Abstract
A series of surfactants derived from l-Proline, the free amine esters, the ester hydrochlorides and the quaternary ammonium compounds with varying chain lengths (C8–C14) were synthesised. The physicochemical and biological properties were determined in both single and sodium dodecyl sulphate (SDS) mixed systems with a view of enhancing the properties of the individual surfactants as potential ingredients in detergent formulations. The mode of action of the proline surfactants were investigated by their ability to form mixed micelles with the phospholipid 1,2-dimyristoyl-sn-glycero-3-phosphatidylcholine (DMPC). The presence of a quaternary ammonium moiety and an increase in alkyl chain length were found to enhance the antibacterial activity of the proline QUAT derivatives. The SDS-C14 QUAT mixed system displayed good antibacterial activity with optimum activity at mole fractions αQUAT: 0.4 and 0.6. The antibacterial activity of the mixed system was found to be governed by the monomers rather than the micelles. The SDS-C14 QUAT mixed system also showed moderate irritancy which makes them potential candidates as detergents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surfactants constitute an important class of chemicals widely used in almost every sector of modern industry, and are considered to be a key ingredient in detergents, comprising 15 to 40 % of the total detergent formulations [1, 2]. With this view, the demand of the detergent industry for the development of formulations with improved detergency in the past few years has been the driving force for the synthesis of new surfactants as potential ingredients in cleaning agents. Alkylbenzenesulfonates, alkyl sulphates [1], alcohol ethoxylates [3], quaternary ammonium compounds [4] and betaine surfactants [5] are commonly used in detergent compositions, and their physicochemical behaviour such as good interfacial, surface activity and foaming abilities is well understood.
The increase in environmental awareness among consumers has led to the development of biodegradable and less toxic surfactants having good interfacial and biological properties for potential use in biomedical applications. In this perspective, amino acid surfactants as alternatives to conventional surfactants have attracted widespread attention over the last decade [6–10].
Amino acid surfactants, especially those of a cationic nature, are known to exhibit good antibacterial activity against a broad spectrum of microorganisms [11–13]. The mechanism of action of these surfactants on bacteria is known to involve electrostatic and hydrophobic interaction between the cationic agent and the phospholipid bilayer of the bacterial cell wall [14, 15]. These agents are also believed to form mixed micelles with the membrane phospholipid molecules [16]. These lead to the disruption of the membrane and permit release of electrolytes and nucleic materials, leading to cell death.
Proline-based surfactants have been found to possess interesting physicochemical as well as biological activities. Proline is a cyclic secondary amine which gives it an exceptional conformational rigidity compared to other amino acids. Optically active as well as racemic mixtures of N-acyl proline-based surfactants have been found to aggregate spontaneously in aqueous solutions [17]. Proline-based surfactants bearing an ester and amide linkage have been found to interact strongly with DNA [18, 19]. However, there have been limited studies on antibacterial properties of proline-based surfactants.
This study deals with the synthesis and physicochemical characteristics of a range of surfactants derived from proline esters. Their physicochemical and biological activities were determined as both single and mixed surfactant systems with SDS, in order to study their effectiveness as potential ingredients in detergents. The antibacterial activity against gram-positive and gram-negative bacteria were also investigated. In order to investigate the possible mechanism of the surfactant for their antimicrobial activity, mixed micelle-forming ability of selected proline surfactants with 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) were studied. The foaming characteristics of selected proline surfactants were investigated to evaluate their use as detergent formulations.
Materials and Methods
l-Proline, octanol, decanol, dodecanol, and tetradecanol were obtained from Sigma-Aldrich (USA). p-Toluene sulfonic acid (PTSA) was obtained from Merck, Germany. Mueller–Hinton agar and broth were purchased from Oxoid Ltd., United Kingdom. The different bacterial strains were obtained from Microbiologics®, USA and Oxoid Ltd., United Kingdom. 1,2-Dimyristoyl-sn-glycero-3-phosphocholine (DMPC) was purchased from Avanti Polarlipids, Inc., USA. Cetyl trimethyl ammonium bromide (CTAB) and sodium dodecyl sulphate (SDS) were obtained from BDH Laboratory Supplies, England.
