian varieties of olive oil. Jemri oils have the highest mean values of SFA (≈ 21.5%) followed by Touffehi (18.18%) and Fakhari (17.37%). The rate of this sum was found to decrease during maturation in all the studied varieties. It seems that the phenomenon of lipid accumulation during the maturation of olives is accompanied by an increase at the beginning, followed by a drop in saturated fatty acid levels in favor of mono and polyunsaturated fatty acids.
Triacylglycerol Composition
The different molecular species of TAG were separated according to their equivalent carbon number (ECN), Table 3. In the oils analyzed, there were essentially fourteen molecular species. In order of quantitative importance, the main triacylglycerols were the triolein ‘OOO’ followed by the dioleopalmitin ‘POO’, ‘LOO + PLnP’, ‘PLO + SLL’, ‘POP’ and ‘SOO’. These Six predominant TAG species account for 90% of the total peak areas in the chromatogram. Other species are poorly represented (< 2%). These results are comparable to those found for other Tunisian [34] and French olive oils [35].
The examination of the same table shows that for all the studied olive varieties, the oils are characterized by the abundance of triolein (OOO) whose rate is very high in Fakhari variety (39.43%) followed by Touffehi and Jemri. The rate of this TG increases during fruit ripening. For example, it ranges between 19.10% in October and 27.01% in December for Jemri oil. There are two opposite behaviors for (OOP). Actually, the Jemri oils show a rise in the levels of this TG, while a significant decrease is marked for the Toffehi and Fakhari oils between the first and last maturity stage. These differences are probably related to the difference in maturity stage of olives at harvest and the varietal factor [34]. The studied olive oils were very rich in oleic acid and poor in acids: linoleic, linolenic, stearic, and palmitic. This can be explained by the presence in olive oil of high triglyceride levels including oleic acid such as triolein (OOO) and conversely low levels of triglycerides containing linoleic acid, such as trilinolein (LLL). This last TG is a favorable index of oil authenticity [36]. In the case of the studied oils, the LLL content varied according to the varieties but always obeyed the IOC limit (< 0.5%) for all varieties except the Jemri oil at the first stage of maturation (0.86% in October). It is the same sample with oleic acid content below the IOC limit.
Squalene content
Squalene is a precursor in the pathway of cholesterol biosynthesis. Studies have repeatedly shown that olive oil has the highest squalene content compared with other vegetable oils. The presence of squalene in HOV has a protective effect on oxidation stability by heating. The data obtained from Fig. 2a. show that the squalene contents are varied for the three cultivars of olive oils during the different consecutive maturity stages. In fact, the concentrations vary between 0.24 and 1.29%, with Touffehi oils having the highest values of this hydrocarbon, followed by those of Fakhari and Jemri. Indeed, there is a general tendency of decrease from the first to the last harvesting time. In a previous study, Cuesta et al. [37] have reported that the content of squalene in olive oil is the result of two different biosynthetic pathways. The authors suggested that the concentration of squalene in the oil depends not only on its content in the fruit, but also on the oil content in the olive. In fact, the content of squalene in the fruit decreases during maturation due to its participation in the biosynthesis of other compounds, such as sterols and triterpenes. Furthermore, the change in oil content in the olives during ripening causes variation in the concentration of this triterpene.
Total phenol content
Olive oils are known for their high content of phenolic compounds compared to other refined vegetable oils. The obtained results showed that the total polyphenol (PT) content of the olive oils analyzed varied between varieties (Fig. 2b). Thus, the Fakhari variety was characterized by the highest level (312.49 mg/kg), while the Touffehi and Jemri varieties have the lowest values. Indeed, the PT values of the olive oils extracted from green stage olives were significantly higher than other olive oil samples and decreased with the ripening of the fruits. For example, in the Toffehi variety, PT content decreased from 275.54 in October to 226.43 mg/kg in December. This result is in agreement with that obtained by Bengana et al. [38]. In this context Mraicha et al. [26] have also confirmed that the highest PT amount corresponds to olives with the lowest maturity index. As a result, the variations in the observed polyphenol levels may be due to the difference in the degree of maturity of olives before trituration (early olive harvest), and depend on the varietal profile and the geographical area [39].
