Abstract
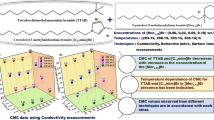
The self-aggregation of sodium dodecylsulphate (SDS), an anionic surfactant, in aqueous solutions of tetraalkylammonium bromide salts (R4NBr, where R = propyl, butyl and pentyl) was determined at various temperatures in the range 288.15–318.15 K. The critical micelle concentration (CMC) determined from conductivity data was used to study the thermodynamics of the surfactant. The presence of bromide salts was found to affect the micellization of SDS in accordance with the hydrophobicity of the tetraalkylammonium cations, thus the CMC values follow the order no additive > Pr4NBr > Bu4NBr > Pen4NBr. The results from conventional conductivity methods were combined with those of spectroscopic techniques like fluorescence and UV–Vis studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Quaternary ammonium compounds (QUATS) have been extensively studied owing to their hydrophobic character, weak surface activity and low aqueous toxicity. They have been widely used as phase transfer catalysts [1–3] and in detergents [4], disinfectants [5, 6] and synthetic reagents [7, 8]. QUATS, especially those containing long alkyl chains e.g. cetylpyridinium chloride, are used as hygienic adjuncts against bacterial growth in various industrial and clinical formulations [9, 10]. Among the different QUATS, tetraalkylammonium bromides are an interesting group owing to their symmetric structure having four short alkyl chains in one molecule providing a large hydrophobic volume. Hence the hydrophobic interactions between alkyl chains allow for the penetration of some alkyl chains at the micellar surface into another micelle. Qualitatively, they act as spacers between the surfactant head groups and promote self-association and micelle formation [11, 12].
Basic data on surfactant–QUATS interactions is essential for understanding the effects of interplay of solute–solute, solute–solvent and solvent–solvent interactions on micelle formation [11–15]. Such information is useful to industrial chemists, especially in the optimization and determination of various characteristic properties of surfactants. A detailed investigation of the literature reveals that studies on the effect of QUAT salts on aggregation of surfactants are scarce. In our previous study, we analysed the dependence of micellization of the cationic surfactant dodecyltrimethylammonium bromide (DTAB) on the alkyl chain length of tetraalkylammonium bromides [16]. The results show the importance of hydrophobic interactions in surfactant micellization in the presence of bromide salts. The present work analyses the effect of tetraalkylammonium bromide salts (R4NBr, where R = propyl, butyl and pentyl) on the micellar behaviour of the anionic surfactant sodium dodecylsulphate (SDS) by employing conventional conductivity methods in combination with spectroscopic techniques like fluorescence and UV–Vis probe studies. For conductivity measurements, temperatures ranging from 288.15 K to 318.15 K at a regular interval of 5 K were selected, whereas the spectroscopic studies were carried out at room temperature (i.e. 298.15 K).
Experimental
Materials
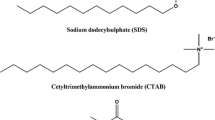
Deionized distilled water with a conductivity of 2–3 × 10−6 S cm−1 and pH of 6.8–7.0 (at 298.15 K) was obtained from a Millipore–Elix system and was used for all the experiments. SDS of A.R. grade was obtained from Himedia (India) and was used after purification as mentioned in the literature [17]. Tetrapropylammonium bromide (C12H28NBr) from Fluka (Switzerland), tetrabutylammonium bromide (C16H36NBr) from SRL (India) and tetrapentylammonium bromide (C20H44NBr) from Acros Organics (Belgium) were dried in a vacuum oven at 333.15 K for 24 h before use. The probe used for the spectroscopic techniques was pyrene (A.R. grade) provided by Merck (Germany) and was used as received. The specifications of all the samples used are provided in Table 1 and their structures are given in Fig. 1.