Conductivity measurements were carried out using a Jenway 4320 conductivity meter. Proton nucler magnetic resonance ( 1H NMR) and carbon-13 ( 13C) NMR spectra were recorded at 250 MHz and 62.9 MHz on a Bruker electro spin NMR spectrometer using CDCl3 as the solvent.
Synthesis and Characterization of l-Proline Esters (1a–d) and Their Hydrochloride Derivatives (2a–d)
The l-proline esters were synthesised according to a modified procedure described by our group [14] by the reaction of l-proline (1.37 g, 12.1 mmol), PTSA (2.30 g, 12.1 mmol) and alcohol (14.5 mmol) in refluxing toluene (100 ml) for 48 h. The crude product was purified by column chromatography (Hexane/DCM 1:1) to give the esters (1a–d) as yellow oils. The proline esters were dissolved in EtOAc and then HCl gas was bubbled through the mixture and after evaporation of the solvent in vacuo, the proline ester hydrochloride derivatives (2a–d) were obtained as solids.
Synthesis of Proline QUAT Derivatives (3a–d)
The l-proline ester (2.32 mmol) and methyl iodide (4 mL) were stirred in acetonitrile in the presence of K2CO3 (1.20 g, 8.7 mmol) for 24 h at room temperature (25°C). The mixture was filtered and the filtrate was evaporated in vacuo to yield the product as white or yellow solid.
Octyl pyrrolidine-2-carboxylate (1a) Yield: 67%. IR, νmax (cm−1): 2956, 1741. 1H NMR (CDCl3), δ (ppm): 0.86 (t, J 7.0 Hz, 3H, O(CH2)7CH 3 ), 1.25 (m, 10H, OCH2CH2(CH 2)5CH3), 1.55 (m, 2H, OCH2CH 2(CH2)5CH3), 1.72 (m, 2H, CH2), 1.87 (m, 2H, CH2), 2.88 (m, 1H, CHH), 3.08 (m, 1H, CHH), 3.62 (t, J 7.0 Hz, 1H, CH), 4.10 (t, J 7.0 Hz, 2H, OCH 2(CH2)6CH3). 13C NMR (CDCl3), δ (ppm): 14.1 (O(CH2)7 CH3), 22.7–30.3 (OCH2(CH2)6CH3), 25.7 (CH2), 31.8 (CH2), 47.0 (N-CH2), 59.8 (CH), 63.1 (OCH2(CH2)6CH3), 175.6 (C=O).
Decyl pyrrolidine-2-carboxylate (1b) Yield: 78%. IR, νmax (cm−1): 2954, 1743. 1H NMR (CDCl3), δ (ppm): 0.86 (m, 3H, O(CH2)9CH 3), 1.24 (m, 14H, OCH2CH2(CH 2)7CH3), 1.54 (m, 2H, OCH2CH 2(CH2)7CH3), 1.74 (m, 2H, CH2), 1.83 (m, 2H, CH2), 2.98 (m, 1H, CHH), 3.15 (m, 1H, CHH), 3.61 (t, J 7.0 Hz, 1H, CH), 4.10 (t, J 7.0 Hz, 2H, OCH 2(CH2)8CH3). 13C NMR (CDCl3), δ (ppm): 14.1 (O(CH2)9 CH3), 22.7–30.3 (OCH2(CH2)8CH3), 25.7 (CH2), 31.9 (CH2), 47.0 (N-CH2), 59.8 (CH), 65.1 (OCH2(CH2)8CH3), 175.6 (C=O).
