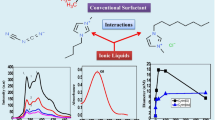
Abstract
The purpose of this study is to investigate the influence of a morpholinium-based ionic liquid, N-pentadecyl-N-methylmorpholinium bromide [Mor1,15]Br on the aggregation behavior of a conventional cationic surfactant, tetradecyltrimethylammonium bromide, and imidazolium-based surface-active ionic liquid, 1-tetradecyl-3-methylimidazolium bromide [C14mim]Br in the aqueous medium. The aggregation behavior has been investigated in (0.00, 0.03, 0.06, and 0.10) wt% of [Mor1,15]Br by employing conductometry, refractive index and surface-tension measurements. The obtained data leads to the determination of the critical micelle concentration values, which have been further used to find various thermodynamic and surface-active parameters of micellization for the investigated system. The modulations observed in the aggregation behavior and the values of obtained parameters are indicative of various interactions prevailing among different constituents of the solution. The knowledge of these interactions is important for the utilization of such systems in various industrial applications such as foams, froths, emulsions, suspensions, and surface coatings to enhance their quality and performance.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The vast applications of surface-active ionic liquids (SAILs) in different sectors, such as nanomaterials synthesis and separation processing, organic synthesis, micellar catalysis, enhanced oil recovery, water treatment technologies, etc., have piqued researchers’ interest [1,2,3,4,5,6]. Ionic liquids (ILs) have several beneficial and intriguing properties, such as low volatility, exceptional thermal stability, high electric conductivity and wide liquid range [7,8,9,10]. The existence of a long hydrophobic chain in ILs makes them surface-active, hence termed SAILs [11,12,13]. Because SAILs generally contain long hydrophobic alkyl chains, they can self-aggregate into micelles. Micellar aggregation characteristics of SAILs and surfactants have recently gotten a lot of interest from academia and practice [14,15,16,17]. Due to the higher stability of SAILs, at high temperatures, than conventional surfactants, the long-chain ILs are able to widen their application regions by mixing the surfactants and ILs. As a result, the self-assembly and aggregation behavior of surfactants or SAILs aided by the ILs addition has a broader use in a variety of domains, including extraction, absorption, separation, and many other practical applications [18,19,20,21].
The aggregation of surface-active molecules takes place over a narrow range of concentrations, recognized as the critical micelle concentration (CMC), below which these amphiphilic molecules are primarily distributed as monomers. The value of CMC is very much important in examining the aggregation behavior of surfactants and SAILs, as it provides a great deal of information concerning surface activities and their technological implications. The surface activity of surfactants and SAILs may be altered by the addition of ILs in the solution, which will result in the formation of micelles with interesting features [22,23,24]. From a practical standpoint, research into such micellar systems incorporating different surfactants and SAILs would overcome various limitations of traditionally used surfactant systems. This will lead us to broaden the scope of our IL research to include relevant micelle features.
Also, an organic cation and a suitable inorganic or organic anion were usually found in SAILs. Their physicochemical properties may usually be altered for specific applications by introducing appropriate cations and anions into their structure. The diverse organic cation rings, such as alkylmethylimidazolium ([Cnmim]+), alkylpyridinium ([CnPy]+), alkylmethylpiperidinium ([Cnmpip]+), alkylmethylpyrrolidinium ([CnmPyrr]+) and morpholinium ([CnMorph]+) can be present in SAILs as reported in the literature, which incorporates unique properties depending upon their cation [25,26,27]. Our research team has studied the aggregation behavior of several surfactants and SAILs with various additives such as drugs, carbohydrates, vitamins, salts, amino acids, alkylmethylimidazolium-based ILs, etc.[28,29,30,31] This, established that the nature of additives and the type of their interactions with the concerned surfactant and SAILs play a significant role in their aggregation. There is no existing report available in the literature that investigates the aggregation behavior of surfactants and SAILs with morpholinium-based SAILs as additives.
Variations in the aggregation behavior of TTAB and [C14mim]Br with (0.00, 0.03, 0.06, and 0.10) wt% [Mor1,15]Br at temperatures i.e., 293.15–308.15 K with temperature intervals of 5 K, have been observed. The evaluation of CMC, thermodynamic and surface-active parameters revealed the nature of interactions occurring in the micellar systems. The morpholinium-based SAILs i.e., [Mor1,15]Br can drastically modify the aggregation of TTAB and [C14mim]Br, resulting in their improved performance and application in diverse areas.
2 Experimental
2.1 Materials
Chemical names with CAS numbers, structures, sources, and purities of all the compounds used in the study have been enlisted in Table 1.
2.1.1 Synthesis of [C14mim][Br]
For the synthesis of [C14mim][Br], the drop-by-drop addition of 1-bromotetradecane to 1-methylimidazole taken in an RB flask was carried out. To facilitate the reaction, acetonitrile was added as a solvent. The reaction mixture was then kept on constant stirring with heating and refluxing at 80 °C for around 48 h. Thin-layer chromatography (TLC) was used to track the reaction’s progression to completion. The excess acetonitrile and volatile impurities were removed by using a rotary evaporator. To remove unreacted reactants, the obtained reaction mixture was rinsed with hexane. The obtained product was kept in vacuum desiccators for a few days before use [32,33,34]. The 1H-NMR technique has been utilized for its characterization. Figure S1 (SI) shows the obtained 1H-NMR spectra of synthesized [C14mim][Br].
2.1.2 Synthesis of [Mor1,15]Br
For the synthesis of [Mor1,15][Br], 1-bromopentadecane and N-methylmorpholine in a 1:1.2 ratio have been taken in the round bottom flask. The toluene was added to the reaction mixture as solvent. Then the reaction mixture was kept on continuous stirring and refluxing at approximately 80–90 °C for 48 h under a nitrogen atmosphere. The reaction progress has been monitored by TLC. After the reaction completion, the rotary evaporator was used to evaporate the excess solvent present in the reaction mixture. The resulting product was washed with ethyl acetate. Then the product was placed in desiccators for a few days before use [35, 36]. 1H-NMR spectra obtained for the [Mor1,15][Br], are shown in Fig. S2 (SI). The synthetic pathways for the [C14mim][Br] and [Mor1,15][Br] synthesis have been indicated in Fig. 1.