Oxidative stability
Oxidative stability is an important property of olive oil, which depends on the lipid composition and antioxidant effects of phenolic compounds [5]. In our study, the highest stability was found in the Fakhari variety (14–23 h), which may be due to its low polyunsaturated fatty acid composition (≈ 10%) conjugated to its high total phenols content (260–312 mg/kg). The oxidation stability also varies according to the ripening stage (Fig. 2c). There is a significant decrease for all studied olive oils from the beginning to the end of harvest time. As previously shown, Jemri oils decreased from 17.22 h in green fruits to 9.32 h in ripe ones. In the same context, Nieto et al. [40] have also found that the oxidative stability and the total phenol content of the Picual, Hojiblanca, and Arbequina (Spain) oils decrease as a function of MI during the ripening process. In addition, the study of correlations between the total phenol concentrations, the C18:1/C18:2 ratio and the oxidative stability of olive oil from the three studied cultivars at the first stage of maturity (Fig. 3) reveals a strong correlation between total phenols and oxidative stability, as well as between the C18:1/C18:2 ratio and stability. Oxidative stability is related to the concentration of the oil in monounsaturated and polyunsaturated fatty acids, pigments and phenol content. It is also influenced by several other factors, such as variety, extraction systems and storage conditions [5, 13].
Chemometric analysis
Principal component analysis (PCA)
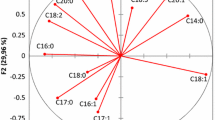
To characterize the effect of ripening stage and cultivars on the composition of extra virgin olive oil, a PCA, including oxidative stability, total phenols content, TAG composition and fatty acid profile, was performed. The obtained two main components explained 81.54% of the total variance (62.68% PC1 and 18.85% PC2). Figure 4a depicts the vector arrows of the variables, showing their contribution to PC1 and PC2. The first component was mainly explained by the fatty acid profile, with C16:0, C18:1w9, C18:1w7, C18:1/C18:2, SFA MUFA and PUFA, producing the greatest influence (loadings of − 0.971, 0.993, − 0.9734, 0.975, 0.962, 0.992 and − 0.983, respectively). TAG profile, with LLL, OOO and SOO were also an important contributor to PC1 (loading − 0.927, 0.992, and 0.961, respectively). The second component was clearly dominated by squalene, oxidative stability and total phenols (loadings of 0.890, − 0.824 and − 0.746, respectively). Figure 4b represents the spatial distribution of the oils according to PC1 and PC2. As regards these factors, the PCA of the samples revealed the separation of the three cultivars depending on their ripening stage into three distinct clusters. With respect to the first factor, we can observe that sample 4 is located in the extreme left hand side of the graph, followed by samples 4 and 5 in Jemri oils, while there is evident proximity among the Fakhari oils 1, 2 and 3, which are all located on the right-hand side of the graph. This distribution is associated with the total phenol content, oxidative stability and fat composition, particularly the oleic acid content and OOO percentage. Taking into account factor 1, Touffehi samples 7, 8 and 9 are located in the higher part of the graph, mainly due the TAG composition of the oils and squalene content.
Conclusion
-
The characteristics of Jemri, Touffehi and Fakhari olive oils from southern Tunisia have revealed that the cultivar and the ripening process may affect the physicochemical properties and composition of olive oils. Palmitic acid, oleic acid, and linoleic acid were the major fatty acids that conformed to standards, except Jemri oils in the first harvesting time. The ratios of MUFA/PUFA and oleic acid/linoleic acid decreased during ripening. Oxidative stability, total pigments contents and total phenol content of olive oils decreased with ripening. PCA analyses of olive oils indicated that different TAG and fatty acid components were responsible for characterization and classification of olive oils according to ripening stages. By sampling and monitoring the ripeness index monthly, it would be possible to determine an optimal harvesting time for olives according to the industrial yield and the physicochemical and nutritional properties of the olive oil.
