Abstract
Marine phospholipids (MPL) have attracted a great deal of attention recently as they are considered to have a better bioavailability, a better resistance towards oxidation and a higher content of eicosapentaenoic (EPA) and docosahexaenoic acids (DHA) than oily triglycerides (fish oil) from the same source. Due to their tight intermolecular packing conformation at the sn-2 position and their synergism with α-tocopherol present in MPL extracts, they can form stable liposomes which are attractive ingredients for food or feed applications. However, MPL are still susceptible to oxidation as they contain large amounts polyunsaturated fatty acids and application of MPL in food and aquaculture industries is therefore a great challenge for researchers. Hence, knowledge on the oxidative stability of MPL and the behavior of MPL in food and feed systems is an important issue. For this reason, this review was undertaken to provide the industry and academia with an overview of (1) the stability of MPL in different forms and their potential as liposomal material, and (2) the current applications and future prospects of MPL in both food and aquaculture industries with special emphasis on MPL in the liposomal form.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The present imbalance in the intake of n-3 and n-6 polyunsaturated fatty acids (PUFA) has a serious negative impact on health in the general population [1–3] and there is a strong desire to improve the situation by introducing new products on the market with a higher level of n-3 PUFA and a lower level of n-6 PUFA. Currently, the global food and dietary supplement market for n-3 fatty acids (EPA and DHA) is estimated to be 15,000–20,000 tons, derived from a total world production of fish oil of approximately 300,000 tons per year. Marine phospholipids (MPL) from, e.g., krill represents an alternative source of n-3 PUFA, but the market for MPL is still in its infancy even though an increasing activity in this field has been observed recently [4]. A number of companies are preparing market introduction of either natural MPL, derivatives of natural MPL, or synthetic MPL. The leading MPL product on the market at the moment is a krill extract with approximately 35% PL [5]. There are also MPL products that are made from fish processing by-products and salmon roe. It is expected that the MPL market will follow the general trends of n-3 fish oils. MPL are new on the market and their range of applications has yet to be determined. However, MPL are believed to have potential applications in human and animal nutrition, in pharmacology, and in drug delivery. The most well-documented applications of MPL are related to liposomes. Liposomes made from MPL have been developed as a test system for antioxidants and as model systems for oxidation of biological membranes [6–9].
Many studies have been performed on n-3 triacyglycerols (TAG) enriched functional foods [10] while limited studies have been carried out on MPL enriched functional foods either in their pure form or in liposomal form. Furthermore, the current applications of phospholipid liposomes are limited to lecithin from soy bean or phosphatidylcholine (PC) from egg yolk and no attempts to use MPL based liposomes for food purposes have been reported in the literature [11–13]. However, some studies [14–19] have investigated the use of MPL such as herring roe or krill PL for larvae feed in the aquaculture industry. The limited application of MPL and liposomes in both food and aquaculture industries can be attributed to several reasons (1) lack of knowledge especially related to the behavior of MPL in food and feed systems, (2) limitations in large scale production of liposomes without using organic solvents and (3) the requirement of expensive equipment for liposome production. Nevertheless, there is ongoing research in this area [20–28]. With the growing understanding of the following areas regarding (1) the physicochemical properties of MPL, (2) the oxidative stability of MPL or MPL based liposomes under gastrointestinal condition and (3) emerging technologies for liposome production without using organic solvents such as microfluidization and pro-liposomes method [29], it may soon become feasible to use MPL in both the food and aquaculture industries. This review gives an overview of our current knowledge on the above mentioned aspects.
Classification and Sources of MPL
PL can be divided into three classes: glycerophospholipids, ether glycerolipids and sphingophospholipids. Glycerophospholipids represent the most widespread phospholipid class and they differ in their polar head groups. For example, phosphatidylcholine (PC) has choline as a head group, while phosphatidylethanolamine (PE) has ethanolamine as a head group, etc. as shown in Fig. 1. In addition, PL from different sources also have different fatty acid profiles in the sn-1 and sn-2 positions (Fig. 2a). Thereby, the chain length and degree of unsaturation may vary from source to source. For example, PL originating from plants such as soy bean do not have fatty acid chain lengths longer than 18 carbon atoms and contain only one to three double bonds, while PL originating from egg yolk or marine sources additionally have chain lengths of 20 and 22 carbon atoms with four to six double bonds e.g. as found in fatty acids of EPA and DHA. However, egg yolk only contains small amounts of EPA and DHA while marine sources are high in EPA and DHA. As far as marine sources are concerned, PL are found relatively abundant in roe, fish heads and offal such as viscera [30]. The most predominant PL in marine source such as salmon, tuna, rainbow trout and blue mackerel is phosphatidycholine (PC) as shown in Table 1. The second most abundant is phosphatidylethanolamine (PE). Phosphatidylinositol (PI), phosphatidylserine (PS), sphingomyelin (SPM) and lysophosphatidylcholine (LPC) are usually found in smaller amounts in marine sources, except for the relatively high level of sphingomyelin (SPM) found in tuna species [31–36]. Furthermore, krill such as Euphausia superba and Euphausia pacifica are other rich source of MPL [37, 38]. Almost half the lipid content of both types of krill is present in phospholipid form, mainly around 35% PC and 16% PE in Euphausia superba and 29% PC and 26% PE in Euphausia pacifica, respectively. Currently, Neptune Krill oil (a concentrate of MPL from Euphausia superba) is a leading commercial krill oil on the market.
Similar to the production of egg yolk PL, production of MPL in industry uses a combination of organic solvents such as hexane and acetone, isopropanol and ethanol for extraction of wet or dried biomass [36]. Non-polar solvents are used to extract TAG while polar solvents are used to extract PL. However, extraction of lipids using organic solvents may bring adverse health effects. Recently, a more promising method without using an organic solvent, supercritical fluid extraction (SFE) has been used for the extraction and fractionation of lipids [39–42]. The extraction can be carried out at low temperature by using CO2. However, CO2 can only extract neutral lipids from lipid mixtures, and a generally recognized as safe (GRAS) co-solvent such as ethanol must also be used to extract PL for the food industry. For instance, the addition of about 5–10% of ethanol to CO2 is necessary to achieve the extraction of PL from egg yolk [42–44]. Additionally, krill oil has been extracted by a patented cold vacuum extraction process that can protect the biomass from exposure to heat, light or oxygen. Thereby, the oil is protected throughout the production process and the original nutrients of the krill are maintained intact.
Health Benefits of MPL
Many studies have shown that MPL are more efficient carriers of n-3 PUFA than TAG (normal fish oils) in terms of n-3 PUFA absorption in different tissues [45–47]. Thus, MPL not only contains more n-3 PUFA than TAG from the same source [31, 48, 49], but also provide better absorption in most tissues. This may be due to the amphiphilic properties of PL resulting in better water dispersability and their greater reactivity towards phospholipases compared to the glycerolysis of triglycerides [49]. For this reason, supplementation of foods with n-3 PUFA rich PL has recently emerged as an interesting way of increasing the assimilation and thereby the health benefits of EPA and DHA. EPA and DHA have numerous well-documented health benefits, which have been reviewed extensively by Narayan et al. [50]. The more recent studies on these health benefits include a reduction of coronary heart diseases, inflammation, autoimmune diseases, hypertension, cancer, diabetes, susceptibility to mental illness and neurological diseases such as depression and Alzheimer’s disease, as well as improved brain and eye functions in infants [51–59].
Apart from the benefits obtained from their favorable fatty acid composition, MPL may also provide health benefits due to their polar head groups [60, 61] or to a unique combination of the two in the same molecules. The latter explanation is supported by the following observations; the use of n-3 fatty acids (EPA and DHA) in PL form (either from marine or synthetic origin), instead of the triglyceride form, together with a vegetable oil containing n-6 fatty acids in a nutritive lipid emulsion, gave even lower blood triglyceride and cholesterol levels of patients as compared to the same amount of n-3 fatty acids given as fish oil [62]. The same observation was also obtained by Bunea et al. [63] who investigated the effect of krill oil (mainly present as PL) on hyperlipidemia. In addition, they reported that high doses of krill oil significantly reduced low-density lipoproteins (LDL) level and increased high-density lipoproteins (HDL). Their study concluded that krill oil was more effective at improving blood lipids and lipoproteins than fish oil. Apart from that, several studies have also shown that krill oil has many beneficial health effects such as it may has therapeutic value for metabolic syndrome, non-alcoholic fatty liver disease, attention deficit/hyperactivity deficit disorder (AD/HD), premenstrual syndrome (PMS) and it also showed anti-inflammatory effect [64–68]. Sampalis et al. [67] reported that phospholipid krill oil was more effective than triglyceride fish oil at improving both the physical and emotional symptoms of PMS while Deutsch [66] reported that the intake of krill oil at a daily dose of 300 mg can significantly inhibit inflammation and reduce arthritic symptoms within a short treatment period of 7 and 14 days. According to Maki et al. [64], 4 weeks of krill oil supplementation increased plasma EPA and DHA of overweight and obese men and women and was well tolerated without adverse effects on safety parameters. Besides that, Hayashi et al. [69] also showed that n-3 PUFA from salmon roe phosphatidylcholine may be beneficial in treatment of chronic liver diseases while Taylor et al. [70] showed that MPL is a promising new dietary approach to tumor-associated weight loss. Due to these numerous health benefits, there is an increasing desire to offer MPL containing n-3 PUFA to a wider market, e.g. for human foods and also to the general feed and aquaculture industry.
Introduction to Liposomes
Liposomes or lipid vesicles are aggregates formed from aqueous dispersions of amphiphylic molecules such as polar lipids that tend to produce bilayer structures [71]. They are useful microscopic carriers for nutrients and have a great potential for applications in both food and aquaculture industries. Besides that, liposomes have been recognized as a powerful tool in the treatment of diseases by the pharmaceutical industry. Their use as drug delivery vesicles and their medical applications such as in anticancer therapy, vaccination, gene therapy, and diagnostics have been reported in literature [72]. According to Watwe et al. [73], liposomes can be divided into three main classes: (a) multilamellar vesicles (MLV), contain more than a single bilayer membrane with a size range of 0.1–6.0 μm, (b) small unilamellar vesicles (SUV) and (c) large unilamellar vesicles (LUV) which both contain only a single bilayer membrane with sizes range of 0.02–0.05 μm and >0.06 μm, respectively. LUV are the most useful liposomes because they are more homogeneous than MLV and have higher encapsulation efficiency [74]. MPL or MPL based liposomes have obtained considerable attention and their oxidative stability has been studied extensively as shown in Table 2. Generally, MPL have been found to have a higher oxidative stability than TAG as will be discussed in the following.