Methods
Conductivity measurements were carried out with a Cyberscan CON-510 digital conductivity meter. The calibration of the conductivity cell was done with 0.01 M KCl calibration solution provided by Merck Chem Ltd. The reproducibility of the conductance measurements was estimated to be ±5 µS cm−1. The temperature was maintained constant at ±0.01 K by circulating thermostated water through a double-walled conductivity vessel containing the solution. The specific conductance (κ) of SDS solution in aqueous solutions of 0.01 mol kg−1 Pr4NBr, Bu4NBr and Pen4NBr were measured over a wide range of temperature (288.15–318.15 K) at an interval of 5 K.
Both fluorescence and UV–Vis spectra were recorded at room temperature (298.15 K). Pyrene solution of concentration approx. 2 × 10−6 mol kg−1 in ethanol was used as a probe. Fluorescence measurements were carried out using a Perkin Elmer LS 55 fluorescence spectrometer at room temperature (298.15 K). The emission spectra of the solutions were recorded in the wavelength range of 350–450 nm and the excitation and emission slit widths were 8 nm and 2.5 nm, respectively. UV–Vis absorption spectra of SDS-R4NBr solutions were recorded using a Genesys 10S spectrophotometer supplied by Thermoscientific, USA using 10-mm-path-length quartz cuvettes. The absorbance spectra were obtained in the 200–400 nm wavelength range and at room temperature (298.15 K).
Results and Discussion
Conductivity Measurements
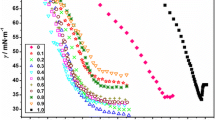
The conductivity data for the SDS in the absence and presence of 0.01 mol kg−1 tetraalkylammonium bromide salts R4NBr are reported in Table S1 of the supplementary data. The specific conductance (κ) varies linearly with [SDS] in both pre- and post-micellar regions (Fig. 2), with the slope (\(S_{1}\)) in pre-micellar region always greater than that in post-micellar region (\(S_{2}\)). The intersection point between two straight lines is the critical micellar concentration (CMC) while the ratio \({{S_{2} } \mathord{\left/ {\vphantom {{S_{2} } {S_{1} }}} \right. \kern-0pt} {S_{1} }}\) gives counterion dissociation (α). Linear regression analysis of the conductivity data was used to calculate \(S_{1}\) and \(S_{2}\) values and, in this case, the correlation factor was found to be better than 0.995.
The CMC and corresponding \(X_{\text{CMC}}\) (CMC in mole fraction units) values for aqueous SDS in the absence and presence of R4NBr are recorded in Table 2 and reveal that CMC values for the SDS (8.20 mmol kg−1 at 298.15 K) in water are closer to the values reported in the literature i.e. 8.10 mmol kg−1 for SDS [18].
The effect of temperature on CMC values of SDS is presented in Fig. 3, indicating that CMC values first decrease to a minimum around 298.15 K and then increase with rising temperature. For most of the ionic surfactants such as alkyl sulphates and some non-ionic surfactants like N-decanoyl-N-methylglucamine (MEGA 10), the minimum in the CMC vs. temperature plot is a usual trend which is well documented in the literature [19–23]. In general, the temperature dependence of CMC is analysed by considering two opposite processes [24]. As the temperature increases, the hydrophilicity of the surfactant decreases as a result of the dehydration of the monomers, which favours micellization. However, the rise in temperature also causes the disruption of water structure around the hydrophobic groups, increasing the solubilisation of surfactant monomers and hence inhibits the micelle formation.
Also as the temperature rises, the thermal motions of surfactant and solvent molecules are enhanced so that the formation of ordered micelle structures becomes difficult. The increase of temperature increases the kinetic energies and destroys the ordered micellar structures leading to an increase in the CMC value. Therefore, the higher the temperature is, the greater the degree of disaggregation and the higher the CMC are. In the case of SDS, the gradual decrease in CMC at lower temperatures and then similar increase at higher temperatures may be due to the dominance of the first and second factors, respectively.