2.2 Sample Preparation
The stock solutions of TTAB, [C14mim][Br], and [Mor1,15][Br] have been prepared in the double-distilled water. Double distilled water with the conductivity value ≤ 5 µS·cm−1 was obtained using a specialized water purification system i.e., Millipore Milli-Q Academic water purification system. The stock solutions with concentrations (0.00, 0.03, 0.06, and 0.10) wt% of [Mor1,15]Br have been prepared. These concentrations of [Mor1,15]Br molecules were chosen so that there may not be the self-assembly of [Mor1,15]Br molecules in the solution. The accurate weighing of compounds has been carried out using Sartorius CPA 225 D electronic balance (precision ±0.00001 g). The analysis of obtained data has been conducted using Origin 2018 and MS Excel.
2.3 Methods used
2.3.1 Conductivity Measurements
The specific conductivity of several samples has been measured with a digital conductivity meter (Systronics 306) by using a platinum electrode dipping cell (cell constant = 1.0 cm−1). Aqueous potassium chloride (KCl) solutions in the concentration range (0.01–1.0 mol·kg−1) were used for the instrument’s calibration. By multiplying the cell constant by the measured conductivity values, the specific conductivity (κ) values were obtained. Since the cell constant = 1.0 cm−1, the calculated and measured values of specific conductivity (κ) coincide perfectly. The refrigerated circulated water thermostat (accuracy ± 0.1 °C) from Macro Scientific Works Pvt. Ltd. Delhi has been used to sustain the solution temperature. To do this, double walled jacketed cells containing sample solutions are used through which water kept at a certain temperature was circulated. This allows measuring the electrical conductivity of solutions at that particular temperature. To ensure accuracy, all the measurements were carried out in triplicate.
2.3.2 Refractive Index Measurements
Anton Paar’s Abbemat 300 refractometer (resolution 0.00001 nD) is used to measure the refractive index values. Before performing actual measurements, the equipment is calibrated by measuring the refractive index value of water which is 1.3325 nD at 298.15 K. Internally maintaining the temperature with a temperature probe precision of ± 0.05 °C, the measurements were made at four distinct temperatures (293.15, 298.15, 303.15, 308.15) K. Each measurement is repeated at least three times, with the mean result treated as the final reading (accuracy ± 0.0001 nD).
2.3.3 Surface Tension Measurements
DCAT 15 tensiometer from DataPhysics Instruments, Germany, was used to measure the values of surface tension at 298.15 K. The DCAT 33 software, combined with the LDU 25 dosing equipment and Wilhelmy plate PT 11, is specifically intended for automatic CMC determination. The temperature is sustained at 298.15 K using a liquid temperature control device TV 70 and a refrigerated circulating water thermostat. The cleaning of plate PT 11 was done by red-burning it with butane gas. Before taking actual measurements, the instrument is calibrated with water, which has a surface tension of 71.99 mN·m−1 at 298.15 K. Each measurement is made in triplicate, with the average value being used (accuracy ± 0.01 mN·m−1).
3 Results and Discussion
3.1 Conductivity Measurements
The conductivity data obtained has been utilized to obtain the CMC values and thermodynamic parameters for the aggregation of TTAB and [C14mim]Br with (0.00, 0.03, 0.06, and 0.10) wt% [Mor1,15]Br at various temperatures. The data has been analyzed to understand the influence of [Mor1,15]Br at various concentrations and temperatures on the aggregation of TTAB and [C14mim]Br.
3.1.1 Critical Micelle Concentration (CMC)
The obtained specific conductivity values for the aqueous solutions of TTAB and [C14mim]Br with (0.00, 0.03, 0.06, and 0.10) wt% [Mor1,15]Br at various temperatures are utilized to determine the CMC values. The obtained values of specific conductivity for various solutions at (293.15, 298.15 303.15, and 308.15) K have been reported in Table S1–3 shown in supporting information. The CMC values have been obtained by plotting the specific conductivity versus the concentration for various solutions, which have been shown in Figs. 2 and 3. It can be detected from the obtained plots, that specific conductivity values increase with the concentration of TTAB and [C14mim]Br in the solution. When the concentration approaches the specific concentration i.e., CMC, the micelle formation takes place. Then, the rate of change in conductivity decreases with the increasing concentration of TTAB and [C14mim]Br with (0.00, 0.03, 0.06, and 0.10) wt% [Mor1,15]Br, resulting in a lower slope for the line in the post-micellar region than in the pre-micellar region. The slope of the pre-micellar region (\({S}_{1}\)) and the post-micellar region (\({S}_{2}\)) have been employed to determine the degree of counterion dissociation (α) by using Eq. 1.
The variation in the slopes is due to the fact that the charge carriers become fewer on micelle formation [22, 37]. The loss of charge carriers after micelle formation is because the counterions get attached to the micellar surface. Also, the formed micelles exhibit lower mobility as compared to free surfactant monomers, further slowing the charge transport. Thus, a breakpoint is observed in conductivity versus concentration plots [38, 39].
The concentrations of surface-active molecules corresponding to the breakpoint at different temperatures give the values of CMC for TTAB and [C14mim]Br with (0.00, 0.03, 0.06, and 0.10) wt% [Mor1,15]Br, as indicated in Table 2. The addition of [Mor1,15]Br at various concentrations and temperatures has been shown to significantly modulate the micellization behavior of TTAB and [C14mim]Br in aqueous media.