Oxidative Stability of MPL
Mechanism of Oxidation for MPL
The PUFA chains in PL are the primary targets of oxidation. Similar to the oxidation of TAG, phospholipid oxidation may occur through radical and non-radical reactions involving enzymes such as lipoxygenase and myeloperoxidase or non-enzymatic systems such as ·OOH, ·OH, Fe2+, Cu+ and radiation [75]. Due to the low dissociation energy of bisallylic carbon–hydrogen in double bonds of PUFA, a hydrogen atom can easily be removed. The first steps in the lipid peroxidation consist of hydrogen abstraction, rearrangement of double bonds and addition of triplet oxygen leading to highly reactive peroxyl radicals. These radicals can undergo a large variety of consecutive reactions including further reaction with other PL, fragmentation and generation of truncated PL and different types of low molecular weight compounds such as aldehydes and ketones. However, enzymatic oxidation of PL can be eliminated in the MPL during their thermal production. Besides that, different PL oxidation products can be formed depending on the predominating oxidative process [76]. Oxidation products can be classified into three main categories such as: (1) long chain products that preserve the PL skeleton, and which may result from insertion of oxygen followed by rearrangement or cleavage of the PL hydroperoxides leading to epoxy, polyhydroxy, hydroxy, or keto derivatives of PL, (2) short-chain or truncated products, formed by cleavage of the unsaturated fatty acids. These products include ketones, aldehydes, unsaturated carboxylic acids, (keto)hydroxyl-aldehydes, (keto)hydroxyl-carboxylic acids, lyso-phospholipids and lyso-phospholipid halohydrins, and (3) adducts, formed by reaction between oxidation products and molecules containing nucleophilic groups, this include the products usually formed by cross-linking reactions between PL oxidation products with carbonyl groups and amino groups present in neighboring biomolecules such as peptides, proteins and phosphatidylethanolamine.
Dangers of Auto-Oxidation of MPL
Oxidation of MPL can not only deteriorate the quality of MPL enriched foods and affect the flavor, but also promote the development of neurodegenerative diseases. Many reported studies [75, 77–83] have shown that oxidized PL cause harmful effects to human health as they play physiopathological roles in developing diseases such as age-related and chronic diseases, acute lung injury, atherosclerosis, inflammation and decrease immune response. PL oxidation products such as hydroperoxyl, hydroxyl, aldehyde and epoxy groups that are potentially important in the progression of atherosclerosis and inflammation [80]. For instance, by activating the receptor for the platelet-activating factor (PAF), oxidized PL induce platelet aggregation [84–86]. Oxidized PL can also induce monocyte adhesion to endothelial cells, accumulate in atherosclerotic lesions, and play a role in inflammation and signaling inflammatory response. The dangers of the oxidized PL have been reviewed extensively and will not be further discussed in this review.
Antioxidant Effect of PL
King et al. [87] investigated the role of PL and the degree of fatty acid unsaturation on lipid oxidation in a salmon oil model system. Their findings showed that addition of a 2.5% (wt/wt) or a 5% (wt/wt) PL fraction extracted from bluefish to salmon oil increased its stability during heating at 55 and 180 °C as compared to the control salmon oil, or salmon oil to which 0.02% (wt/wt) of BHT or 5% (wt/wt) of other lipid fractions from bluefish such as total lipid or neutral lipid had been added. The PL fraction with 34% DHA was found to exhibit higher oxidative stability than other lipid fractions with 15% DHA. Subsequently, they investigated the antioxidant properties of individual PL in a salmon oil model system [88]. They found that nitrogen-containing PL such as PE, PC, LPC, and SPM were equally effective as antioxidants and they were more effective than PS, PG and PI. Their studies did not postulate any mechanism or reasons for the antioxidant properties of the different PL classes. In both studies by King and colleagues, the oxidative stability of the salmon oil model system was investigated through 2-thiobarbituric acids (TBARS) assay and the decreases in the ratio of DHA to PA (C22:6/C16:0). Boyd et al. [89] investigated the effect of 0.5% (by weight) PL toward lipid oxidation of 2.5 g salmon oil and menhaden oil model systems respectively, through the more sensitive headspace gas chromatographic analysis. Their study also showed that addition of PL significantly reduced the production of volatile compounds in both oil model systems.
Conformations of PUFA at the sn-2 Position of PL
Miyashita et al. [90] showed that salmon roe PC had a higher oxidative stability than soybean PC in an aqueous solution dispersed with chicken egg albumin although the degree of unsaturation in the salmon roe PC was higher than in the soybean PC. They suggested that the high stability of salmon roe PC was mainly correlated with the conformation of the PC molecule and the phase behavior of PC aggregation. The main molecular species of soybean PC was 1,2-dilinoleoyl-phosphatidylcholine (1,2-diLA-PC), while for salmon roe PC it was 1-palmitoyl-2-PUFA-phosphatidylcholine (1-PA-2-PUFA-PC) as shown in Fig. 2b. Hence, the presence of this main molecular species in salmon roe PC (with most of the PUFA located at the sn-2 position of PC) may provide a more tightly packed molecular conformation as compared to the soybean PC and thereby increase resistance of PC towards oxidation. The findings of Miyashita et al. [90] corroborated the original work of Applegate and Glomset [91] who reported that DHA in the sn-2 position of diacylglycerol (DAG) containing a saturated acyl chain in the sn-1 position could form a tighter intermolecular packing conformation as will be further discussed below.
Conformations of DHA at the sn-2 Position in a DAG Model
Applegate and Glomset (1986) used a molecular modeling approach to search for conformations of DHA that might uniquely influence acyl chain packing in cell membranes. Their DHA conformations of lowest energy as shown in Fig. 3 were extended conformations in which six double bonds projected outward from the methylene axis (a) in two nearly perpendicular planes to form an extend angle-iron shaped structure or (b) at nearly 90° intervals to form a helical structure, respectively. Studies of packed arrays of these hexaenes with or without saturated hydrocarbons showed that tight packing arrangements were possible especially for angle iron-shaped molecules as a consequence of back-to-back, intermolecular contacts involving these chains. Applegate and Glomset [92, 93] further concluded that different unsaturated fatty acids at the sn-2 position of sn-1,2-diacylglycerols (DAG) may promote different packing and conformations. For instance, 1-stearoyl-2-DHA-DAG and 1-stearoyl-2-AA-DAG can assume a regular shape and tight packing while 1-stearoyl-2-oleoyl-DAG adopt a highly irregular shape and much looser packing. The simulations by Applegate and Glomset were done without reference to potential effects of polar headgroups, water of hydration and applied thermal energy. However, the molecular areas obtained for the model of DAG are in good agreement with that of the sn-2 polyunsaturated phosphoglycerides [94, 95]. This raises the possibility that corresponding natural phosphoglycerides may be able to pack closely together in monolayers and bilayers if their headgroups do not interfere. The findings of Applegate and Glomset were supported by Albrand et al. [96] who also agreed with the existence of the extended-helical conformations of DHA in PL. However, they also suggested several coiled conformations for DHA, tightly back-folded helical conformations with 1.2 and 1.5 spirals appearing to be the most stable as shown in Fig. 3.
More Recent Studies on the Conformation of PUFA at the sn-2 Position of PL
Nara et al. [6, 7] further compared the oxidative stability of PC from salmon roe, soybean and chicken egg in aqueous micelles and also in the form of liposomes with and without encapsulation of lipophilic substances. In aqueous micelles, salmon roe PC was found to have the highest oxidative stability as evaluated by the highest content of un-oxidized PUFA, followed by chicken egg PC and soybean PC. Their findings are in agreement with the findings of Miyashita et al. [90]. No significant difference was found in oxidative stability between chicken egg PC and salmon roe PC when in the pure form of liposomes. However, for liposomes encapsulating with DHA enriched TAG resulted in the highest oxidative stability of both TAG and PC when salmon roe PC was used as the encapsulation material [7]. This unusual order of oxidative stability could be expected to be closely related to the conformation of PUFA at the sn-2 position in PC molecules as mentioned earlier [91]. Consequently, it is difficult for free radicals and oxygen to attack PUFA in bilayers of tighter conformation in salmon roe PC liposomes. Nara et al. [7] also suggested the possibility of using salmon egg PC as a liposomal material for the prevention of the oxidation of encapsulated fish oils.
Furthermore, Araseki et al. [8] also reported the characteristic oxidative stability of PC liposomes prepared from synthesized PC containing palmitic acid (PA), linoleic acid (LA), arachidonic acid (AA) and docosahexaenoic acid (DHA) in known positions. When the oxidative stability of 1-PA-2-LA-PC or 1-PA-2-AA-PC was compared with that of a 1:1 (mol ratio) mixture of 1,2-diPA-PC + 1,2-diLA-PC, or 1,2-diPA-PC + 1,2-diAA-PC respectively, the PC were more oxidatively stable than the latter corresponding PC mixtures in all oxidation systems despite the fact that the degree of unsaturation was the same in 1-PA-2-PUFA-PC and the corresponding mixture of PC. This was suggested to be due to the different conformation of PC bilayers which refer to the location of PUFA at the sn-2 position and the different rate of hydrogen abstraction by free radicals from intermolecular and intramolecular acyl groups. Their finding did not support a study by Lyberg et al. [9] who reported that the stability of DHA was improved independent of its position (sn-1 or sn-2) in PC or PE. Besides that, the more recent experiments and simulations [97–102] emphasized various degrees of flexibility of the DHA chain that gives looser packing of lipids bilayer. Their NMR analysis showed that the mobility of the hydrophobic part of the DHA molecule is higher than that of LA in liposome formation. These two competing views were portrayed in a review by Gawrish et al. [103]. However, according to Saiz and Klein [100], the flexibility of DHA chain conformation gives looser packing of the membrane at the lipid water interface and causes high water permeability. The presence of water molecules near DHA molecules lowers the density of the bisallylic hydrogen and inhibits the hydrogen abstraction from double bonds of PUFA during the propagation stage of auto-oxidation. As a conclusion, the higher water permeability of DHA and its specific conformation may be a reason for higher oxidative stability of DHA or other PUFA containing liposomes.