The addition of tetraalkylammonium bromide salts decreased the surfactant CMC in the following order: C12H28NBr > C16H36NBr > C20H44NBr. The presence of four alkyl chains in addition to the positive charge on the nitrogen atom in the tetraalkylammonium cation allows it to interact electrostatically as well as hydrophobically with the micellar surface of anionic SDS. The strong electrostatic interactions between the anionic micelles and cationic counterions neutralize the effective charge on the head groups of the surfactant, thereby reducing the repulsions between polar head groups. These interactions result in an increase of dispersion forces and therefore promote micelle formation. The dominance of this factor has been observed in the literature [25–27]. However, the presence of hydrocarbon chains in the case of quaternary ammonium salts may result in penetration of some of the alkyl chains into the micellar core of the surfactant as a result of hydrophobic interactions. The remarkable decrease in CMC of the surfactant as the size of the alkyl chain increases also supports the dominance of hydrophobic interactions [11, 28–30].
Thermodynamics of Micellization
In order to further interpret the surfactant–tetraalkylammonium bromide salt interactions, various thermodynamic parameters of micellization have been calculated and explained. According to the charged pseudo-phase model of micellization, the standard free energy (\(\Delta G_{m}^{o}\)) of micelle formation per mole of surfactant is given by
where \(R\) is the gas constant and T is the temperature in kelvin (K).
The standard enthalpy of micelle formation (\(\Delta H_{m}^{o}\)) can be derived by Van’t Hoff equation
where \(d(\ln X_{\text{CMC}} )/dT\) is the slope of the plot of \(\ln X_{\text{CMC}}\) against temperature at each temperature calculated by fitting \(\ln X_{\text{CMC}}\) versus T data to a second-order polynomial and differentiation.
The standard entropy of micelle formation (\(\Delta S_{m}^{o}\)) was calculated from the relation
The thermodynamic parameters of micellization for SDS in different aqueous solutions of tetraalkylammonium bromides at different temperatures are presented in Table 3.
The \(\Delta H_{m}^{o}\) values for SDS were endothermic when T < T* (where T* is the temperature at which CMC was minimum) and became exothermic and larger in magnitude when T > T* for all the studied systems (Table 3). At low temperatures, the positive \(\Delta H_{m}^{o}\) values were probably due to the destruction of structured (or hydrogen-bonded) water molecules around hydrophobic alkyl chains of surfactant monomers. However, such hydrophobic interactions become increasingly insignificant and the hydration of water molecules around the hydrophilic head groups may result into negative \(\Delta H_{m}^{o}\) values at higher temperatures. The positive \(\Delta H_{m}^{o}\) values show the dominance of hydrophobic interactions in micellization, whereas negative \(\Delta H_{m}^{o}\) values suggest the importance of London dispersion interactions as an alternative force for micellization [28, 31]. The tetraalkylammonium salts seem to add to this effect which is clearly reflected by the large negative \(\Delta H_{m}^{o}\) values for these salts.
The \(\Delta S_{m}^{o}\) values are positive and show a decrease with rise in temperature. This can be explained by considering two opposing processes that occur during aggregation viz. (1) disruption of three-dimensional water structure around the hydrocarbon tails of surfactant monomers due to aggregation into micelles resulting in increased randomness and hence increase in the entropy of the system and (2) the arrangement of disordered monomers into more ordered surfactant aggregates leading to a negative change in entropy [31, 32]. As can be seen from Table 3, the decrease in \(\Delta S_{m}^{o}\) values with temperature may be due to the dominance of the latter process. Hence, it appears that the process of micellization of SDS is entropy controlled at low temperatures but enthalpy controlled at high temperatures in all the studied systems. Such an effect of temperature on \(\Delta H_{m}^{o}\) and \(\Delta S_{m}^{o}\) values of ionic surfactants has also been reported in the literature [19, 21, 22, 24].
From Table 3 it can be inferred that \(\Delta G_{m}^{o}\) values are negative in all the studied cases suggesting the spontaneous micellization of SDS [19]. Temperature as well as type of electrolytes seems to have a negligible effect on \(\Delta G_{m}^{o}\) values. However, \(\Delta G_{m}^{o}\) is the sum of the enthalpic (\(\Delta H_{m}^{o}\)) and entropic (\({ - }T\Delta S_{m}^{o}\)) contributions. As the temperature rises, the contribution of entropy to free energy decreases, whereas that of enthalpy increases (Fig. 4).