3.1.1.1 Effect of Adding [Mor1,15]Br at Different Concentrations
The micelle formation tendency of TTAB and [C14mim]Br enhances with the concentration of [Mor1,15]Br in solution at all investigated temperatures. This is due to the existence of favorable interactions among the monomers of TTAB and [C14mim]Br when [Mor1,15]Br is added to the solution. The strong hydrophobic interactions occur between the hydrophobic chains of TTAB, which lead to the formation of micellar aggregates in the solution. The existence of strong electrostatic attraction among the trimethylammonium (–N+(CH3)3) head groups of TTAB and Br− counterions facilitates micelle formation by masking the electrostatic repulsive interactions among the similarly charged cationic head groups of TTAB [40]. The addition of [Mor1,15]Br in the solution of TTAB facilitates micelle formation as the long alkyl chains of [Mor1,15]Br starts interacting with the alkyl chains of TTAB, thereby reducing the CMC values of TTAB with the increase in concentrations of [Mor1,15]Br in solution at all temperatures [41]. Similarly, the CMC values of [C14mim]Br in aqueous media also decrease with the addition of [Mor1,15]Br in the solution. The existence of electrostatic repulsive interactions among similarly charged imidazolium cation-based head groups of [C14mim]Br gets compensated with the existence of counterions. The hydrophobic interactions occurring among the alkyl chains of [C14mim]Br and [Mor1,15]Br and hydrogen bonding interactions prevailing between the head groups of [C14mim]Br and [Mor1,15]Br over the micellar surface contribute to enhancing the micellization tendency of [C14mim]Br with [Mor1,15]Br as an additive at various concentrations in solution [42]. The increase in concentrations of [Mor1,15]Br leads to enhance the micellization tendency of TTAB and [C14mim]Br in aqueous media as evident from the obtained CMC values at all investigated temperatures.
3.1.1.2 Effect of Varying Temperature on Micellization
The temperature has been shown to influence the micellization tendency of TTAB and [C14mim]Br in a significant way. As observed in Table 2, the CMC values of TTAB decrease with an increase in temperature as the temperature is raised from 293.15 to 303.15 K. After 303.15 K, the CMC value for TTAB starts increasing as the temperature is increased to 308.15 K. Similar trend in the CMC variation of TTAB in (0.00, 0.03, 0.06 and 0.10) wt% of [Mor1,15]Br in solution with the temperature change has been observed. Thus, the CMC values of TTAB represent a U-type behavior in the range of 293.15 to 308.15 K. On the other side, the values of CMC for [C14mim]Br increases with temperature increase in the range of 293.15 to 308.15 K as observed in the presence of (0.00, 0.03, 0.06, and 0.10) wt% of [Mor1,15]Br in solution. The observed behavior in the micelle forming tendency of investigated systems with the temperature variation can be explained based on two opposing effects. (i) As the temperature rises, there may be a decrease in the hydration of hydrophilic head groups present in surface-active monomers. This results in the enhancement of micellization tendency, which results in the CMC value decrease. (ii) However, as the temperature rises, the structured water surrounding the hydrophobic group is also disrupted, making micellization more difficult. This is due to the solubilization of surface-active molecules in water and the value of CMC increases. Thus, in the case of a particular surface-active agent, the CMC variation with temperature depends upon the dominance of any one of the effects over the other [43, 44]. In the case of TTAB, the first effect dominates in the range 293.15–303.15 K, whereas the second effect dominates above 303.15 K. Thus, the CMC values of TTAB with [Mor1,15]Br in solution initially decrease from 293.15 to 303.15 K, and then start increasing as the temperature rises above 303.15 K as indicated in Fig. 4a. In the case of [C14mim]Br with [Mor1,15]Br in solution, the second effect dominates in the entire investigated temperature range i.e., from 293.15 to 308.15 K, leading to retard the micelle formation of [C14mim]Br with temperature elevation in (0.00, 0.03, 0.06 and 0.10) wt% [Mor1,15]Br in solution. Thus, the CMC values of [C14mim]Br with [Mor1,15]Br in solution increase with the increase in temperature as indicated in Fig. 4b.
3.1.2 Thermodynamic Parameters of Micellization: Standard Free Energy of Micellization ( \(\Delta G_{m}^{0}\) ), Standard Enthalpy of Micellization ( \(\Delta H_{m}^{0}\) ), Standard Entropy of Micellization ( \(\Delta S_{m}^{0}\) )
The obtained values of CMC and α have been further employed to calculate different thermodynamic parameters of micellization i.e., standard free energy of micellization (\({\Delta }{G}_{m}^{0}\)), standard enthalpy of micellization (\({{\Delta }H}_{m}^{0}\)), and standard entropy of micellization (\({\Delta }{S}_{m}^{0}\)) [45, 46].