However, as compared to the study mentioned earlier by Miyashita et al. [90], contradictory results have also been reported by Monroig et al. [15, 16, 19] in their efforts to develop PUFA-rich liposomes for fish feed. They found that liposomes made from krill PL with 67% PC, 9% PE and a high content of PUFA showed lower oxidative stability as compared to liposomes made from soybean lecithin with 95% PC. The contradictory findings may be due to the different experimental conditions in the two studies, liposomes in model system versus liposomes in Artemia enrichment condition. In the model system, liposomes were formulated with pure PC containing fatty acid chains in known positions of the glycerol moiety and the oxidation was carried out in a very well-defined condition (temperature of 37 °C, in the dark and without agitation). On the contrary, the Artemia enrichment conditions were as follows: enrichment was carried out at 28 °C with strong aeration and 21 h of incubation.
Synergism Between PL and α-Tocopherol
Many studies have shown that the higher stability of PL may be due to the presence of antioxidants such as α-tocopherol in the PL mixture or synergistic effects of PL together with α-tocopherol [21, 25, 87, 88, 104–107]. The mechanism responsible for the synergy of tocopherols and PL is not very well understood. However, Hildebrand et al. [108] postulated that the mechanism involved in synergism of PE, PC and PI with tocopherol in the autoxidation of soybean oils were as follows: (1) amino groups of organic bases in PE and PC molecules and reducing sugar in the PI molecule facilitate hydrogen or electron donation to tocopherol and (2) these PL extend the antioxidant efficacy of tocopherol by delaying the irreversible oxidation of tocopherol to tocopherylquinone. Additionally, Saito et al. [106] reported that antioxidant activity of PL was found to be attributable not only to side chain amino groups such as choline and ethanolamine, but also to the hydroxyl group in the side chain.
Oshima et al. [105] studied the oxidative stability of sardine and mackerel lipids with respect to synergism between phospholipids and α-tocopherol. They investigated the oxidative stability of lipid fractions from different parts of sardine and mackerel; tissue from white and red muscles, viscera and skin of the fish. The oxidative stability was determined through the measured changes of the peroxide value (PV), fatty acid composition, α-tocopherol content and the oxygen uptake of lipids during an incubation period at 37 °C. Muscle lipids, which contain α-tocopherol and larger amounts of PL (PE and PC) than other tissues, showed good oxidative stability despite their high content of PUFA. It was postulated that the synergistic effect of PE with α-tocopherol was the main reason for this phenomenon. Cho et al. [21] compared the oxidative stability of lipid fractions from marine organisms, squid muscle total lipids (TL), squid viscera TL, squid eye TL, tuna orbital TL, trout egg TL and bonito TAG. The fatty acid compositions, lipid classes, tocopherol contents and average number of bisallylic positions in each lipid fraction are shown in Table 3. Higher oxidative stabilities of three kinds of squid tissue TL and trout egg TL compared to those of bonito TAG and tuna orbital TL were observed as shown in Fig. 4. The authors suggested that the presence of PL in lipid fractions from squid tissue and trout egg was responsible for this increased oxidative stability. In addition, bonito TAG was found to be less susceptible to oxidation than tuna orbital TL and this could be due to the presence of a higher tocopherol content in bonito TAG.
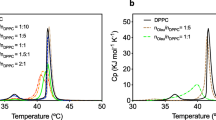
a Changes in the peroxide value (PV) and b unoxidized PUFA in lipids from marine organisms during auto-oxidation at 37 °C. (open triangle) Squid viscera total lipids (TL); (open circle) squid muscle TL; (open square) squid eye TL; (filled circle) tuna orbital TL; (filled triangle ) trout egg TL; (filled square) bonito oil. Reproduced from Cho et al. [18] with permission from John Wiley & Sons Ltd
Moriya et al. [25] compared the oxidative stability of fish roe lipids (salmon roe and herring roe) with that of lipids from commercial fish oils (crude tuna oil and crude sardine oil). As shown in Table 4, fish roe lipids contain higher levels of PL, EPA and DHA, and lower levels of tocopherol while lipids from commercial fish oils contain higher levels of TAG, tocopherol and lower EPA and DHA levels. Judging from these data, fish roe lipids were presumed to have lower oxidative stability. However, the opposite was observed as shown in Fig. 5 and it was proposed that the higher oxidative stability of fish roe lipids was mainly due to their high content of PL. It was also suggested that the synergistic effect of PL on the antioxidant activity of tocopherol was the main reason for this phenomenon. The higher oxidative stability of herring roe as compared to salmon roe was suggested to be due to synergism between PE and tocopherol. As shown in Table 4, the PE content in herring roe lipids was 6.6%, but there was no PE in salmon roe. Furthermore, herring roe also contained higher levels of PS and lysoPC than salmon roe and this may also have caused differences in their oxidative stability. The presence of antioxidants other than tocopherols in fish roe lipids such as astaxanthin, coenzyme Q10 and lutein might contribute to this extraordinary stability as well.
a Oxygen consumption during the oxidation of fish lipids at 37 °C in the dark. (open diamond) fish-1; (filled diamond) fish-2; (open triangle) salmon roe lipids; (open circle) herring roe lipids. b Propanal formation during the oxidation of fish lipids at 37 °C in the dark. (filled diamond) fish-2; (open triangle) salmon roe lipids; (open circle) herring roe lipids. Reproduced from Moriya et al. [22] with permission from John Wiley & Sons Ltd.
Other studies [104, 107, 109] reported that the synergistic effect of PE with α-tocopherol was higher than that of PC. Bandarra et al. [104] investigated the antioxidant synergy of α-tocopherol (0.04%) with several PL fractions (0.5%) such as PE, PC and cardiolipin (CL) in a refined sardine oil model system. Their results showed that PC was the most effective individual antioxidant when it was compared to PE, CL and α-tocopherol while PE provided the highest synergistic effect with α-tocopherol. Higher synergism of PE as compared with that of PC could be due to the easier hydrogen transfer from the amino group of PE to tocopheroxyl radical and regeneration of tocopherol or the secondary antioxidant action of PE in reducing quinones formed during oxidation of tocopherols [109]. Since MPL may contribute to better oxidative stability than marine TAG, it can be expected that enrichment of foods or food emulsions with MPL could lead to n-3 PUFA enriched foods that have better oxidative stability than foods enriched with n-3 TAG.
Stability of MPL Based Liposomes Under Gastrointestinal Conditions
MPL based liposomes were designed with the purpose of increasing the PUFA bioavailability and also to protect entrapped compounds from digestive degradation. However, liposome characterization with respect to vesicle composition and membrane integrity under various gastrointestinal conditions are needed before considering liposomes as a useful oral dosage form. Many studies have shown that MPL liposomes could be used as an oral administration vector [6, 7, 20, 26–28]. This is because bilayer structures of MPL based liposomes were still maintained even under acid stress or gastrointestinal conditions despite of slight morphological modifications. Nacka et al. [28] investigated the in vitro behavior of MPL based liposomes under the influence of pH from 1.5–2.5 (stomach) to 7.4 (intestine) at physiological temperature (37 °C) in the presence of bile salts and phospholipase A2 (Table 2). Their study showed that acidification induced instantaneous vesicle aggregation of MPL based-liposomes, which was partially reversed when the external medium was neutralized. Acidification also caused a complex morphological bilayer rearrangement and led to the formation of small aggregates. Nevertheless, Nacka et al. [27, 28] reported that the pH and temperature dependent structural rearrangement is mainly due to the osmotic shock and chemical lipid alterations such as oxidation and hydrolysis. Hydrolysis of the liposomes was amplified under the influence of an acid medium and high temperatures (Table 2).
Cansell et al. [20] investigated the physical stability of MPL-based liposomes containing vitamin B1 under acidic conditions simulating the stomach conditions. Encapsulation of vitamin B1 in the liposomes was carried out through passive encapsulation and active loading methods. They observed that vitamin B1 was totally released from liposomes after 24 h storage in a neutral medium and the time of release was shortened to 1 h in acidic condition (pH 1.5). According to their study, this liposome instability could result from the external medium osmolarity that forced water to flow out of the liposomes and simultaneously dragged vitamin B1 molecules through the bilayer. Furthermore, protons may also destabilize the lipid membrane by their interaction with PL via structural membrane rearrangement as previously mentioned. However, their study also proved that addition of xanthan gum improved the encapsulation efficiency and also the retention of vitamin B1 in liposomes regardless of the encapsulation method used. They suggested that this increase is due to the adsorption of hydrocolloid to the outer surface of the liposomes that not only trapped part of the external vitamin but also formed a strong xanthan gum coating around the liposome surface. They postulated that this coating resulted from strong lipid–hydrocolloid interactions occurring during the centrifugation steps of liposome preparation.
The Effect of Lamellarity, pH, Temperature, Ionic Strength, Presence of Pro-oxidants and Chelators on MPL-Based Liposomes’ Stability
Chemical and physical stability of liposomes are closely related to the mechanical strength and lipid bilayer conformation. Strong and well-packed lipid bilayers or multilamellar layers can protect the entrapped substance, decrease the changes of size distribution, fusion or other changes in the mechanical properties of lipid bilayers. For this reason, factors such as lamellarity, pH, temperature, ionic strength, dissolved oxygen content within the formulation, the presence of antioxidants and chelators are believed to affect mechanical properties of lipid bilayers and thereby affect the physical and chemical stability of MPL-based liposomal products [22, 23].
Nacka et al. [27] showed that the sensitivity of MPL based liposomes towards harsh condition such as acidic condition depends on their size and lamellarity (Table 2). They found that filtered liposomes with higher lamellarity and a protective effect against aggregation showed a slower size rearrangement. This finding supported a study by Monroig et al. [19] who, in addition, reported that liposomes with multilamellar vesicles seem to be more suitable than liposomes with unilamellar vesicles in the encapsulation of free methionine. They found that methionine dissolved in the more internal intermembrane spaces of multilamellar liposomes would remain encapsulated, whereas methionine from the aqueous compartments located between the more outer membranes would leak out into the external medium when the liposomes were subjected to harsh conditions. However, this result contradicts another study by this group [15] where unilamellar liposomes were found to be more stable than multilamellar liposomes. The apparent discrepancy in these two studies is probably due to different experimental conditions and materials used for the liposomes preparation.