Enthalpy–Entropy Compensation for SDS Micellization
The dependence of the enthalpy of micellization on the entropy of micellization follows a straight line equation described by the relation
where T c, the compensation temperature, quantifies the de-solvation due to the micellization and \(\Delta H_{m}^{*}\) measures the chemical aggregation of the surfactant monomers to form the micelles. The slope, T c from \(\Delta H_{m}^{0}\) versus \(\Delta S_{m}^{0}\) plots, characterizes both solute–solvent and solute–solvent interactions, whereas \(\Delta H_{m}^{*}\) interprets the solute–solute interactions [33].
In the present study, a good correlation between \(\Delta H_{m}^{0}\) and \(\Delta S_{m}^{0}\) values of SDS in all the cases was observed with the correlation coefficient lying near to 0.998 (Fig. 5). The parameter \(\Delta H_{m}^{*}\) is the enthalpy when \(\Delta S_{m}^{0}\) = zero and indicates the stability of micelles. The stability of micelles increases with the increase in the negative values of \(\Delta H_{m}^{*}\). It is clear from Table 4 that the value of \(\Delta H_{m}^{*}\) shows a slight increase as the length of alkyl chain of tetraalkylammonium bromide salt increases. In other words, the increase in the steric hindrance with longer chains probably reduces the contribution of the chemical part towards micellization [34]. The value for compensation temperature T c lies in the well-documented range of approximately 301–307 K for various sodium alkylsulphate surfactants [35].
Spectroscopic Measurements
UV–Vis spectroscopy provides supporting evidence about the formation of micelles in solution. The spectra with varying concentration of surfactant yield important information regarding the interaction between pyrene and surfactant. The simple UV–Vis absorbance spectrum of pyrene in water gives four strong peaks at 242, 272, 320, and 336 nm due to multiple rings [36]. The total absorbance (A T) i.e. the sum of absorbance of all four strong peaks is plotted against the concentration of surfactant in Fig. 6. At low surfactant concentration, there is a very small increase in the absorbance as pyrene resides in a polar aqueous environment. When the concentration reaches the CMC value, there is a sudden increase in absorbance because pyrene resides in a non-polar environment provided by SDS micelles [37]. Also the lack of hydrophilicity of pyrene helps it to stay at the interface. This reduces the hydrophobic repulsions between water and pyrene [38]. It also reveals the importance of ionic interactions between the pyrene molecules and ionic headgroup of surfactant. After the micellization, there is a slight increase in absorbance. It has been observed that the absorbance of peaks increases with concentration of surfactant. In all the absorbance–surfactant concentration profiles, A T increases sigmoidally with concentration and thus a sigmoidal Boltzmann equation (SBE) [39] can be fitted to evaluate the CMC values reported in Table 5.
Fluorescence Probe Study
Fluorescence probe analysis is a useful method for studying micellar aggregates and membranes [40, 41]. In the present study, pyrene has been used as a fluorescence probe to obtain the CMC of SDS in the absence and presence of tetraalkylammonium bromide salts. The ratio of the fluorescence intensity of the highest vibrational band energy to that of the third highest vibrational band energy i.e. (I 1/I 3) has been taken as a measure of polarity of the environment and hence has been used to study the formation of the surfactant micelles [42, 43]. Figure 7 shows the plots of I 1/I 3versus [SDS] in the presence of tetraalkylammonium bromide salts R4NBr. Pyrene senses the polar environment at low concentration of surfactant, resulting in high values of ratio I 1/I 3. The further addition of surfactant makes the environment around pyrene hydrophobic due to the formation of micelle, resulting in a drastic decrease in I 1/I 3. Thus the fitting of Boltzmann equation to these sigmoidal curves allows the determination of the CMC. An interesting feature is that the I 1/I 3 values follow the order Pr4NBr > Bu4NBr > Pen4NBr. These results showed that as the hydrophobicity of the salt increased, the electrostatic interactions decreased as discussed in conductivity studies and hence the values of I 1/I 3.