The standard free energy of micellization (\({\Delta }{G}_{m}^{0}\)) can be calculated as
The standard enthalpy of micellization (\({{\Delta }H}_{m}^{0}\)) can be calculated as
The standard entropy of micellization (\({\Delta }{S}_{m}^{0}\)) can be calculated as
where R is the universal gas constant, T represents temperature, \({X}_{\text{CMC}}\) denotes the CMC in the mole fraction units. The determined values for \({\Delta }{G}_{m}^{0}\), \({{\Delta }H}_{m}^{0}\) and \({\Delta }{S}_{m}^{0}\)at different temperature for the micellization of TTAB and [C14mim]Br in (0.00, 0.03, 0.06, and 0.10) wt% [Mor1,15] are reported in Table 2. Figures S3–5 given in supporting information indicate the change in the values of these parameters with the variation in temperature and concentrations of [Mor1,15]Br in solution. The negative \({\Delta }{G}_{m}^{0}\) values obtained for the micellization of TTAB and [C14mim]Br at all investigated temperatures and concentrations of [Mor1,15]Br indicate the spontaneous micelle formation. The more negative values of \({\Delta }{G}_{m}^{0}\) at higher concentrations of [Mor1,15]Br have been obtained indicating greater spontaneity and feasibility in the micellization of TTAB and [C14mim]Br with the addition of [Mor1,15]Br in solution. The monomers of [Mor1,15]Br interacts with the monomers of TTAB and [C14mim]Br in such a way that leads to enhance their micellization tendency. An analogous trend has been obtained for the \({{\Delta }H}_{m}^{0}\) values, where the obtained negative values \({{\Delta }H}_{m}^{0}\) specify the exothermic nature for the micellization of TTAB and [C14mim]Br with and without [Mor1,15]Br in solution. The\({{\Delta }S}_{m}^{0}\) values have been obtained positive in all the cases, whose values decrease with the rise in temperature for both TTAB and [C14mim]Br at various [Mor1,15]Br concentrations. The values of \({{\Delta }S}_{m}^{0}\) have been observed to increase for TTAB, whereas these values decrease for [C14mim]Br with the increase in [Mor1,15]Br concentration. The obtained negative values of \({{\Delta }H}_{m}^{0}\) and positive values of \({{\Delta }S}_{m}^{0}\) contribute to the negative values obtained for \({{\Delta }G}_{m}^{0}\) in case of TTAB and [C14mim]Br in the absence and presence of [Mor1,15]Br in solution. The higher contribution of \({{\Delta }S}_{m}^{0}\) as compared to \({{\Delta }H}_{m}^{0}\) towards the obtained negative values of \({{\Delta }G}_{m}^{0}\) has been observed in all the cases. This is because − T\({{\Delta }S}_{m}^{0}\) has been obtained higher as compared to \({{\Delta }H}_{m}^{0}\), which signifies the entropy-driven process of micellization. It means that the structured water clusters around the hydrophobic tails of TTAB and [C14mim]Br monomers in solution get distorted upon micelle formation, due to the “hiding” of non-polar hydrocarbon alkyl chains inside the micellar core. This leads to the release of water molecules which results in more positive \({{\Delta }S}_{m}^{0}\) values for the micellization of TTAB and [C14mim]Br with and without [Mor1,15]Br in solution.
3.2 Refractive Index Measurements
The refractive index is the quantity that measures how a light beam bends as it goes through one medium to the other. The measurement of refractive index at different concentrations of surface-active molecules can also be used to calculate their CMC values. This is owing to the fact that the refractive index of monomers is lower than that of produced micelles. Thus, the refractive index values for TTAB and [C14mim]Br in (0.00, 0.03, 0.06, and 0.10) wt% [Mor1,15]Br have been measured at different temperatures as reported in Tables S4–6 given in supporting information.
To obtain the CMC values, the refractive index values have been used, which were plotted against the concentration of TTAB and [C14mim]Br in (0.00, 0.03, 0.06, and 0.10) wt% [Mor1,15]Br as indicated in Figs. 5 and 6, respectively.
Figures show that the refractive index values increase rapidly with the concentration of TTAB and [C14mim]Br in (0.00, 0.03, 0.06, and 0.10) wt% [Mor1,15]Br, when the concentration is below CMC value. But as the concentration approaches CMC, the rate of increase of refractive index with the increase in concentration decreases [47,48,49]. As a result, the breakpoint appears in the refractive index versus concentration plots of TTAB and [C14mim]Br with and without [Mor1,15]Br. The values of CMC for the investigated systems have been obtained at concentration consistent with the breakpoint. Table 3 displays the comparison between the CMC values acquired using this method and those obtained by conductivity measurements. The measurements of CMC values using the refractive index measurement technique correlate well with conductivity-based estimates.
3.3 Surface Tension Measurements
The surface tension measurement technique is another convenient approach that aids in examining the aggregation behavior of surface-active molecules and also provides crucial details regarding the adsorption process occurring at the interface. Thus, the self-assembly, aggregation and interfacial behavior along with the probable interactions which occur among various components of the solution have been investigated using the surface tension method [14, 50]. The surface tension (γ) values of the aqueous TTAB and [C14mim]Br in the [Mor1,15]Br solutions were measured to determine their surface activity and CMC values. The surface tension (γ) values for TTAB and [C14mim]Br in (0.00, 0.03, 0.06, and 0.10) wt% [Mor1,15]Br have been reported in Tables S7 and S8, respectively, given in supporting information. Figure 7 represents the surface tension versus the logarithm of TTAB and [C14mim]Br concentration plots with and without the addition of various concentrations of [Mor1,15]Br in solution. It is evident from the figure that, at higher concentrations of surface-active molecules, the surface tension gradually decreases as the concentration rises; this suggests that surface-active molecules are adsorbed at the air/solution interface. As the concentration of surface-active molecules approaches the CMC value, the γ values become approximately constant yielding a breakpoint in the plot. Thus, the CMC value is obtained at the concentration coinciding with the breakpoint [51, 52]. The obtained data for surface tension has been further employed to determine various surface-active parameters for the concerned systems as indicated in Table 4.
The surface pressure at CMC \(\left( {\Pi _{\text{CMC}} } \right)\) has been calculated as follows.
where \({{\gamma }}_{0}\) represents surface tension of solvent in pure state and \({{\gamma }}_{\text{CMC}}\) represents the surface tension of solution at CMC. The maximum surface excess concentration (\({{\Gamma }}_{\text{max}}\)) and minimum area of surfactant monomer at the air/solution interface (\(A_{{\min }}\)) have been evaluated by applying the following equations.
where \(T\) is the temperature, \(R\) is the universal gas constant, \(C\) is the concentration of the solution, \({{N}}_{\text{A}}\) represents the Avogadro’s Number i.e., 6.022 × 1023 mol−1.
The standard Gibbs free energy of adsorption (\({\Delta }{G}_{\text{ad}}^{0}\)) and the free energy of a surface at equilibrium (\(G_{{\min }}^{s}\)), proposed by Sugihara et al. have been calculated as follows.
where \({\Delta }{G}_{m}^{0}\) is the standard Gibbs free energy of micellization as determined by using Eq. 2.