Mozuraityte et al. [22] examined the lipid oxidation rate of liposomes made from cod PL under influence of factors such as the temperature, the amount of added Fe2+, the lipid concentration, pH, the concentration of NaCl, and the dissolved oxygen. Their study showed that the rate of lipid oxidation was proportional to the iron and lipid concentrations. Furthermore, lipid oxidation was dependent on pH, with a maximum observed between pH 4 and 5. Addition of NaCl decreased the rate of lipid oxidation. However, contradictory results were reported in another study [110] which showed that addition of NaCl had no effect or even increased iron-catalyzed oxidation of a sodium dodecyl sulfate-stabilized salmon oil emulsion.
Mozuraityte et al. [23] examined the effect of zeta potential on the lipid oxidation rate of liposomes made from cod PL under the influence of pH and different cations such as Na+, K+, Ca+, Mg+ and anions such as H2PO4 − and Cl− (Table 2). Their data showed that cations did not influence the rate of oxidation in the tested range of the ionic strength from 0 to 0.14 M whereas the opposite was the case for anions. Both phosphate and chlorides have an additive antioxidative effect on the oxidation in liposomes. Phosphate was shown to be more effective in reducing the oxidation rate than chloride. The inhibition of Fe2+ induced oxidation of liposomes by phosphate might be due to the phosphate chelation of iron [111, 112]. Furthermore, they also concluded that addition of salts and changes in pH affected the zeta potential of the liposomes. However, absolute values of the zeta potential alone cannot be used to predict oxidation rates.
Improvement of MPL Based Liposomes’ Oxidative Stability
Many studies have been conducted to improve the oxidative stability of liposomes. Most of the studies focus on the use of cholesterol in improving the oxidative stability of liposomes [12, 113–115]. For example, a study conducted by Nara et al. [7] showed that addition of cholesterol and ingredients such as diacetyl phosphate (DP) and stearylamine (SA) improved the oxidative stability of salmon roe PC liposomes. Furthermore, in the effort of developing liposomes as feed supplement in larva culture. Monroig et al. [15] also showed that addition of cholesterol to liposomes made from krill PL or 1,2-PA-PC or soy PC improved the oxidative stability of the liposomes. Cholesterol has a condensing effect on the PC bilayer arrangement over its phase transition temperature and thus improves the physical stabilization of PC liposomes [116]. Addition of cholesterol can increase the rigidity of ‘fluid state’ liposomal bilayers and the retention of entrapped hydrophilic substances [117]. It counteracts lipids phase transition and increases resistance to in vivo liposomes degradation [118–120]. An interaction mechanism between bilayer forming PL and cholesterol has been proposed. This is due to the formation of hydrogen bonds between the three hydroxyl group of cholesterol and fatty acyl esters of PL at both sn-1 and sn-2 positions [121, 122]. These physico-chemical effects of cholesterol on liposomes may contribute to the increased oxidative stability in liposomes with cholesterol.
α-Tocopherol is widely known for its antioxidative effect [123]. However addition of high concentrations of α-tocopherol may also cause prooxidative effects [124, 125]. The most effective concentration of α-tocopherol in the prevention of lipid oxidation in salmon roe PC liposome suspensions was 0.25 μM in a study conducted by Nara et al. [7]. Nacka et al. [26] investigated the most efficient amount of α-tocopherol for liposomes incorporation under gastrointestinal-like conditions. Their findings showed that the best oxidative stability was obtained for liposomes that were prepared at a ratio of 5 mol% of α-tocopherol of the total marine lipids. This concentration of α-tocopherol produced liposomes with the lowest concentration of propanal as an oxidation product of n-3 PUFA and required the longest time of oxidation induction phase. They also found that incorporation of α-tocopherol induced liposome structural modifications, evidenced by turbidity and the production of lysophospholipids from PL chemical hydrolysis.
Nara et al. [6, 7] investigated the effect of addition of diacetyl phosphate (DP), stearylamine (SA) and chicken egg albumin, and soybean protein on improving the oxidative stability of MPL based-liposomes. DP and SA give a negative or positive charge to the liposomes respectively and thus protect the liposomes from aggregation. An improved oxidative stability of liposomes after addition of this ingredient was observed and suggested that it was due to the physical stabilization of the PC liposomes. Furthermore, added proteins such as chicken egg albumin and soybean protein improved the oxidative stability of liposomes by protecting the PC bilayer from the attack of free radicals. Proteins have the ability to absorb at PC–water interfaces and this adsorption of proteins would closely relate to its antioxidant activity [6]. However, albumin acted as a more effective inhibitor of the oxidation of PC containing DHA than PC containing LA [90].
Determination of Oxidation Products from MPL
As discussed above MPL has been found to exert antioxidative effects toward lipids oxidation. However, many of the lipid oxidation studies [6–8, 21, 90, 105] were performed using simple analyses such as TBARS, PV, determination of the un-oxidized lipids (PUFAs) content through gas chromatography, or determination of only one secondary volatile compound, propanal (as a marker of n-3 PUFA oxidation) by headspace GC–MS analysis [25], etc. In many of these oxidative stability studies, there is a lack of determination of the entire spectrum of volatile oxidation products or identification of specific oxidation products which are responsible for sensory off-flavors of the marine lipids. Furthermore, there are no studies providing the sensory data or statistical correlation between instrumental analysis and sensory data for oxidation of MPL. These data are particularly important in the studies of MPL for foods enrichment and additional studies in this area are clearly needed. Due to the low odor threshold, the presence of volatile secondary oxidation products, even at low concentrations, can significantly decrease the sensory quality of marine lipids or marine lipids containing foods.
In the recent years, the oxidation products of PL have attracted intensive research interest due to their biological functions in human pathophysiology. Similar to other lipids such as TAG, many methods can be used to study the oxidation of PUFA containing PL such as (1) measurement of lipid hydroperoxides through spectrophotometric determination of PV or conjugated dienes (CD). Lipid hydroperoxides may also be determined by sample derivatization followed by HPLC with chemiluminescence detection, (2) measurement of breakdown products of hydroperoxides, such as the aldehydes, malondialdehyde, etc. through anisidine value (AV), 2-thiobarbituric acid value (TBARS), etc., (3) measurement of secondary volatile compounds through more sensitive instrumental methods such as GC–MS, (4) measurement of long chain oxidation derivatives of PL through MS. Electrospray ionization (ESI) is gaining in popularity in this area nowadays for this purpose [76]. ESI is a soft ionization technique that does not cause fragmentation and allows detection of intact PL classes without sample derivatization. ESI can readily be coupled to reverse phase LC and allow the analysis of oxidized PL [126–129]. Interfacing reverse phase LC to ESI–MS has the advantage as oxidized PL elutes earlier than their native counterparts due to their higher hydrophilicity. Spickett et al. [127] used the positive ion ESI–MS for detection of hydroperoxide in PC vesicles after treatment with tert-butylhydroperoxide and Fe2+ while Yin et al. [129] used ultra performance liquid chromatography (UPLC) coupled with negative ion electrospray ion trap MS to identify the intact oxidation products of glycerophospholipids in vitro and in vivo such as hydroxyeicosatetraenoates (HETE) and isoprostanes (IsoP). Other soft ionization methods include matrix-assisted laser desorption ionization (MALDI) and tandem mass spectrometry (MS/MS). As a conclusion, the future direction for research and development could focus on the investigation of oxidative stability for MPL by using advanced MS analysis.
Potential of MPL as Liposomal Material
A variety of liposome preparation methods are available nowadays ranging from traditional methods using solvent extraction such as thin film hydration, detergent dialysis, reverse-phase evaporation, etc. to emerging technologies without using an organic solvent such as pro-liposome, supercritical fluid extraction, and microfluidization. Each method has its own advantages and drawbacks as reviewed by Taylor et al. [130]. Among these technologies, pro-liposome and microfluidization are recommended to produce liposomes for food applications. Pro-liposome is a simple method for mass production of liposomes without using large amounts of energy, solvents and complex equipment. This method is based on the idea that addition of water to an appropriate mixture of ingredients leads to the spontaneous formation of liposomes [29]. On the other hand, microfluidization is a method using a microfluidizer (a high pressure homogenizer) that can rapidly produce large volumes of liposomes in a continuous and reproducible manner. The average size of the liposomes can be adjusted through this technology and the solutes to be encapsulated are not exposed to sonication, detergents or organic solvents. Furthermore, this technology enables the production of stable liposomes with high encapsulation efficiency [74]. Recently, Thompson et al. [131–133] used a microfluidization technique to produce liposomes from milk fat globule membrane PL in the food industry. Studies showed that liposomes prepared via microfluidization have high encapsulation efficiencies, smaller size, a narrower size distribution and a higher proportion of unilamellar vesicles as compared to methods such as thin film hydration. PL from soybean and egg yolk, either in purified form, crude form or hydrogenated form are widely used for liposome production in both the food and aquaculture industries. The use of MPL-based liposomes has gained attention recently in the aquaculture industry and there is much ongoing research in this area as shown in Table 5. Several studies have shown the use of MPL such as herring roe or krill PL for larvae feed in the aquaculture industry [14–19] but no attempts to use MPL based liposomes for food purposes have been reported in the literature so far.
One potential advantage of using MPL-based liposomes for food application is that they may provide better bioavailability of encapsulated nutrients [26, 134, 135] as compared to TAG. Nacka et al. [26] showed that MPL-based liposomes facilitated α-tocopherol uptake after oral delivery as compared to sardine oil digestion. Furthermore, Hossain et al. [136] also showed that MPL-based PC liposomes (squid PC and starfish PC) enhanced the permeability, transportation and uptake of PL in Caco-2-cells. It is also known that the fluidity of liposomes increases with increasing contents of highly unsaturated PUFA such as AA and DHA, showing the advantage of PC containing AA or DHA for use in drug or nutrient delivery systems [100, 101].