The CMC values of SDS in the absence and presence of tetraalkylammonium bromide salts obtained from the fluorescence studies are reported in Table 5 along with those from literature [44–46]. The values obtained by this method were close to those obtained by conductivity and UV–Vis spectroscopic methods. The small difference in the CMC values may be due to the different methods adopted. However, the CMC values followed the same trend: no additive > Pr4NBr > Bu4NBr > Pen4NBr. These results also showed that the addition of tetraalkylammonium bromide salts effectively decreased the CMC value for aqueous solution of SDS as a result of increased hydrophobicity of the salts.
Conclusion
The addition of cationic tetraalkylammonium bromide salts to anionic SDS micelles promotes micellization of the surfactant. This may be due to the (1) strong ionic attractions between the oppositely charged ions of the surfactant and the bromide salt and (2) hydrophobic interactions due to the penetration of long alkyl chains of QUATS into the hydrophobic core of the micelles. It has been also confirmed that the longer the hydrocarbon chain of the bromide salt are, the stronger the interactions are. Furthermore, the investigation of thermodynamic parameters reveals that the micellization of SDS is spontaneous and is mainly enthalpy driven in the presence of QUATS. The enthalpy of micellization correlates well with the entropy of micellization. The results from the conductivity measurements are strongly supported by those obtained from fluorescence and UV–Vis probe studies.
References
Starks CM. Phase-transfer catalysis. I. Heterogeneous reactions involving anion transfer by quaternary ammonium and phosphonium salts. J Am Chem Soc. 1971;93:195–9.
Halpren M, Sasson Y, Robinovitz M. Hydroxide ion initiated reactions under phase transfer catalysis conditions–IV: effect of catalyst. Tetrahedron. 1982;38:3183–7.
Lygo B, Andrews BI, Crosby J, Peterson JA. Asymmetric alkylation of glycine imines using in situ generated phase-transfer catalysts. Tetrahedron Lett. 2002;43:8015–8.
Barney R, Carroll J IV, Delaet D. Surfactant studies of quaternary ammonium compounds: critical surfactant concentration. J Surfact Deterg. 2006;9:137–40.
Taylor RB, Toasaksiri S, Raid RG. Determination of antibacterial quaternary ammonium compounds in lozenges by capillary electrophoresis. J Chromat A. 1998;798:335–43.
Lopaz JR, Videa M. Study of the ion transfer of quaternary ammonium ions by SWV. J Mex Chem Soc. 2012;56:417–25.
Deakyne CA, Meot-Ner M. Unconventional ionic hydrogen bonds. 2. NH+···Π-complexes of onium ions with olefins and benzene derivatives. J Am Chem Soc. 1985;107:474–9.
Ghammani S, Sajabi SA. Tetrabutylammonium fluorochromate(VI) (TBAFC): a mild and efficient reagent for oxidation of organic substrates. J Serb Chem Soc. 2005;70:1243–8.
Vashkov VI. Antimicrobial agents and desinfection methods against infectious diseases. Moscow: Meditsina; 1997.
McBain AJ, Ledder RG, Moore LE, Catrenich CE, Gilbert P. Effects of quaternary-ammonium-based formulations on bacterial community dynamics and antimicrobial susceptibility. Appl Environ Microbiol. 2004;70:3449–56.
Patel J, Varade D, Bahadur P. Effect of tetraalkylammonium bromides on the micellar behaviour of ionic and non-ionic surfactants. IJC. 2004;43A:715–21.
Mata J, Varade D, Ghosh G, Bahadur P. Effect of tetrabutylammonium bromide on the micelles of sodium dodecyl sulphate. Colloid Surf A. 2004;245:69–73.
Patist A, Huibers PDT, Deneka B, Shah DO. Effect of tetraalkylammonium chlorides on foaming properties of sodium dodecyl sulfate solutions. Langmuir. 1998;14:4471–4.