References
del Monaco G, Officioso A, D’Angelo S et al (2015) Characterization of extra virgin olive oils produced with typical Italian varieties by their phenolic profile. Food Chem 184:220–228. https://doi.org/10.1016/j.foodchem.2015.03.071
Gouvinhas I, de Almeida JMMM, Carvalho T et al (2015) Discrimination and characterisation of extra virgin olive oils from three cultivars in different maturation stages using Fourier transform infrared spectroscopy in tandem with chemometrics. Food Chem 174:226–232. https://doi.org/10.1016/j.foodchem.2014.11.037
Ben Brahim S, Amanpour A, Chtourou F et al (2018) Gas chromatography–mass spectrometry–olfactometry to control the aroma fingerprint of extra virgin olive oil from three tunisian cultivars at three harvest times. J Agric Food Chem 66:2851–2861. https://doi.org/10.1021/acs.jafc.7b06090
Oueslati I, haddada M, Nouairi F I, et al (2009) Changes in the biochemical composition of the tataouine virgin olive oils during thermal oxidation. J Arid Land Stud 19:93–96
Borges TH, Pereira JA, Cabrera-Vique C et al (2017) Characterization of Arbequina virgin olive oils produced in different regions of Brazil and Spain: physicochemical properties, oxidative stability and fatty acid profile. Food Chem 215:454–462. https://doi.org/10.1016/j.foodchem.2016.07.162
Köseoğlu O, Sevim D, Kadiroğlu P (2016) Quality characteristics and antioxidant properties of Turkish monovarietal olive oils regarding stages of olive ripening. Food Chem 212:628–634. https://doi.org/10.1016/j.foodchem.2016.06.027
International Olive Council (IOC) (2013) Trade standard applying to olive oil and olive-pomace oil. IOC: Madrid, Spain, May 2013; COI/ T.15/NC No 3/Rev
Rhouma A, Gharsallaoui M, Khlif M (2005) Etude de l’effet de stockage des olives des deux variétés chemleli et chetoui sur les caractéristiques de leur huile. Rev Ezzitouna 10:52–59
Oueslati I, Anniva C, Daoud D et al (2009) Virgin olive oil (VOO) production in Tunisia: the commercial potential of the major olive varieties from the arid Tataouine zone. Food Chem 112:733–741
Ben Brahim S, Kelebek H, Ammar S et al (2017) LC–MS phenolic profiling combined with multivariate analysis as an approach for the characterization of extra virgin olive oils of four rare Tunisian cultivars during ripening. Food Chem 229:9–19. https://doi.org/10.1016/j.foodchem.2017.02.025
Aguilera MP, Beltran G, Sanchez-Villasclaras S et al (2010) Kneading olive paste from unripe ‘Picual’ fruits: I. Effect on oil process yield. J Food Eng 97:533–538
Jiménez B, Sánchez-Ortiz A, Lorenzo ML, Rivas A (2013) Influence of fruit ripening on agronomic parameters, quality indices, sensory attributes and phenolic compounds of Picudo olive oils. Food Res Int 54:1860–1867. https://doi.org/10.1016/j.foodres.2013.08.016
Ben Brahim S, Marrakchi F, Gargouri B, Bouaziz M (2015) Optimization of malaxing conditions using CaCO3 as a coadjuvant: a method to increase yield and quality of extra virgin olive oil cv. Chemlali. LWT Food Sci Technol 63:243–252. https://doi.org/10.1016/j.lwt.2015.03.013
Brahim SB, Gargouri B, Marrakchi F, Bouaziz M (2016) The effects of different irrigation treatments on olive oil quality and composition: a comparative study between treated and olive mill wastewater. http://pubs.acs.org/doi/abs/10.1021/acs.jafc.5b05030. Accessed 20 Feb 2018
Hermoso M, Uceda M, García A, Morales B, Frias ML, Fernández A (1991) Elaboración de Aceite de Calidad; Consejeria de Agricultura y Pesca. Sevilla, Spain, Serie Apuntes 5/92
Lazzez A, Perri E, Caravita MA et al (2008) Influence of olive maturity stage and geographical origin on some minor components in virgin olive oil of the chemlali variety. J Agric Food Chem 56:982–988. https://doi.org/10.1021/jf0722147
Mínguez-Mosquera MI, Gandul-Rojas B, Montaño-Asquerino A, Garrido-Fernández J (1991) Determination of chlorophylls and carotenoids by HPLC during live lactic fermentation. Res Gate 585:259–266. https://doi.org/10.1016/0021-9673(91)85086-U
Bouaziz M, Jemai H, Khabou W, Sayadi S (2010) Oil content, phenolic profiling and antioxidant potential of Tunisian olive drupes. J Sci Food Agric 90:1750–1758
Bouaziz M, Feki I, Ayadi M et al (2010) Stability of refined olive oil and olive-pomace oil added by phenolic compounds from olive leaves. Eur J Lipid Sci Technol 112:894–905
Talhaoui N, Gómez-Caravaca AM, Roldán C et al (2015) Chemometric analysis for the evaluation of phenolic patterns in olive leaves from six cultivars at different growth stages. J Agric Food Chem 63:1722–1729
Bouchaala FC, Lazzez A, Jabeur H et al (2014) Physicochemical characteristics of extra virgin olive oil in function of tree age and harvesting period using chemometric analysis. Sci Hortic 180:52–58. https://doi.org/10.1016/j.scienta.2014.09.056