Dodecyl pyrrolidine-2-carboxylate (1c) Yield: 56%. IR, νmax (cm−1): 3377, 2957, 1737. 1H NMR (CDCl3), δ (ppm): 0.88 (m, 3H, O(CH2)11CH 3), 1.26 (m, 18H, OCH2CH2(CH 2)9CH3), 1.61 (m, 2H, OCH2CH 2(CH2)9CH3), 1.74 (m, 2H, CH2), 1.80 (m, 2H, CH2), 2.88 (m, 1H, CHH), 3.06 (m, 1H, CHH), 3.75 (m, 1H, CH), 4.11 (t, J 7.0 Hz, 2H, OCH 2(CH2)10CH3). 13C NMR (CDCl3), δ (ppm): 14.1 (O(CH2)9 CH3), 22.7–30.3 (OCH2(CH2)8CH3), 25.9 (CH2), 31.9 (CH2), 47.0 (N-CH2), 59.8 (CH), 65.1 (OCH2(CH2)8CH3), 175.6 (C=O).
Tetradecyl pyrrolidine-2-carboxylate (1d) Yield: 75%. IR, νmax (cm−1): 3420, 2921, 1739. 1H NMR (CDCl3), δ (ppm): 0.88 (m, 3H, O(CH2)13CH 3), 1.26 (m, 22H, OCH2CH2(CH 2)11CH3), 1.52 (m, 2H, OCH2CH 2(CH2)11CH3), 1.78 (m, 2H, CH2), 1.86 (m, 2H, CH2), 3.03 (m, 2H, CH2), 3.64 (t, J 7.0 Hz, 1H, CH), 4.13 (t, J 7.0 Hz, 2H, OCH 2(CH2)12CH3). 13C NMR (CDCl3), δ (ppm): 14.1 (O(CH2)9 CH3), 22.7–30.3 (OCH2(CH2)8CH3), 25.7 (CH2), 31.9 (CH2), 46.6 (N-CH2), 59.5 (CH), 65.8 (OCH2(CH2)8CH3), 173.1 (C=O).
Octyl pyrrolidine-2-carboxylate hydrochloride (2a) Yield: 49%. Elem. Anal. Found: C, 58.93; H, 9.66; N, 5.00 Calcd. for C13H26NO2Cl: C, 59.19; H, 9.93; N, 5.31. 1H NMR (CDCl3), δ (ppm): 0.88 (m, 3H, O(CH2)7CH 3), 1.27 (m, 10H, OCH2CH2(CH 2)5CH3), 1.62 (m, 2H, OCH2CH 2(CH2)5CH3), 2.04 (m, 2H, CH2), 3.54 (m, 2H, CH2), 4.10 (m, 2H, OCH 2(CH2)6CH3), 4.50 (m, 1H, CH).
Decyl pyrrolidine-2-carboxylate hydrochloride (2b) Yield: 52%. Elem. Anal. Found: C, 62.14; H, 10.32; N, 4.53; Calcd. for C15H30NO2Cl: C, 61.73; H, 10.36; N, 4.80. 1H NMR (CDCl3), δ (ppm): 0.86 (m, 3H, O(CH2)9CH 3), 1.27 (m, 14H, OCH2CH2(CH 2)7CH3), 1.64 (m, 2H, OCH2CH 2(CH2)7CH3), 2.03 (m, 2H, CH2), 3.53 (m, 2H, CH2), 4.12 (m, 2H, OCH 2(CH2)8CH3), 4.48 (m, 1H, CH).
Dodecyl pyrrolidine-2-carboxylate hydrochloride (2c) Yield: 63%. Elem. Anal. Found: C, 64.17; H, 11.14; N, 4.75; Calcd. for C17H34NO2Cl: C, 63.82; H, 10.71; N, 4.38. 1H NMR (CDCl3), δ (ppm): 0.87 (m, 3H, O(CH2)11CH 3), 1.26 (m, 18H, OCH2CH2(CH 2)9CH3), 1.66 (m, 2H, OCH2CH 2(CH2)9CH3), 2.04 (m, 2H, CH2), 3.53 (m, 2H, CH2), 4.12 (m, 2H, OCH 2(CH2)10CH3), 4.48 (m, 1H, CH).