Matsouka K, Chiba N, Yoshimura T, Takeuchi E. Effect of double quaternary ammonium groups on micelle formation of partially fluorinated surfactant. J Colloid Interf Sci. 2011;356:624–9.
Yoshimura T, Chiba N, Matsouka K. Supra-long chain surfactants with double or triple quaternary ammonium headgroups. J Colloid Interf Sci. 2012;374:157–63.
Chauhan S, Kaur M, Kumar K, Chauhan MS. Study of the effect of electrolyte and temperature on the critical micelle concentration of dodecyltrimethylammonium bromide in aqueous medium. J Chem Thermodyn. 2014;78:175–81.
Chauhan S, Kumar K, Singh K, Jyoti J. Volumetric, compressibility, and surface tension studies on micellization behavior of SDS in aqueous medium: effect of sugars. J Surfact Deterg. 2014;17:169–75.
Das D, Ismail K. Aggregation and adsorption properties of sodium dodecyl sulfate in water–acetamide mixtures. J Colloid Interf Sci. 2008;327:198–203.
Kang KH, Kim HU, Lim KH. Effect of temperature on critical micelle concentration and thermodynamic potentials of micellization of anionic ammonium dodecyl sulfate and cationic octadecyl trimethyl ammonium chloride. Colloid Surf A. 2001;189:113–21.
Mata J, Varade D, Bahadur P. Aggregation behavior of quaternary salt based cationic surfactants. Thermochim Acta. 2005;428:147–55.
Mehta SK, Bhasin KK, Chauhan R, Dham S. Effect of temperature on critical micelle concentration and thermodynamic behavior of dodecyldimethylethylammonium bromide and dodecyltrimethylammonium chloride in aqueous media. Colloid Surf A. 2005;255:153–7.
Noudeh GD, Housaindokht M, Bazzaz BSF. The effect of temperature on thermodynamic parameters of micellization of some surfactants. J Appl Sci. 2007;7:47–52.
Prasad M, Chakraborty I, Rakshit AK, Moulik SP. Critical evaluation of micellization behavior of nonionic surfactant MEGA 10 in comparison with ionic surfactant tetradecyltriphenylphosphonium bromide studied by microcalorimetric method in aqueous medium. J Phys Chem B. 2006;110:9815–21.
Chauhan S, Sharma K. Effect of temperature and additives on the critical micelle concentration and thermodynamics of micelle formation of sodium dodecyl benzene sulfonate and dodecyltrimethylammonium bromide in aqueous solution: a conductometric study. J Chem Thermodyn. 2014;71:205–11.
Behera K, Pandey S. Modulating properties of aqueous sodium dodecyl sulphate by adding hydrophobic ionic liquid. J Colloid Interf Sci. 2007;316:803–14.
Ali A, Ansari NH. Studies on the effect of amino acids/peptide on micellization of SDS at different temperatures. J Surfact Deterg. 2010;13:441–9.
Javadian S, Nasiri F, Heydari A, Yousefi A, Shahir AA. Modifying effect of imidazolium-based ionic liquids on surface activity and self-assembled nanostructures of sodium dodecyl sulfate. J Phys Chem B. 2014;118:4140–50.
Das C, Das B. Effect of tetraalkylammonium salts on the micellar behavior of lithium dodecyl sulfate: a conductometric and tensiometric study. J Mol Liq. 2008;137:152–8.
Sinha S, Bahadur P. Effect of organic counter-ions on the surface activity, micellar formation and dye solubilization behaviour of cationic surfactants. IJC. 2002;41A:914–20.
Kabir-ud-din, Kumar S, Parveen N. The clouding phenomenon for anionic sodium dodecyl sulfate + quaternary bromides in polar nonaqueous-water-mixed solvents. J Surfact Deterg. 2008;11:335–41.
Yan Z, Bai X, Liu R, Wu S, Wang J. Effect of dipeptides on the micellization and thermodynamic parameters of sodium dodecyl sulfonate: conductometric and fluoimetric studies. J Mol Liq. 2013;177:78–84.