The value of the packing parameter (p) has also been evaluated as follows.
where \({V}_{0}\) indicates the volume occupied by the hydrophobic group in the micellar core, \({l}_{c}\) represents the length of the hydrophobic core. The values of \({V}_{0}\) and \({l}_{c}\) can be determined by using Tanford’s formulae.
Here, \({n}_{c}\) represents the total number of carbons in the hydrocarbon chain of the surface active agent.
The careful analysis of Table 4 indicates the proximity in the CMC values obtained by different techniques. The addition of [Mor1,15]Br at various concentrations in the solutions of TTAB and [C14mim]Br results in a decrease in values of \({{\upgamma }}_{\text{CMC}}\), indicating a rise in surface activity of the solution. The greater surface-activity of [C14mim]Br has been detected than TTAB as specified by the obtained lesser values of \({{\upgamma }}_{\text{CMC}}\) in case of [C14mim]Br as compared to TTAB in (0.00, 0.03, 0.06 and 0.10) wt% [Mor1,15]Br. The values of \(\Gamma _{{\max }}\) and \(A_{{\min }}\) provides the knowledge of orientation and packing of surface-active monomers at the air/solution interface [53, 54]. The addition of [Mor1,15]Br at higher concentrations in the solution of TTAB and [C14mim]Br results in the decrease in values of \({{\Gamma }}_{\text{max}}\) and the increase in the values of \(A_{{\min }}\). This trend in the values of \(\Gamma _{{\max }}\) and \(A_{{\min }}\) indicate loose packing of TTAB and [C14mim]Br monomers at air/solution interface with the rise in [Mor1,15]Br concentration. Also, the lesser value of \(\Gamma _{{\max }}\) for [C14mim]Br as compared to TTAB with and without [Mor1,15]Br indicates loose packing of [C14mim]Br monomers at the interface as compared to that of TTAB. The values of \(\Gamma _{{\max }}\) and \(A_{{\min }}\) primarily depend on (a) the attractive or van der Waals interactions that exist between the hydrophobic parts of the [Mor1,15]Br with TTAB and [C14mim]Br in TTAB/[Mor1,15]Br and [C14mim]Br/[Mor1,15]Br system, respectively and (b) the steric hindrance brought on by the bulky groups present in various interacting species. The hydrophobic part of [Mor1,15]Br monomers can be used to explain the observed decrease in \(\Gamma _{{\max }}\) as the concentration of [Mor1,15]Br increases in the solution. The hydrophobic alkyl chains of [Mor1,15]Br avoid making contact with polar water molecules, thus, tend to concentrate in substantial amounts at the interface, and obstruct the adsorption in TTAB/[Mor1,15]Br and [C14mim]Br/[Mor1,15]Br system, respectively. Thus, the loose packing of TTAB and [C14mim]Br in respective TTAB/[Mor1,15]Br and [C14mim]Br/[Mor1,15]Br systems has been observed at the interface as evident from the obtained values of \(\Gamma _{{\max }}\) and \(A_{{\min }}\). The negative values of \({\Delta }{G}_{\text{ad}}^{0}\) have been obtained for pure TTAB, pure [C14mim]Br, TTAB/[Mor1,15]Br mixtures and [C14mim]Br/[Mor1,15]Br mixtures. On comparing \({\Delta }{G}_{\text{ad}}^{0}\) and \({\Delta }{G}_{m}^{0}\), it is observed that the former has a higher negative value than the latter, indicating that the adsorption process is preferable to the micellization process [51]. Thus, adsorption is the primary process, whereas micellization is the secondary process as some work required the transportation of surfactant monomers from their monomeric form at the surface to their micellar form in the aqueous solution. The \({\Delta }{G}_{\text{ad}}\) values have been observed to attain more negative values in the case of TTAB/[Mor1,15]Br mixtures and [C14mim]Br/[Mor1,15]Br mixtures as compared to pure TTAB and pure [C14mim]Br solutions. This indicates the greater spontaneity of monomer adsorption for TTAB and [C14mim]Br monomers in TTAB/[Mor1,15]Br and [C14mim]Br/[Mor1,15]Br mixtures, respectively. The obtained positive values of \(G_{{\min }}^{s}\) in the investigated systems indicates the formation of thermodynamically stable surfaces and micelles in solutions of TTAB/[Mor1,15]Br and [C14mim]Br/[Mor1,15]Br. The values of the packing parameter (p) indicate the formed micelles in the concerned systems to be spherically shaped as the value of p lies between 0 and 0.33 [55].
4 Conclusion
The aggregation behavior of TTAB and [C14mim]Br in (0.00, 0.03, 0.06, and 0.10) wt% [Mor1,15]Br has been analyzed to get insight into the interactions prevailing in TTAB/[Mor1,15]Br and [C14mim]Br/[Mor1,15]Br mixtures. Conductivity, refractive index, and surface tension measurement techniques have been used to evaluate various parameters such as critical micelle concentration (CMC), the standard free energy of micellization (\({\Delta }{G}_{m}^{0}\)), standard enthalpy of micellization (\({{\Delta }H}_{m}^{0}\)), and standard entropy of micellization (\({\Delta }{S}_{m}^{0}\)), surface pressure at cmc \(\left( {\Pi _{\text{CMC}} } \right)\), maximum surface excess concentration (\(\Gamma _{{\max }}\)), the minimum area of surfactant monomer at the air/solution interface (\(A_{{\min }}\)), standard Gibbs free energy of adsorption (\({\Delta }{G}_{\text{ad}}^{0}\)), the free energy of a surface at equilibrium (\(G_{{\min }}^{s}\)) and packing parameter (p). The values of CMC and other evaluated parameters for the investigated systems get altered with the temperature and the concentration of [Mor1,15]Br in the solution. The values of all the parameters obtained for TTAB/[Mor1,15]Br and [C14mim]Br/[Mor1,15]Br mixtures have been compared to analyze the aggregation behavior of TTAB and [C14mim]Br with [Mor1,15]Br at various concentrations in aqueous media. The superior surface activity has been observed for [C14mim]Br as compared to TTAB with and without [Mor1,15]Br in solution at various concentrations and temperatures.