Application of PL Liposomes in the Food Industry
The uses of liposomes in the food industry can be summarized as follows (1) use of liposomes to encapsulate food ingredients in order to provide better protection or to hide the bitter taste of entrapped substances and (2) use of liposomes to control the delivery of functional components by delaying the release of the encapsulated materials. Liposomes have been used to entrap thermally sensitive compounds such as vitamins, enzymes, flavorings, PUFA from fish oils, antimicrobial peptides (lysozyme, nisin) and other nutrients [13, 137–144]. Hydrophilic substances can be entrapped in the internal water core of the liposomes while lipophilic compounds can be efficiently enclosed in the PL bilayer at the same time through a pro-liposomes approach [29]. For this reason, liposomes can be used for the formulation of functional foods or drinks such as energy drinks, sport drinks, fortified milk, etc. Arnaud et al. [145] reported that PC from egg or soybean has been used in development of liposome-based functional drinks. With the use of PC-based liposomes in food industry, consumers not only benefit from the health benefits of water soluble nutrients that are entrapped in the liposomes but also benefit from the nutritional benefits of PL in liposomes. In the production of cheese, PL liposomes may be used to delay the release of encapsulated proteinases [146, 147] or to protect encapsulated enzyme such as protease and lipases with the purpose of improving the texture and sensory properties of cheese [148–152]. Liposomes have also been used to encapsulate vitamin D with the purpose of increasing the vitamin D content of cheese [153].
Application of PL Liposomes in the Aquaculture Industry
Besides food incorporation, recent studies have also indicated that liposomes rich in n-3 PUFA can offer a range of benefits when used for fish larvae feed. Due to the high consumer demand and limited natural stocks of fish species such as salmon, trout and eel, much effort has recently been spent by researchers on developing cost effective aquaculture methods for farming such species. Generally, the main problems faced by aquaculture industry are low survival rate of the hatched fish larvae of the farmed species and the difficulty in supplying live prey organisms which provide nutritionally adequate feed for these larvae. Live prey such as Rotifers Brachionus plicatilis and Artemia nauplii provide adequate amounts of protein and energy. However, they do not provide lipid profiles that cover the requirements for EPA and DHA, which are essential for optimum survival, growth and development of larvae [154–157]. Thus, to provide prey organisms with such a composition of n-3 PUFA, it is necessary to cultivate these organisms in the presence of enrichment products with high EPA and DHA contents, preferably in an easily digestible, highly bio available form, such as MPL. During the enrichment process, enrichment products are passively filtered by Artemia nauplii and their digestive tract becomes loaded with these enrichment products. A wide variety of enrichment products are available nowadays such as microalgae, microcapsules [158] and oil emulsion products [159].
PL especially MPL are considered to be a better way for providing EPA and DHA for larvae than TAG fish oil due to reasons such as: (1) marine fish larvae commonly ingest and assimilate better natural diets rich in PL than TAG [160–162]. The ratio of DHA:EPA in the PL naturally consumed by larvae is generally higher as compared to the corresponding ratio in TAG fish oil [156], (2) studies also showed that PL facilitate the absorption of lipids in the larvae gut [163] and thus promote growth and survival of larvae [164], and (3) PL have been shown to exert antioxidant properties against oxidation [87, 88].
Mcevoy et al. [14, 165] showed the advantage of using PC from soybean and marine fish eggs in enrichment of Artemia nauplii. They found that a mixture of DHA rich fish oil and PC (90:10) resulted in Artemia nauplii which were markedly enriched in DHA, and with minimal peroxidation in an aerated mixture during 18 h of enrichment. This is because the added PC functions as a natural emulsifying agent and a natural protectant against oxidation. They also showed that PC from marine egg sources was superior to soy PC in terms of n-3 PUFA content. This is presumably due to the presence of readily assimilable DHA and EPA in a ratio of 2:1 in marine roe lipids as compared to LA in soy PC. Their study corroborated the original work of Kanazawa et al. [166] using soy and bonito PC as feed supplements for larval sea bream and aye.
As mentioned earlier, there are several forms of enrichment products commercially available nowadays for live prey. However, as compared to an emulsion, liposomes provide more advantages. This is due to their ability to encapsulate lipids as well as water soluble components. For example, liposomes have been successfully used to encapsulate vitamin C [19] or water soluble antibiotics [167] in Artemia nauplii enrichment. In addition, liposomes can also be used to encapsulate hydrophobic components such as vitamin A [19] and free amino acids such as methionine [19, 168] or glycine [169]. Many studies have also shown that it is possible to encapsulate considerable amounts of n-3 PUFA into liposomes for Artemia enrichment [14, 15, 170].
Future Prospects and Conclusion
MPL may offer more advantages to consumer, food, and aquaculture industries as compared to fish oils. Particularly, the use of MPL-based liposomes is expected to provide benefits such as better oxidative stability, higher bioavailability and higher fluidity as compared to other PL-based liposomes. However, the use of MPL-based liposomes is just starting to be explored in both aquaculture and food industries and no current use of MPL-based liposomes for food applications has been reported. The next frontier in liposome application in the food industry will probably focus on the use of MPL for the development of n-3 PUFA enriched functional foods or the use of MPL-based liposomes as nutrient delivery system in foods and feed. Additionally, another area of study that needs further exploration is the use of liposomes for encapsulation of flavor, aroma and natural coloring compound in foods. However, due to the high content of n-3 PUFA in MPL, foods containing MPL are highly susceptible to lipid oxidation, which results in oxidative products that not only cause deterioration of food quality but also increase the risk of certain degenerative diseases as mentioned earlier. Therefore, it is expected that many more studies will be carried out in the future to explore the oxidative stability and sensory properties of MPL or MPL liposomes prior their potential uses in both food and aquaculture industries.
Abbreviations
- AA:
-
Arachidonic acid
- BHT:
-
Butylated hydroxytoluene
- CHO:
-
Cholesterol
- CL:
-
Cardiolipin
- DAG:
-
Diacyglycerols
- DHA:
-
Docosahexaenoic acid
- DP:
-
Diacetyl phosphate
- EE:
-
Encapsulation efficiency
- EFA:
-
Essential fatty acid
- EPA:
-
Eicosapentaenoic acid
- LA:
-
Linoleic Acid
- LPC:
-
Lysophosphatidylcholine
- LUV:
-
Large unilamellar vesicles
- MLV:
-
Multilamellar vesicles
- MPL:
-
Marine phospholipids
- n-3 PUFA:
-
Omega-3 polyunsaturated fatty acid(s)
- PA:
-
Palmitic acid
- PC:
-
Phosphatidylcholine(s)
- PE:
-
Phosphatidylethanolamine
- PG:
-
Phosphatidylglycerol
- PI:
-
Phosphatidylinositol
- PL:
-
Phospholipid(s)
- PS:
-
Phosphatidylserine
- SA:
-
Stearylamine
- SPM:
-
Sphingomyelin
- TAG:
-
Triacyglycerols
- TL:
-
Total lipids
- NL:
-
Neutral lipids
References
Okuyama H (2001) High n-6 to n-3 ratio of dietary fatty acids rather than serum cholesterol as a major risk factor for coronary heart disease. Eur J Lipid Sci Technol 103:418–422
Hibbeln JR, Nieminen LRG, Blasbalg TL, Riggs JA, Lands WEM (2006) Healthy intakes of n-3 and n-6 fatty acids: estimations considering worldwide diversity. Am J Clin Nutr 83:1483S–1493S
Simopoulos AP (2008) The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med 233:674–688
Løvaas E (2006) Marine phospholipids (MPL) resources, applications and markets. In: Luten JB, Jacobsen C, Bekaert K, Saebo A, Oehlenschlager J (eds) Seafood research from fish to dish, 1st ed. edn. Wageningen Academic Publishers, The Netherlands, pp 17–28
Neptune Technologies & Bioresources (2001) Natural phospholipids of marine origin containing flavonoids and polyunsaturated phospholipids and their uses [EP 1417211]
Nara E, Miyashita K, Ota T (1997) Oxidative stability of liposomes prepared from soybean PC, chicken egg PC, and salmon egg PC. Biosci Biotechnol Biochem 61:1736–1738
Nara E, Miyashita K, Ota T, Nadachi Y (1998) The oxidative stabilities of polyunsaturated fatty acids in salmon egg phosphatidylcholine liposomes. Fish Sci 64:282–286
Araseki M, Yamamoto K, Miyashita K (2002) Oxidative stability of polyunsaturated fatty acid in phosphatidylcholine liposomes. Biosci Biotechol Biochem 66:2573–2577
Lyberg AM, Fasoli E, Adlercreutz P (2005) Monitoring the oxidation of docosahexaenoic acid in lipids. Lipids 40:969–979
Jacobsen C (2008) Omega-3s in food emulsions: overview and case studies. Agro Food Ind Hi Tech 19:9–12