Tetradecyl pyrrolidine-2-carboxylate hydrochloride (2d) Yield: 61%. Elem. Anal. Found: C, 65.83; H, 11.27; N, 4.09; Calcd. for C19H38NO2Cl: C, 65.58; H, 11.01; N, 4.03. 1H NMR (CDCl3), δ (ppm): 0.87 (m, 3H, O(CH2)13CH 3), 1.25 (m, 22H, OCH2CH2(CH 2)11CH3), 1.61 (m, 2H, OCH2CH 2(CH2)11CH3), 2.04 (m, 2H, CH2), 3.55 (m, 2H, CH2), 4.19 (m, 2H, OCH 2(CH2)12CH3), 4.51 (m, 1H, CH).
2-Octyloxycarbonyl-1,1-dimethyl-pyrrolidinium iodide (3a) Yield: 57%. Elem. Anal. Found: C, 47.27; H, 8.03; N, 3.21; Calcd. for C15H30NO2I: C, 47.00; H, 7.89; N, 3.65. 1H NMR (CDCl3), δ (ppm): 0.87 (m, 3H, O(CH2)7CH 3), 1.28 (m, 10H, OCH2CH2(CH 2)5CH3), 1.52 (m, 2H, OCH2CH 2(CH2)5CH3), 2.11 (m, 2H, CH2), 2.50 (m, 1H, CHH), 2.82 (m, 1H, CHH), 3.22 (s, 3H, CH3), 3.63 (s, 3H, CH3), 4.04 (m, 1H, CHH), 4.20 (m, 2H, OCH 2(CH2)6CH3), 4.58 (m, 1H, CHH), 5.31 (m, 1H, CH). 13C NMR (CDCl3), δ(ppm): 14.1 (O(CH2)7 CH3), 22.7–30.3 (OCH2(CH2)8CH3), 25.7 (CH2), 31.9 (CH2), 46.6 (N-CH2), 47.0 (CH3), 51.7 (CH3), 59.5 (CH), 63.2 (OCH2(CH2)8CH3), 165.8 (C=O).
2-Decyloxycarbonyl-1,1-dimethyl-pyrrolidinium iodide (3b) Yield: 43%. Elem. Anal. Found: C, 49.17; H, 8.56; N, 3.03. Calcd. for C17H34NO2I: C, 49.64; H, 8.33; N, 3.40. 1HNMR (CDCl3), δ (ppm): 1H NMR (CDCl3), δ (ppm): 0.86 (m, 3H, O(CH2)9CH 3), 1.25 (m, 14H, OCH2CH2(CH 2)7CH3), 1.52 (m, 2H, OCH2CH 2(CH2)7CH3), 2.14 (m, 2H, CH2), 2.48 (m, 1H, CHH), 2.76 (m, 1H, CHH), 3.21 (s, 3H, CH3), 3.73 (s, 3H, CH3), 4.04 (m, 1H, CHH), 4.18 (m, 2H, OCH 2(CH2)8CH3), 4.58 (m, 1H, CHH), 5.42 (m, 1H, CH). 13C NMR (CDCl3), δ (ppm): 14.1 (O(CH2)13 CH3), 22.7–29.5 (OCH2(CH2)10CH3), 25.9 (CH2), 31.9 (CH2), 47.2 (CH3), 51.9 (CH3), 63.4 (OCH2(CH2)10CH3), 68.4 (N-CH2), 73.6 (CH), 166.0 (C = O).
2-Dodecyloxycarbonyl-1,1-dimethyl-pyrrolidinium iodide (3c) Yield: 37%. Elem. Anal. Found: C, 52.12; H, 9.06; N, 3.24; Calcd. for C19H38NO2I: C, 51.93; H, 8.72; N, 3.19. 1H NMR (CDCl3), δ (ppm): 0.88 (m, 3H, O(CH2)11CH 3), 1.26 (m, 18H, OCH2CH2(CH 2)9CH3), 1.51 (m, 2H, OCH2CH 2(CH2)9CH3), 2.25 (m, 2H, CH2), 2.51 (m, 1H, CHH), 2.83 (m, 1H, CHH), 3.24 (s, 3H, CH3), 3.65 (s, 3H, CH3), 4.04 (m, 1H, CHH), 4.22 (m, 2H, OCH 2(CH2)10CH3), 4.62 (m, 1H, CHH), 5.31 (m, 1H, CH). 13C NMR (CDCl3), δ (ppm): 14.1 (O(CH2)11 CH3), 19.2 (CH2), 22.7–31.9 (OCH2(CH2)11CH3), 25.9 (CH2), 47.3(CH3), 52.3 (CH3), 63.1 (OCH2(CH2)11CH3), 68.0 (N-CH2), 73.1 (CH), 165.8 (C=O).