References
Huang, W., Wu, X., Qi, J., Zhu, Q., Wu, W., Lu, Y., Chen, Z.: Ionic liquids: green and tailor-made solvents in drug delivery. Drug Discov. Today. 25, 901–908 (2020). https://doi.org/10.1016/J.DRUDIS.2019.09.018
Dutta, R., Kundu, S., Sarkar, N.: Ionic liquid-induced aggregate formation and their applications. Biophys. Rev. 10, 861–871 (2018). https://doi.org/10.1007/s12551-018-0408-5
Toledo Hijo, A.A.C., Maximo, G.J., Costa, M.C., Batista, E.A.C., Meirelles, A.J.A.: Applications of ionic liquids in the food and bioproducts industries. ACS Sustain. Chem. Eng. 4, 5347–5369 (2016). https://doi.org/10.1021/acssuschemeng.6b00560
Wasilewski, T.: Ionic liquids in gas sensors and biosensors. In: Green Sustainable Process for Chemical and Environmental Engineering and Science: Ionic Liquids as Green Solvents, pp. 287–318. Elsevier Inc, Amsterdam (2019)
Mahura, T., Bahadur, I., Naidoo, P., Ramjugernath, D.: Probing the ion–dipole interactions between the imidazolium-based ionic liquids and polyethylene glycol 200 using excess thermodynamic and spectroscopy studies. J. Mol. Liq. 350, 118519 (2022). https://doi.org/10.1016/j.molliq.2022.118519
Masilo, K., Singh, S.K., Bahadur, I.: Interactions between 1-butyl-3-methylimidazolium cation with various anions and carboxylic acids: physicochemical and spectroscopic aspects. Colloids Surf. Physicochem. Eng. Asp. 617, 126376 (2021). https://doi.org/10.1016/j.colsurfa.2021.126376
Brennecke, J.F., Maginn, E.J.: Ionic liquids: innovative fluids for chemical processing. AIChE J. 47, 2384–2389 (2001). https://doi.org/10.1002/aic.690471102
Chiappe, C., Pieraccini, D.: Ionic liquids: solvent properties and organic reactivity. J. Phys. Org. Chem. 18, 275–297 (2005). https://doi.org/10.1002/poc.863
Giernoth, R.: Task-specific ionic liquids. Angew. Chem. Int. Ed. 49, 2834–2839 (2010). https://doi.org/10.1002/anie.200905981
Rogers, R.D., Seddon, K.R.: Ionic liquids–solvents of the future? Science 80(302), 792–793 (2003). https://doi.org/10.1126/science.1090313
Garcia, M.T., Ribosa, I., González, J.J., Comelles, F.: Surface activity, self-aggregation and antimicrobial activity of catanionic mixtures of surface active imidazolium- or pyridinium-based ionic liquids and sodium bis(2-ethylhexyl) sulfosuccionate. J. Mol. Liq. 303, 112637 (2020). https://doi.org/10.1016/j.molliq.2020.112637
Kumar, H., Kaur, G.: Scrutinizing self-assembly, surface activity and aggregation behavior of mixtures of imidazolium based ionic liquids and surfactants: a comprehensive review. Front. Chem. (2021). https://doi.org/10.3389/fchem.2021.667941
El Seoud, O.A., Keppeler, N., Malek, N.I., Galgano, P.D.: Ionic liquid-based surfactants: recent advances in their syntheses, solution properties, and applications. Polymers (Basel) 13, 1100 (2021). https://doi.org/10.3390/polym13071100
Pal, A., Saini, M.: Thermodynamic and micellization behavior of long chain 1-octyl-2,3-dimethylimidazolium bromide [odmim][Br] in aqueous solution in the absence and in presence of a series of alkali salts. J. Dispers. Sci. Technol. 40, 1197–1204 (2019). https://doi.org/10.1080/01932691.2018.1503546
Srinivasa Rao, K., Gehlot, P.S., Trivedi, T.J., Kumar, A.: Self-assembly of new surface active ionic liquids based on Aerosol-OT in aqueous media. J. Colloid Interface Sci. 428, 267–275 (2014). https://doi.org/10.1016/j.jcis.2014.04.062
Dong, B., Li, N., Zheng, L., Yu, L.: Surface adsorption and micelle formation of surface active ionic liquids in aqueous solution. Langmuir. 23, 4178–4182 (2007). https://doi.org/10.1016/j.jcis.2012.09.073
Chauhan, S., Kaur, M., Singh, K., Chauhan, M.S., Kohli, P.: Micellar and antimicrobial activities of ionic surfactants in aqueous solutions of synthesized tetraalkylammonium based ionic liquids. Colloids Surf. Physicochem. Eng. Asp. 535, 232–241 (2017). https://doi.org/10.1016/j.colsurfa.2017.09.042
Bera, A., Agarwal, J., Shah, M., Shah, S., Vij, R.K.: Recent advances in ionic liquids as alternative to surfactants/chemicals for application in upstream oil industry. J. Ind. Eng. Chem. 82, 17 (2020)
Mqoni, N., Singh, S., Bahadur, I., Hashemi, H., Ramjugernath, D.: Ionic liquids, the mixture of ionic liquids and their co-solvent with N,N-dimethylformamide as solvents for cellulose using experimental and COSMO study. Results Eng. 15, 100484 (2022). https://doi.org/10.1016/j.rineng.2022.100484
Negadi, L., Bahadur, I., Masilo, K., Negadi, A.: Excess molar volumes of (2-butoxyethanol or 1-methoxy-2-propanol + benzene or cyclohexane) at (283.15-343.15) K. Thermochim. Acta 686, 178539 (2020). https://doi.org/10.1016/j.tca.2020.178539
Govinda, V., Reddy, P.M., Bahadur, I., Attri, P., Venkatesu, P., Venkateswarlu, P.: Effect of anion variation on the thermophysical properties of triethylammonium based protic ionic liquids with polar solvent. Thermochim. Acta. 556, 75–88 (2013). https://doi.org/10.1016/j.tca.2013.02.002