Rodriguez-Nogales JM, Perez-Mateos M, Busto MD (2004) Application of experimental design to the formulation of glucose oxidase encapsulation by liposomes. J Chem Technol Biotechnol 79:700–705
Xia SQ, Xu SY (2005) Ferrous sulfate liposomes: preparation, stability and application in fluid milk. Food Res Int 38:289–296
Taylor TM, Gaysinsky S, Davidson PM, Bruce BD, Weiss J (2007) Characterization of antimicrobial-bearing liposomes by zeta-potential, vesicle size, and encapsulation efficiency. Food Biophys 2:1–9
Mcevoy LA, Navarro JC, Hontoria F, Amat F, Sargent JR (1996) Two novel Artemia enrichment diets containing polar lipid. Aquaculture 144:339–352
Monroig O, Navarro JC, Amat I, Gonzalez P, Amat F, Hontoria F (2003) Enrichment of Artemia nauplii in PUFA, phospholipids, and water-soluble nutrients using liposomes. Aquacult Int 11:151–161
Monroig O, Navarro JC, Amat F, Gonzalez P, Hontoria F (2007) Oxidative stability and changes in the particle size of liposomes used in the Artemia enrichment. Aquaculture 266:200–210
Monroig O, Navarro JC, Amat F, Gonzalez P, Hontoria F (2006) Effects of nauplial density, product concentration and product dosage on the survival of the nauplii and EFA incorporation during Artemia enrichment with liposomes. Aquaculture 261:659–669
Monroig O, Navarro JC, Amat F, Gonzalez P, Bermejo A, Hontoria F (2006) Enrichment of Artemia nauplii in essential fatty acids with different types of liposomes and their use in the rearing of gilthead sea bream (Sparus aurata) larvae. Aquaculture 251:491–508
Monroig O, Navarro JC, Amat F, Hontoria F (2007) Enrichment of Artemia nauplii in vitamin A, vitamin C and methionine using liposomes. Aquaculture 269:504–513
Cansell M, Moussaoui N, Lefrancois C (2001) Stability of marine lipid based-liposomes under acid conditions. Influence of xanthan gum. J Liposome Res 11:229–242
Cho SY, Joo DS, Choi HG, Nara E, Miyashita K (2001) Oxidative stability of lipids from squid tissues. Fish Sci 67:738–743
Mozuraityte R, Rustad T, Storro I (2006) Pro-oxidant activity of Fe2+ in oxidation of cod phospholipids in liposomes. Eur J Lipid Sci Technol 108:218–226
Mozuraityte R, Rustad T, Storro I (2006) Oxidation of cod phospholipids in liposomes: effects of salts, pH and zeta potential. Eur J Lipid Sci Technol 108:944–950
Mozuraityte R, Rustad T, Storro I (2008) The role of iron in peroxidation of polyunsaturated fatty acids in liposomes. J Agric Food Chem 56:537–543
Moriya H, Kuniminato T, Hosokawa M, Fukunaga K, Nishiyama T, Miyashita K (2007) Oxidative stability of salmon and herring roe lipids and their dietary effect on plasma cholesterol levels of rats. Fish Sci 73:668–674
Nacka F, Cansell M, Meleard P, Combe N (2001) Incorporation of alpha-tocopherol in marine lipid-based liposomes: in vitro and in vivo studies. Lipids 36:1313–1320
Nacka F, Cansell M, Gouygou JP, Gerbeaud C, Meleard P, Entressangles B (2001) Physical and chemical stability of marine lipid-based liposomes under acid conditions. Colloids Surf B 20:257–266
Nacka F, Cansell M, Entressangles B (2001) In vitro behavior of marine lipid-based liposomes, influence of pH, temperature, bile salts, and phospholipase A(2). Lipids 36:35–42
Arnaud JP (1995) Liposomes in the agro food-industry. Agro Food Ind Hi Tech 6:30–36
Falch E, Rustad T, Jonsdottir R, Shaw NB, Dumay J, Berge JP, Arason S, Kerry JP, Sandbakk M, Aursand M (2006) Geographical and seasonal differences in lipid composition and relative weight of by-products from gadiform species. J Food Compost Anal 19:727–736
Gbogouri GA, Linder M, Fanni J, Parmentier M (2006) Analysis of lipids extracted from salmon (Salmo salar) heads by commercial proteolytic enzymes. Eur J Lipid Sci Technol 108:766–775
Striby L, Lafont R, Goutx M (1999) Improvement in the Iatroscan thin-layer chromatographic-flame ionisation detection analysis of marine lipids. Separation and quantitation of monoacylglycerols and diacylglycerols in standards and natural samples. J Chromatogr A 849:371–380
Medina I, Aubourg SP, Martin RP (1995) Composition of phospholipids of white muscle of 6 tuna species. Lipids 30:1127–1135
Body DR, Vlieg P (1989) Distribution of the lipid classes and eicosapentaenoic (20-5) and docosahexaenoic (22-6) acids in different sites in blue mackerel (Scomber australasicus) fillets. J Food Sci 54:569–572
Hazel JR (1985) Determination of the phospholipid-composition of trout gill by Iatroscan TLC/FID—effect of thermal-acclimation. Lipids 20:516–520
Schneider M (2008) Major sources, composition and processing. In: Gunstone FD (ed) Phospholipid technoloy and applications. The Oily Press, Bridgewater, pp 21–40
Saito H, Kotani Y, Keriko JM, Xue CH, Taki K, Ishihara K, Ueda T, Miyata S (2002) High levels of n-3 polyunsaturated fatty acids in Euphausia pacifica and its role as a source of docosahexaenoic and icosapentaenoic acids for higher trophic levels. Mar Chem 78:9–28
Le Grandois J, Marchioni E, Zhao MJ, Giuffrida F, Ennahar S, Bindler F (2009) Investigation of natural phosphatidylcholine sources: separation and identification by liquid chromatography-electrospray ionization-tandem mass spectrometry (LC–ESI-MS2) of molecular species. J Agric Food Chem 57:6014–6020
Boselli E, Caboni MF (2000) Supercritical carbon dioxide extraction of phospholipids from dried egg yolk without organic modifier. J Supercrit Fluids 19:45–50
Kang KY, Ahn DH, Jung SM, Kim DH, Chun BS (2005) Separation of protein and fatty acids from tuna viscera using supercritical carbon dioxide. Biotechnol Bioprocess Eng 10:315–321
Letisse M, Rozieres M, Hiol A, Sergent M, Comeau L (2006) Enrichment of EPA and DHA from sardine by supercritical fluid extraction without organic modifier—I. Optimization of extraction conditions. J Supercrit Fluids 38:27–36
Aro H, Jarvenpaa E, Konko K, Sihvonen M, Hietaniemi V, Huopalahti R (2009) Isolation and purification of egg yolk phospholipids using liquid extraction and pilot-scale supercritical fluid techniques. Eur J Lipid Sci Technol 228:857–863
Froning GW, Wehling RL, Cuppett SL, Pierce MM, Niemann L, Siekman DK (1990) Extraction of cholesterol and other lipids from dried egg-yolk using supercritical carbon dioxide. J Food Sci 55:95–98
Rossi M, Spedicato E, Shiraldi A (1990) Improvement of supercritical carbon dioxide extraction of egg lipids by means of ethanolic entrainer. Ital J Food Sci 2:249–256
Lemaitre-Delaunay D, Pachiaudi C, Laville M, Pousin J, Armstrong M, Lagarde M (1999) Blood compartmental metabolism of docosahexaenoic acid (DHA) in humans after ingestion of a single dose of [C−13]DHA in phosphatidylcholine. J Lipid Res 40:1867–1874
Amate L, Gil A, Ramirez M (2001) Feeding infant piglets formula with long-chain polyunsaturated fatty acids as triacylglycerols or phospholipids influences the distribution of these fatty acids in plasma lipoprotein fractions. J Nutr 131:1250–1255
Wijendran V, Huang MC, Diau GY, Boehm G, Nathanielsz PW, Brenna JT (2002) Efficacy of dietary arachidonic acid provided as triglyceride or phospholipid as substrates for brain arachidonic acid accretion in baboon neonates. Pediatr Res 51:265–272
Peng JL, Larondelle Y, Pham D, Ackman RG, Rollin X (2003) Polyunsaturated fatty acid profiles of whole body phospholipids and triacylglycerols in anadromous and landlocked Atlantic salmon (Salmo salar L.) fry. Comp Biochem Phys B 134:335–348
Phares Pharmaceutical Research N.V. (2004) Marine Lipid Compositions [WO/2004/047554]
Narayan B, Miyashita K, Hosakawa M (2006) Physiological effects of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)—a review. Food Rev Int 22:291–307
Leaf A (2008) Historical overview of n-3 fatty acids and coronary heart disease. Am J Clin Nutr 87:1978S–1980S
Virtanen JK, Mozaffarian D, Chiuve SE, Rimm EB (2008) Fish consumption and risk of major chronic disease in men. Am J Clin Nutr 88:1618–1625
Calon F, Cicchetti F (2009) Omega-3 fatty acid in Parkinson disease. Agro Food Ind Hi Tech 20:7–9
Fotuhi M, Mohassel P, Yaffe K (2009) Fish consumption, long-chain omega-3 fatty acids and risk of cognitive decline or Alzheimer disease: a complex association. Nat Clin Pract Neurol 5:140–152
Ramakrishnan U, Imhoff-Kunsch B, DiGirolamo AM (2009) Role of docosahexaenoic acid in maternal and child mental health. Am J Clin Nutr 89:958S–962S
Boudrault C, Bazinet RP, Ma DWL (2009) Experimental models and mechanisms underlying the protective effects of n-3 polyunsaturated fatty acids in Alzheimer’s disease. J Nutr Biochem 20:1–10
Tinoco SMB, Sichieri R, Setta CL, Moura AS, Do Carmo MGT (2009) n-3 polyunsaturated fatty acids in milk is associate to weight gain and growth in premature infants. Lipids Health Dis 8:23
Adkins Y, Kelley DS (2010) Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J Nutr Biochem 21:781–792
Lopez-Huertas E (2010) Health effects of oleic acid and long chain omega-3 fatty acids (EPA and DHA) enriched milks. A review of intervention studies. Pharmacol Res 61:200–207
Wilson TA, Meservey CM, Nicolosi RJ (1998) Soy lecithin reduces plasma lipoprotein cholesterol and early atherogenesis in hypercholesterolemic monkeys and hamsters: beyond linoleate. Atherosclerosis 140:147–153
Zeisel SH (1992) Choline—an important nutrient in brain-development, liver-function and carcinogenesis. J Am Coll Nutr 11:473–481
Pharmacia AB (1995) Phospholipids containing omega-3 fatty acids [US Patent 5434183]
Bunea R, El Farrah K, Deutsch L (2004) Evaluation of the effects of Neptune krill oil on the clinical course of hyperlipidemia. Altern Med Rev 9:420–428