Dag A, Kerem Z, Yogev N et al (2011) Influence of time of harvest and maturity index on olive oil yield and quality. Sci Hortic 127:358–366. https://doi.org/10.1016/j.scienta.2010.11.008
Beltrán G, Aguilera MP, Del Rio C et al (2005) Influence of fruit ripening process on the natural antioxidant content of Hojiblanca virgin olive oils. Food Chem 89:207–215
Salvador MD, Aranda F, Fregapane G (2001) Influence of fruit ripening on ‘Cornicabra’ virgin olive oil quality a study of four successive crop seasons. Food Chem 73:45–53. https://doi.org/10.1016/S0308-8146(00)00276-4
Criado MN, Romero MP, Casanovas M, Motilva MJ (2008) Pigment profile and colour of monovarietal virgin olive oils from Arbequina cultivar obtained during two consecutive crop seasons. Food Chem 110:873–880. https://doi.org/10.1016/j.foodchem.2008.02.075
Mraicha F, Ksantini M, Zouch O et al (2010) Effect of olive fruit fly infestation on the quality of olive oil from Chemlali cultivar during ripening. Food Chem Toxicol 48:3235–3241
Psomiadou E, Tsimidou M (2001) Pigments in Greek virgin olive oils: occurrence and levels. J Sci Food Agric 81:640–647
Criado MN, Motilva MJ, Goñi M, Romero MP (2007) Comparative study of the effect of the maturation process of the olive fruit on the chlorophyll and carotenoid fractions of drupes and virgin oils from Arbequina and Farga cultivars. Food Chem 100:748–755
Dabbou S, Chehab H, Faten B et al (2010) Effect of three irrigation regimes on Arbequina olive oil produced under Tunisian growing conditions. Agric Water Manag 97:763–768
Kesen S, Kelebek H, Selli S (2013) Characterization of the volatile, phenolic and antioxidant properties of monovarietal olive oil obtained from cv. Halhali. J Am Oil Chem Soc 90:1685–1696. https://doi.org/10.1007/s11746-013-2327-8
Lazzerini C, Domenici V (2017) Pigments in extra-virgin olive oils produced in Tuscany (Italy) in different years. Foods. https://doi.org/10.3390/foods6040025
Zarrouk W, Haddada FM, Baccouri B et al (2008) Characterization of virgin olive oil from Southern Tunisia. Eur J Lipid Sci Technol 110:81–88. https://doi.org/10.1002/ejlt.200700085
Laroussi-Mezghani S, Vanloot P, Molinet J et al (2015) Authentication of Tunisian virgin olive oils by chemometric analysis of fatty acid compositions and NIR spectra. Comparison with Maghrebian and French virgin olive oils. Food Chem 173:122–132. https://doi.org/10.1016/j.foodchem.2014.10.002
Ouni Y, Flamini G, Youssef NB et al (2011) Sterolic composition and triacylglycerols of Oueslati virgin olive oil: comparison among different geographic areas. Int J Food Sci Technol 46:1747–1754
Ollivier D, Artaud J, Pinatel C et al (2006) Differentiation of French virgin olive oil RDOs by sensory characteristics, fatty acid and triacylglycerol compositions and chemometrics. Food Chem 97:382–393
Baccouri B, Temime SB, Taamalli W et al (2007) Analytical characteristics of virgin olive oils from two new varieties obtained by controlled crossing on Meski variety. J Food Lipids 14:19–34
Fernández-Cuesta A, León L, Velasco L, De la Rosa R (2013) Changes in squalene and sterols associated with olive maturation. Food Res Int 54:1885–1889
Bengana M, Bakhouche A, Lozano-Sánchez J et al (2013) Influence of olive ripeness on chemical properties and phenolic composition of Chemlal extra-virgin olive oil. Food Res Int 54:1868–1875
Laroussi-Mezghani S, Dréau YL, Molinet J et al (2016) Biodiversity of Tunisian virgin olive oils: varietal origin classification according to their minor compounds. Eur Food Res Technol 242:1087–1099. https://doi.org/10.1007/s00217-015-2613-9
Nieto LM, Hodaifa G, Lozano Peña JL (2010) Changes in phenolic compounds and Rancimat stability of olive oils from varieties of olives at different stages of ripeness. J Sci Food Agric 90:2393–2398
Acknowledgements
The authors acknowledge the help of Pr. Jacques Artaud for the TAG analysis. The authors acknowledge also National Funds through Ministry of Higher Education-Tunisia for financing MedOOmics Project “Mediterranean Extra Virgin Olive Oil Omics: profiling and fingerprinting”—“Arimnet2/0001/2015”. The authors also extend thanks to Leila Mahfoudhi, Major teacher of English in the Sfax Faculty of Sciences, for having proofread and polished the language of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ben Brahim, S., Bouaziz, M. Characterization of rare virgin olive oils cultivated in southern Tunisia during fruits development process: major compounds and oxidative state in tandem with chemometrics. Eur Food Res Technol 245, 939–949 (2019). https://doi.org/10.1007/s00217-019-03230-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-019-03230-2