Wagle VB, Kothari PS, Gaikar VG. Effect of temperature on aggregation behavior of aqueous solutions of sodium cumene sulfonate. J Mol Liq. 2007;133:68–76.
Lumry R, Rajender S. Enthalpy–entropy compensation phenomena in water solutions of proteins and small molecules: a ubiquitous property of water. Biopolymers. 1970;9:1125–7.
Perez AG, Castillo JLD, Czapkiewicz J, Rodriguez JR. Micellization of decyl- and dodecyldimethylbenzylammonium bromides at various temperatures in aqueous solutions. Colloid Polym Sci. 2002;280:503–8.
Chen LJ, Lin SY, Huang CC. Effect of hydrophobic chain length of surfactants on enthalpy-entropy compensation of micellization. J Phys Chem B. 1998;102:4350–6.
Tanhaei B, Saghatoleslami N, Chenar MP, Ayati A, Hesampour M, Manttari M. Experimental study of CMC evaluation in single and mixed surfactant systems, using the UV–Vis spectroscopic method. J Surfact Deterg. 2013;16:357–62.
Kumar K, Chauhan S. Surface tension and UV-visible investigations of aggregation and adsorption behaviour of NaC and NaDC in water-amino acid mixtures. Fluid Phase Equilib. 2015;394:165–74.
Khan AM, Shah SS. A UV-visible study of partitioning of pyrene in an anionic surfactant sodium dodecyl sulfate. J Dispers Sci Techn. 2008;29:1401–7.
Hait SK, Moulik SP, Palepu R. Refined method of assessment of parameters of micellization of surfactants and percolation of W/O microemulsions. Langmuir. 2002;18:2471–6.
Aguiar J, Carpena P, Molina-Bolívar JA, Carnero Ruiz C. On the determination of the critical micelle concentration by the pyrene 1:3 ratio method. J Colloid Interf Sci. 2003;258:116–22.
Ali A, Tariq M, Patel R, Ittoo FA. Interaction of glycine with cationic, anionic, and nonionic surfactants at different temperatures: a volumetric, viscometric, refractive index, conductometric, and fluorescence probe study. Colloid Polym Sci. 2008;286:183–90.
Pal A, Chaudhary S. Effect of hydrophilic ionic liquid on aggregation behaviour of aqueous solutions of sodium dodecylsulfate (SDS). Fluid Phase Equilib. 2013;352:42–6.
Yoshimura T, Nagata Y, Esumi K. Interactions of quaternary salt-type gemini surfactants with sodium poly(styrene sulfonate). J Colloid Interf Sci. 2004;275:618–22.
Zhang X, Jackson JK, Burt HM. Determination of surfactant critical micelle concentration by a novel fluorescence depolarization technique. J Biochem Biophys Methods. 1996;31:145–50.
Ruiz CC. Micelle formation and microenvironmental properties of sodium dodecyl sulfate in aqueous urea solutions. Colloid Surf A. 1999;147:349–57.
Dominguez A, Fernandez A, Gonzalez N, Iglesias E, Montenegro L. Determination of critical micelle concentration of some surfactants by three techniques. J Chem Educ. 1997;74:1227–31.
Acknowledgements
S. Chauhan and Maninder Kaur are highly thankful to UGC, New Delhi for financial assistance under the project (F. No. 42-249/2013/SR) and award of Senior Research Fellowship (No. F.17-40/2008(SA-1) dated 31.07.2014), respectively. Financial support from UGC-SAP (DRS-I) (No. F.540/3/DRS/2010 (SAP-1)) to the Department of Chemistry, HPU is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Chauhan, S., Kaur, M. Modulation of Aggregation Behaviour of Anionic Surfactant in the Presence of Aqueous Quaternary Ammonium Salts. J Surfact Deterg 20, 599–607 (2017). https://doi.org/10.1007/s11743-017-1949-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-017-1949-5