Maki KC, Reeves MS, Farmer M, Griinari M, Berge K, Vik H, Hubacher R, Rains TM (2009) Krill oil supplementation increases plasma concentrations of eicosapentaenoic and docosahexaenoic acids in overweight and obese men and women. Nutr Res 29:609–615
Tandy S, Chung RWS, Wat E, Kamili A, Berge K, Griinari M, Cohn JS (2009) Dietary krill oil supplementation reduces hepatic steatosis, glycemia, and hypercholesterolemia in high-fat-fed mice. J Agric Food Chem 57:9339–9345
Deutsch L (2007) Evaluation of the effect of Neptune krill oil on chronic inflammation and arthritic symptoms. J Am Coll Nutr 26:39–48
Sampalis F, Bunea R, Pelland MF, Kowalski O, Duguet N, Dupuis S (2003) Evaluation of the effects of Neptune krill oil on the management of premenstrual syndrome and dysmenorrhea. Altern Med Rev 8:171–179
Ierna M, Kerr A, Scales H, Berge K, Griinari M (2010) Supplementation of diet with krill oil protects against experimental rheumatoid arthritis. BMC Musculoskelet Disorders 11:136
Hayashi H, Tanaka Y, Hibino H, Umeda Y, Kawamitsu H, Fujimoto H, Amakawa T (1999) Beneficial effect of salmon roe phosphatidylcholine in chronic liver disease. Curr Med Res Opin 15:177–184
Taylor LA, Pletschen L, Arends J, Unger C, Massing U (2010) Marine phospholipids—a promising new dietary approach to tumor-associated weight loss. Support Care Cancer 18:159–170
Lasch J, Weissing V, Brandi M (2003) Preparation of liposomes. In: Torchilin VP, Weissing V (eds) Liposomes: a practical approach, 2nd edn. Oxford University Press, New York, pp 3–30
Lasic DD (1998) Novel applications of liposomes. Trends Biotechnol 16:307–321
Watwe RM, Bellare JR (1995) Manufacture of liposomes—a review. Curr Sci 68:715–724
Kim HY, Baiau IC (1991) Novel liposome microencapsulation techniques for food applications. Trends Food Sci Tech 2:55–61
Fruhwirth GO, Loidl A, Hermetter A (2007) Oxidized phospholipids: from molecular properties to disease. BBA Mol Basis Dis 1772:718–736
Domingues MRM, Reis A, Domingues P (2008) Mass spectrometry analysis of oxidized phospholipids. Chem Phys Lipids 156:1–12
Subbanagounder G, Deng YJ, Borromeo C, Dooley AN, Beliner JA, Salomon RG (2002) Hydroxy alkenal phospholipids regulate inflammatory functions of endothelial cells. Vascul Pharmacol 38:201–209
Leitinger N (2003) Oxidized phospholipids as modulators of inflammation in atherosclerosis. Curr Opin Lipidol 14:421–430
Leitinger N (2005) Oxidized phospholipids as triggers of inflammation in atherosclerosis. Mol Nutr Food Res 49:1063–1071
Spickett CM, Dever G (2005) Studies of phospholipid oxidation by electrospray mass spectrometry: from analysis in cells to biological effects. Biofactors 24:17–31
Spiteller G (2006) Peroxyl radicals: inductors of neurodegenerative and other inflammatory diseases. Their origin and how they transform cholesterol, phospholipids, plasmalogens, polyunsaturated fatty acids, sugars, and proteins into deleterious products. Free Radic Bio Med 41:362–387
Bochkov VN (2007) Inflammatory profile of oxidized phospholipids. Thromb Haemost 97:348–354
Imal Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YHC, Wang H, Liu H, Sun Y, Pasparakis M, Kopf M, Mech C, Bavari S, Peiris JS, Slutsky AS, Akira S, Hultqvist M, Holmdahl R, Nicholls J, Jiang C, Binder CJ, Penninger JM (2008) Identification of oxidative stress and toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133:235–249
Subbanagounder G, Leitinger N, Shih PT, Faull KF, Berliner JA (1999) Evidence that phospholipid oxidation products and/or platelet-activating factor play an important role in early atherogenesis—in vitro and in vivo inhibition by WEB 2086. Circ Res 85:311–318
Androulakis N, Durand H, Ninio E, Tsoukatos DC (2005) Molecular and mechanistic characterization of platelet-activating factor-like bioactivity produced upon LDL oxidation. J Lipid Res 46:1923–1932
Gopfert MS, Siedler F, Siess W, Sellmayer A (2005) Structural identification of oxidized acyl-phosphatidylcholines that induce platelet activation. J Vasc Res 42:120–132
King MF, Boyd LC, Sheldon BW (1992) Effects of phospholipids on lipid oxidation of a salmon oil model system. J Am Oil Chem Soc 69:237–242
King MF, Boyd LC, Sheldon BW (1992) Antioxidant properties of individual phospholipids in a salmon oil model system. J Am Oil Chem Soc 69:545–551
Boyd LC, Nwosu VC, Young CL, MacMillian L (1998) Monitoring lipid oxidation and antioxidant effects of phospholipids by headspace gas chromatographic analyses of rancimat trapped volatiles. J Food Lipids 5:269–282
Miyashita K, Nara E, Ota T (1994) Comparative-study on the oxidative stability of phosphatidylcholines from salmon egg and soybean in an aqueous-solution. Biosci Biotechnol Biochem 58:1772–1775
Applegate KR, Glomset JA (1986) Computer-based modeling of the conformation and packing properties of docosahexaenoic acid. J Lipid Res 27:658–680
Applegate KR, Glomset JA (1991) Effect of acyl chain unsaturation on the conformation of model diacylglycerols—a computer modeling study. J Lipid Res 32:1635–1644
Applegate KR, Glomset JA (1991) Effect of acyl chain unsaturation on the packing of model diacylglycerols in simulated monolayers. J Lipid Res 32:1645–1655
Feng SS, Brockman HL, Macdonald RC (1994) On osmotic-type equations of state for liquid-expanded monolayers of lipids at the air–water-interface. Langmuir 10:3188–3194
Brockman HL, Applegate KR, Momsen MM, King WC, Glomset JA (2003) Packing and electrostatic behavior of sn-2-docosahexaenoyl and -arachidonoyl phosphoglycerides. Biophys J 85:2384–2396
Albrand M, Pageaux JF, Lagarde M, Dolmazon R (1994) Conformational-analysis of isolated docosahexaenoic acid (22/6 N-3) and its 14-(S) and 11-(S) hydroxy derivatives by force-field calculations. Chem Phys Lipids 72:7–17
Koenig BW, Strey HH, Gawrisch K (1997) Membrane lateral compressibility determined by NMR and X-ray diffraction: effect of acyl chain polyunsaturation. Biophys J 73:1954–1966
Eldho NV, Feller SE, Tristram-Nagle S, Polozov IV, Gawrisch K (2003) Polyunsaturated docosahexaenoic vs docosapentaenoic acid—differences in lipid matrix properties from the loss of one double bond. J Am Chem Soc 125:6409–6421
Feller SE, Gawrisch K, MacKerell AD (2002) Polyunsaturated fatty acids in lipid bilayers: intrinsic and environmental contributions to their unique physical properties. J Am Chem Soc 124:318–326
Saiz L, Klein ML (2001) Structural properties of a highly polyunsaturated lipid bilayer from molecular dynamics simulations. Biophys J 81:204–216
Huber T, Rajamoorthi K, Kurze VF, Beyer K, Brown MF (2002) Structure of docosahexaenoic acid-containing phospholipid bilayers as studied by H-2 NMR and molecular dynamics simulations. J Am Chem Soc 124:298–309
Everts S, Davis JH (2000) H−1 and C−13 NMR of multilamellar dispersions of polyunsaturated (22:6) phospholipids. Biophys J 79:885–897
Gawrisch K, Eldho NV, Holte LL (2003) The structure of DHA in phospholipid membranes. Lipids 38:445–452
Bandarra NM, Campos RM, Batista I, Nunes ML, Empis JM (1999) Antioxidant synergy of alpha-tocopherol and phospholipids. J Am Oil Chem Soc 76:905–913
Ohshima T, Fujita Y, Koizumi C (1993) Oxidative stability of sardine and mackerel lipids with reference to synergism between phospholipids and alpha-tocopherol. J Am Oil Chem Soc 70:269–276
Saito H, Ishihara K (1997) Antioxidant activity and active sites of phospholipids as antioxidants. J Am Oil Chem Soc 74:1531–1536
Kashima M, Cha GS, Isoda Y, Hirano J, Miyazawa T (1991) The antioxidant effects of phospholipids on perilla oil. J Am Oil Chem Soc 68:119–122
Hildebrand DH, Terao J, Kito M (1984) Phospholipids plus tocopherols increase soybean oil stability. J Am Oil Chem Soc 61:552–555
Weng XC, Gordon MH (1993) Antioxidant synergy between phosphatidyl ethanolamine and alpha-tocopherylquinone. Food Chem 48:165–168
Mei LY, Decker EA, McClements DJ (1998) Evidence of iron association with emulsion droplets and its impact on lipid oxidation. J Agric Food Chem 46:5072–5077
Kuzuya M, Yamada K, Hayashi T, Funaki C, Naito M, Asai K, Kuzuya F (1991) Oxidation of low-density-lipoprotein by copper and iron in phosphate buffer. Biochim Biophys Acta 1084:198–201
Djuric Z, Potter DW, Taffe BG, Strasburg GM (2001) Comparison of iron-catalyzed DNA and lipid oxidation. J Biochem Mol Toxicol 15:114–119
Were LM, Bruce BD, Davidson PM, Weiss J (2003) Size, stability, and entrapment efficiency of phospholipid nanocapsules containing polypeptide antimicrobials. J Agric Food Chem 51:8073–8079
Laridi R, Kheadr EE, Benech RO, Vuillemard JC, Lacroix C, Fliss I (2003) Liposome encapsulated nisin Z: optimization, stability and release during milk fermentation. Int Dairy J 13:325–336
Sulkowski WW, Pentak D, Nowak K, Sulkowska A (2005) The influence of temperature, cholesterol content and pH on liposome stability. J Mol Struct 744:737–747
Finean JB (1990) Interaction between cholesterol and phospholipid in hydrated bilayers. Chem Phys Lipids 54:147–156
Fiorentini D, Landi L, Barzanti V, Cabrini L (1989) Buffers can modulate the effect of sonication on egg lecithin liposomes. Free Radic Res Commun 6:243–250
Kirby C, Clarke J, Gregoriadis G (1980) Effect of the cholesterol content of small unilamellar liposomes on their stability in vivo and in vitro. Biochem J 186:591–598