Ali, A., Farooq, U., Uzair, S., Patel, R.: Conductometric and tensiometric studies on the mixed micellar systems of surface-active ionic liquid and cationic surfactants in aqueous medium. J. Mol. Liq. 223, 589–602 (2016). https://doi.org/10.1016/j.molliq.2016.08.082
Pal, A., Yadav, S.: Effect of a copolymer poly(4-styrenesufonic acid-co-maleic acid) sodium salt on aggregation behaviour of imidazolium based surface active ionic liquid in aqueous solution. J. Mol. Liq. 246, 342–349 (2017). https://doi.org/10.1016/j.molliq.2017.09.081
Cheng, N., Ma, X., Sheng, X., Wang, T., Wang, R., Jiao, J., Yu, L.: Aggregation behavior of anionic surface active ionic liquids with double hydrocarbon chains in aqueous solution: experimental and theoretical investigations. Colloids Surf. Physicochem. Eng. Asp. 453, 53–61 (2014). https://doi.org/10.1016/j.colsurfa.2014.03.083
Kim, M.J., Shin, S.H., Kim, Y.J., Cheong, M., Lee, J.S., Kim, H.S.: Role of Alkyl group in the aromatic extraction using pyridinium-based ionic liquids. J. Phys. Chem. B 117, 14827–14834 (2013). https://doi.org/10.1021/jp409117j
Kamboj, R., Bharmoria, P., Chauhan, V., Singh, G., Kumar, A., Singh, S., Kang, T.S.: Effect of cationic head group on micellization behavior of new amide-functionalized surface active ionic liquids. Phys. Chem. Chem. Phys. 16, 26040–26050 (2014). https://doi.org/10.1039/c4cp04054f
Jain, M., Marfatia, A., Imam, N., Ray, D., Aswal, V.K., Patel, N.Y., Raval, V.H., Kailasa, S.K., Malek, N.I.: Ionic liquid-based catanionic vesicles: a de novo system to judiciously improve the solubility, stability and antimicrobial activity of curcumin. J. Mol. Liq. 341, 117396 (2021). https://doi.org/10.1016/j.molliq.2021.117396
Kaur, R., Kumar, H., Singla, M.: A thermodynamic investigation of the effect of cationic structure on the self-aggregation behavior of surface-active ionic liquids in the presence of an amino acid. J. Mol. Liq. 354, 118904 (2022). https://doi.org/10.1016/j.molliq.2022.118904
Kumar, H., Kaur, R.: Exploration of the soluting-out effect of carbohydrates on the micellization and surface activity of long-chain imidazolium ionic liquid in the aqueous medium. J. Mol. Liq. 319, 114209 (2020). https://doi.org/10.1016/J.MOLLIQ.2020.114209
Kaur, G., Kumar, H., Singla, M.: Mixed micellization behavior of 1-dodecyl-3-methylimidazolium chloride [C12mim][Cl] and benzyldimethyl-n-hexadecylammonium chloride (16-BAC) under the influence of gelatin in aqueous media. J. Mol. Liq. (2022). https://doi.org/10.1016/J.MOLLIQ.2022.118479
Chadha, C., Singh, G., Singh, G., Kumar, H., Kang, T.S.: Modulating the mixed micellization of CTAB and an ionic liquid 1-hexadecyl-3-methylimidazollium bromide via varying physical states of ionic liquid. RSC Adv. 6, 38238–38251 (2016). https://doi.org/10.1039/C6RA05330K
Kumar, H., Kaur, G., Sharma, S.: Investigation of surface adsorption and thermodynamic properties of 1-tetradecyl-3-methylimidazolium bromide in the absence and presence of tetrabutylammonium bromide in aqueous medium. J. Dispers. Sci. Technol. (2020). https://doi.org/10.1080/01932691.2020.1844743
Sharma, R., Mahajan, S., Mahajan, R.K.: Surface adsorption and mixed micelle formation of surface active ionic liquid in cationic surfactants: conductivity, surface tension, fluorescence and NMR studies. Colloids Surf. Physicochem. Eng. Asp. 427, 62–75 (2013). https://doi.org/10.1016/j.colsurfa.2013.03.023
Mahajan, S., Sharma, R., Mahajan, R.K.: An investigation of drug binding ability of a surface active ionic liquid: micellization, electrochemical, and spectroscopic studies. Langmuir 28, 17238–17246 (2012). https://doi.org/10.1021/la303193n
Choi, S., Kim, K.S., Lee, H., Oh, J.S., Lee, B.B.: Synthesis and ionic conductivities of lithium-doped morpholinium salts. Korean J. Chem. Eng. 22, 281–284 (2005). https://doi.org/10.1007/BF02701498
Choi, S., Kim, K., Cha, J., Lee, H., Oh, J.S., Lee, B.: Thermal and electrochemical properties of ionic liquids based on N-methyl-N-alkyl morpholinium cations. Korean J. Chem. Eng. 23, 795–799 (2006)
Rub, M.A., Azum, N., Khan, S.B., Marwani, H.M., Asiri, A.M.: Micellization behavior of amphiphilic drug promazine hydrochloride and sodium dodecyl sulfate mixtures at various temperatures: effect of electrolyte and urea. J. Mol. Liq. 212, 532–543 (2015). https://doi.org/10.1016/j.molliq.2015.09.049
Azum, N., Ahmed, A., Rub, M.A., Asiri, A.M., Alamery, S.F.: Investigation of aggregation behavior of ibuprofen sodium drug under the influence of gelatin protein and salt. J. Mol. Liq. 290, 111187 (2019). https://doi.org/10.1016/j.molliq.2019.111187
Ruiz, C.C.: Thermodynamics of micellization of tetradecyltrimethylammonium bromide in ethylene glycol-water binary mixtures. Colloid Polym. Sci. 277, 701–707 (1999). https://doi.org/10.1007/s003960050443