Senior J, Gregoriadis G (1982) Stability of small unilamellar liposomes in serum and clearance from the circulation—the effect of the phospholipid and cholesterol components. Life Sci 30:2123–2136
Papahadj D, Jacobson K, Nir S, Isac T (1973) Phase-transitions in phospholipid vesicles—fluorescence polarization and permeability measurements concerning effect of temperature and cholesterol. Biochim Biophys Acta 311:330–348
Brockerh H (1974) Model of interaction of polar lipids, cholesterol, and proteins in biological-membranes. Lipids 9:645–650
Huang CH (1977) Structural model for cholesterol–phosphatidylcholine complexes in bilayer membranes. Lipids 12:348–356
Frankel EN (1993) In search of better methods to evaluate natural antioxidants and oxidative stability in food lipids. Trends Food Sci Tech 4:220–225
Cillard J, Cillard P, Cormier M, Girre L (1980) Alpha-tocopherol prooxidant effect in aqueous-media—increased autoxidation rate of linoleic-acid. J Am Oil Chem Soc 57:252–255
Bazin BC, Cillard J, Koskas JP, Cillard P (1984) Arachidonic acid autooxidation in an aqueous media effect of α-tocopherol, cystein and nucleic acids. J Am Oil Chem Soc 61:1212–1215
MacMillan DK, Murphy RC (1995) Analysis of lipid hydroperoxides and long-chain conjugated keto acids by negative ion electrospray mass spectrometry. J Am Soc Mass Spectrom 6:1190–1201
Spickett CM, Pitt AR, Brown AJ (1998) Direct observation of lipid hydroperoxides in phospholipid vesicles by electrospray mass spectrometry. Free Radical Biol Med 25:613–620
Spickett CM, Rennie N, Winter H, Zambonin L, Landi L, Jerlich A, Schaur RJ, Pitt AR (2001) Detection of phospholipid oxidation in oxidatively stressed cells by reversed-phase HPLC coupled with positive-ionization electroscopy MS. Biochem J 355:449–457
Yin HY, Cox BE, Liu W, Porter NA, Morrow JD, Milne GL (2009) Identification of intact oxidation products of glycerophospholipids in vitro and in vivo using negative ion electrospray ion trap mass spectrometry. J Mass Spectrom 44:672–680
Taylor TM, Davidson PM, Bruce BD, Weiss J (2005) Liposomal nanocapsules in food science and agriculture. Crit Rev Food Sci 45:587–605
Thompson AK, Hindmarsh JP, Haisman D, Rades T, Singh H (2006) Comparison of the structure and properties of liposomes prepared from milk fat globule membrane and soy phospholipids. J Agric Food Chem 54:3704–3711
Thompson AK, Haisman D, Singh H (2006) Physical stability of liposomes prepared from milk fat globule membrane and soya phospholipids. J Agric Food Chem 54:6390–6397
Thompson AK, Mozafari MR, Singh H (2007) The properties of liposomes produced from milk fat globule membrane material using different techniques. Lait 87:349–360
Cansell M, Nacka F, Combe N (2003) Marine lipid-based liposomes increase in vivo FA bioavailability. Lipids 38:551–559
Cansell M, Moussaoui N, Petit AP, Denizot A, Combe N (2006) Feeding rats with liposomes or fish oil differently affects their lipid metabolism. Eur J Lipid Sci Technol 108:459–467
Hossain Z, Kurihara H, Hosokawa M, Takahashi K (2006) Docosahexaenoic acid and eicosapentaenoic acid-enriched phosphatidylcholine liposomes enhance the permeability, transportation and uptake of phospholipids in Caco-2 cells. Mol Cell Biochem 285:155–163
Kirby CJ, Whittle CJ, Rigby N, Coxon DT, Law BA (1991) Stabilization of ascorbic-acid by microencapsulation in liposomes. Int J Food Sci Tech 26:437–449
Chang HM, Lee YC, Chen CC, Tu YY (2002) Microencapsulation protects immunoglobulin in yolk (IgY) specific against Helicobacter pylori urease. J Food Sci 67:15–20
Rao DR, Chawan CB, Veeramachaneni R (1995) Liposomal encapsulation of beta-galactosidase—comparison of 2 methods of encapsulation and in vitro lactose digestibility. J Food Biochem 18:239–251
Hsieh YF, Chen TL, Wang YT, Chang JH, Chang HM (2002) Properties of liposomes prepared with various lipids. J Food Sci 67:2808–2813
Lee SC, Yuk HG, Lee DH, Lee KE, Hwang YI, Ludescher RD (2002) Stabilization of retinol through incorporation into liposomes. J Biochem Mol Biol 35:358–363
Lee SK, Han JH, Decker EA (2002) Antioxidant activity of phosvitin in phosphatidylcholine liposomes and meat model systems. J Food Sci 67:37–41
Lee SC, Lee KE, Kim JJ, Lim SH (2005) The effect of cholesterol in the liposome bilayer on the stabilization of incorporated retinol. J Liposome Res 15:157–166
Thapon JL, Brule G (1986) Effects of pH and ionic-strength on lysozyme-caseins affinity. Lait 66:19–30
Arnaud JP (1998) Liposome-based functional drinks. Agro Food Ind Hi Tech 9:37–40
Alkhalaf W, Piard JC, Elsoda M, Gripon JC, Desmazeaud M, Vassal L (1988) Liposomes as proteinase carriers for the accelerated ripening of Saint-Paulin type cheese. J Food Sci 53:1674–1679
Kirby CJ, Brooker BE, Law BA (1987) Accelerated ripening of cheese using liposome-encapsulated enzyme. Int J Food Sci Tech 22:355–375
Picon A, Gaya P, Medina M, Nunez M (1994) The effect of liposome encapsulation of chymosin derived by fermentation on Manchego cheese ripening. J Dairy Sci 77:16–23
Picon A, Gaya P, Medina M, Nunez M (1997) Proteinases encapsulated in stimulated release liposomes for cheese ripening. Biotechnol Lett 19:345–348
Benech RO, Kheadr EE, Laridi R, Lacroix C, Fliss I (2002) Inhibition of Listeria innocua in Cheddar cheese by addition of nisin Z in liposomes or by in situ production in mixed culture. Appl Environ Microbiol 68:3683–3690
Kheadr EE, Vuillemard JC, El Deeb SA (2000) Accelerated Cheddar cheese ripening with encapsulated proteinases. Int J Food Sci Tech 35:483–495
Matsuzaki M, Mccafferty F, Karel M (1989) The effect of cholesterol content of phospholipid-vesicles on the encapsulation and acid resistance of beta-galactosidase from Escherichia coli. Int J Food Sci Technol 24:451–460
Banville C, Vuillemard JC, Lacroix C (2000) Comparison of different methods for fortifying Cheddar cheese with vitamin D. Int Dairy J 10:375–382
Navarro JC, Bell MV, Amat F, Sargent JR (1992) The fatty-acid composition of phospholipids from brine shrimp, Artemia sp., eyes. Comp Biochem Physiol B 103:89–91
Bell MV, Batty RS, Dick JR, Fretwell K, Navarro JC, Sargent JR (1995) Dietary deficiency of docosahexaenoic acid impairs vision at low-light intensities in Juvenile herring (Clupea harengus L.). Lipids 30:443–449
Sargent JR, Mcevoy LA, Bell JG (1997) Requirements, presentation and sources of polyunsaturated fatty acids in marine fish larval feeds. Aquaculture 155:117–127
Navarro JC, Amat F, Sargent JR (1992) Lipid-composition of cysts of the brine shrimp Artemia sp. from Spanish populations. J Exp Mar Biol Ecol 155:123–131
Southgate PC, Lou DC (1995) Improving the eta-3 Hufa composition of Artemia using microcapsules containing marine oils. Aquaculture 134:91–99
Narciso L, Pousao-Ferreira P, Passos A, Luis O (1999) HUFA content and DHA/EPA improvements of Artemia sp. with commercial oils during different enrichment periods. Aquac Res 30:21–24
Salhi M, Hernandez-Cruz CM, Bessonart M, Izquierdo MS, Fernandez-Palacios H (1999) Effect of different dietary polar lipid levels and different n-3 HUFA content in polar lipids on gut and liver histological structure of gilthead seabream (Sparus aurata) larvae. Aquaculture 179:253–263
Izquierdo MS, Tandler A, Salhi M, Kolkovski S (2001) Influence of dietary polar lipids’ quantity and quality on ingestion and assimilation of labelled fatty acids by larval gilthead seabream. Aquacult Nutr 7:153–160
Cahu CL, Infante JLZ, Barbosa V (2003) Effect of dietary phospholipid level and phospholipid: neutral lipid value on the development of sea bass (Dicentrarchus labrax) larvae fed a compound diet. Br J Nutr 90:21–28
Koven W, Barr Y, Hadas E, Ben-Atia I, Chen Y, Weiss R, Tandler A (1999) The potential of liposomes as a nutrient supplement in first-feeding marine fish larvae. Aquacult Nutr 5:251–256
Kanazawa A, Teshima SI, Sakamoto M (1985) Effects of dietary lipids, fatty-acids, and phospholipids on growth and survival of prawn (Penaeus japonicus) larvae. Aquaculture 50:39–49
Mcevoy LA, Navarro JC, Amat F, Sargent JR (1997) Application of soya phosphatidylcholine in tuna orbital oil enrichment emulsions for Artemia. Aquacult Int 5:517–526
Kanazawa A, Teshima S, Sakamoto M (1985) Effects of dietary bonito-egg phospholipids and some phospholipids on growth and survival of the larval ayu, Plecoglossus altivelis. Z Angew Ichthyol 4:156–170
Touraki M, Rigas P, Kastritsis C (1995) Liposome mediated delivery of water soluble antibiotics to the larvae of aquatic animals. Aquaculture 136:1–10
Tonheim SK, Koven W, Ronnestad I (2000) Enrichment of Artemia with free methionine. Aquaculture 190:223–235
Ozkizilcik S, Chu FLE (1994) Uptake and metabolism of liposomes by Artemia nauplii. Aquaculture 128:131–141
Hontoria F, Crowe JH, Crowe LM, Amat F (1994) Potential use of liposomes in larviculture as a delivery system through Artemia nauplii. Aquaculture 127:255–264
Acknowledgments
The authors wish to acknowledge the financial support from the European Regional Development Fund, Væksforum Hovedstaden through Øresund Food's 'Healthy Growth' project and also Technical University of Denmark.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Henna Lu, F.S., Nielsen, N.S., Timm-Heinrich, M. et al. Oxidative Stability of Marine Phospholipids in the Liposomal Form and Their Applications. Lipids 46, 3–23 (2011). https://doi.org/10.1007/s11745-010-3496-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-010-3496-y