Shi, L., Li, N., Yan, H., Gao, Y.A., Zheng, L.: Aggregation behavior of long-chain n-aryl imidazolium bromide in aqueous solution. Langmuir 27, 1618–1625 (2011). https://doi.org/10.1021/la104719v
Qin, L., Wang, X.H.: Surface adsorption and thermodynamic properties of mixed system of ionic liquid surfactants with cetyltrimethyl ammonium bromide. RSC Adv. 7, 51426–51435 (2017). https://doi.org/10.1039/c7ra08915e
Pal, A., Pillania, A.: Modulations in surface and aggregation properties of non-ionic surfactant triton X-45 on addition of ionic liquids in aqueous media. J. Mol. Liq. 233, 243–250 (2017). https://doi.org/10.1016/j.molliq.2017.03.037
Mahmood, M.E., Al-koofee, D.: Effect of temperature changes on critical micelle concentration for tween series surfactant. Glob. J. Sci. Front. Res. Chem. 13, 1–7 (2013)
Kang, K.H., Kim, H.U., Lim, K.H.: Effect of temperature on critical micelle concentration and thermodynamic potentials of micellization of anionic ammonium dodecyl sulfate and cationic octadecyl trimethyl ammonium chloride. Colloids Surf. Physicochem. Eng. Asp. 189, 113–121 (2001). https://doi.org/10.1016/S0927-7757(01)00577-5
Alam, M.S., Siddiq, A.M., Mythili, V., Priyadharshini, M., Kamely, N., Mandal, A.B.: Effect of organic additives and temperature on the micellization of cationic surfactant cetyltrimethylammonium chloride: evaluation of thermodynamics. J. Mol. Liq. 199, 511–517 (2014). https://doi.org/10.1016/j.molliq.2014.09.026
Khan, A.B., Ali, M., Malik, N.A., Ali, A., Patel, R.: Role of 1-methyl-3-octylimidazolium chloride in the micellization behavior of amphiphilic drug amitriptyline hydrochloride. Colloids Surf. B Biointerfaces. 112, 460–465 (2013). https://doi.org/10.1016/j.colsurfb.2013.08.018
Kumar, H., Kaur, G.: Priya: Influence of tetra ethyl ammonium bromide (C2H5)4NBr on the aggregation behavior of surface active ionic liquid 1-tetradecyl-3-methylimidazolium bromide [C14mim][Br]. J. Mol. Liq. (2020). https://doi.org/10.1016/j.molliq.2020.113431
Singh, T., Kumar, A.: Aggregation behavior of ionic liquids in aqueous solutions: effect of alkyl chain length, cations, and anions. J. Phys. Chem. B 111, 7843–7851 (2007). https://doi.org/10.1021/jp0726889
Tan, C.H., Huang, Z.J., Huang, X.G.: Rapid determination of surfactant critical micelle concentration in aqueous solutions using fiber-optic refractive index sensing. Anal. Biochem. 401, 144–147 (2010). https://doi.org/10.1016/j.ab.2010.02.021
Łuczak, J., Latowska, A., Hupka, J.: Micelle formation of tween 20 nonionic surfactant in imidazolium ionic liquids. Colloids Surf. Physicochem. Eng. Asp. 471, 26–37 (2015). https://doi.org/10.1016/j.colsurfa.2015.02.008
Shah, M.U.H., Sivapragasam, M., Moniruzzaman, M., Talukder, M.M.R., Yusup, S.B., Goto, M.: Aggregation behavior and antimicrobial activity of a micellar system of binary ionic liquids. J. Mol. Liq. 266, 568–576 (2018). https://doi.org/10.1016/j.molliq.2018.06.101
Zhang, S., Gao, Y., Dong, B., Zheng, L.: Interaction between the added long-chain ionic liquid 1-dodecyl-3-methylimidazolium tetrafluoroborate and Triton X-100 in aqueous solutions. Colloids Surf. Physicochem. Eng. Asp. 372, 182–189 (2010). https://doi.org/10.1016/j.colsurfa.2010.10.011
Kumar, D., Rub, M.A., Azum, N., Asiri, A.M.: Mixed micellization study of ibuprofen (sodium salt) and cationic surfactant (conventional as well as gemini). J. Phys. Org. Chem. 31, 1–12 (2018). https://doi.org/10.1002/poc.3730
Pino, V., Yao, C., Anderson, J.L.: Micellization and interfacial behavior of imidazolium-based ionic liquids in organic solvent-water mixtures. J. Colloid Interface Sci. 333, 548–556 (2009). https://doi.org/10.1016/j.jcis.2009.02.037
Kumar, D., Azum, N., Rub, M.A., Asiri, A.M.: Aggregation behavior of sodium salt of ibuprofen with conventional and gemini surfactant. J. Mol. Liq. 262, 86–96 (2018). https://doi.org/10.1016/j.molliq.2018.04.053
Acknowledgements
Authors acknowledge the Director and Head, Department of Chemistry Dr. B.R. Ambedkar National Institute of Technology for necessary facilities. One of the authors, Gagandeep Kaur is thankful to Department of Science and Technology (DST) of New Delhi for providing financial support via DST INSPIRE Fellowship (IF170753).
Author information
Authors and Affiliations
Contributions
Gagandeep Kaur planned the experiments, analysed the data and wrote the paper. All authors contributed to the manuscript revision, and read and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaur, G., Kaur, R., Kaur, J. et al. Influence of Morpholinium-Based Ionic Liquid on the Aggregation Behavior of Cationic Surfactant and Imidazolium-Based Ionic Liquid with the same Alkyl Chain in an Aqueous Medium. J Solution Chem 52, 1129–1148 (2023). https://doi.org/10.1007/s10953-023-01299-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-023-01299-8