2-Tetradecyloxycarbonyl-1,1-dimethyl-pyrrolidinium iodide (3d) Yield: 78%. Melting point: 121 °C. Elem. Anal. Found: C, 53.62; H, 9.26; N, 3.09; Calcd. for C21H42NO2I: C, 53.96; H, 9.06; N, 3.00. TOF MS, m/z: 340.32 (without the iodide ion). 1H NMR (CDCl3), δ (ppm): 0.88 (m, 3H, O(CH2)13CH 3), 1.25 (m, 22H, OCH2CH2(CH 2)11CH3), 1.53 (m, 2H, OCH2CH 2(CH2)11CH3), 2.22 (m, 2H, CH2), 2.49 (m, 1H, CHH), 2.84 (m, 1H, CHH), 3.22 (s, 3H, CH3), 3.75 (s, 3H, CH3), 4.12 (m, 1H, CHH), 4.22 (m, 2H, OCH 2(CH2)12CH3), 4.52 (m, 1H, CHH), 4.30 (m, 1H, CH). 13C NMR (CDCl3), δ(ppm): 14.1 (O(CH2)13 CH3), 19.1 (CH2), 22.7–31.9 (OCH2(CH2)12CH3), 25.7 (CH2), 47.3 (CH3), 52.2 (CH3), 63.1 (OCH2(CH2)12CH3), 67.9 (N-CH2), 73.5 (CH), 165.0 (C = O).
Critical Micelle Concentration and Phase Behaviour
The critical micelle concentrations (CMCs) of the proline surfactants were determined by a conductivity method [14]. Mixed SDS-C14 QUAT 3d systems were prepared by mixing precalculated volumes of the stock solutions of both surfactants in water and the solutions were stirred for 1 h. In order to study the counterion effect, the mixed micelle was also studied in 5 mM NaI. The composition of each solution was expressed as a mole fraction of the QUAT:
where [QUAT] and [SDS] are the concentrations of QUAT 3d and SDS in the mixed solutions, respectively.
Mixed systems with varying mole fractions of QUATS (αQUAT = 0, 0.2, 0.4, 0.6, 0.8) were prepared and the CMCs of the different mixed systems were determined by adding successive amounts of the stock solutions to deionised water/5 mM NaI in the form of a titration.
Mixed systems of SDS with varying mole fractions of QUAT 3d (αQUAT = 0 to 1) were prepared by mixing the required volume of equimolar (20 mM) QUAT 3d and SDS in 5 mM NaI. The solutions were stirred for 24 h and the phase behaviour of the different mixed systems was investigated by visual inspection and by conductivity measurements.
Mixed surfactant-phospholipid (DMPC) systems were prepared according to the method previously described by Faustino et al. (2011) [16]. Equimolar stock solutions (500 mM) of proline surfactants (2d and 3d) and DMPC were prepared in deionized water. Binary mixtures of DMPC with varying mole fractions (0, 0.2, 0.4, 0.6, 0.8, 1) of the surfactants were prepared by mixing calculated amounts of the stock solutions of both components in aqueous phase. All the solutions were stirred for about 1 h to ensure complete mixing of the phospholipid and the surfactant. The CMC of the mixed surfactant-phospholipid system was determined as mentioned above.
The mole fractions (αL) of the phospholipid in the mixtures were calculated as per the equation below:
where [lipid] and [surfactant] are the concentrations (M) of the phospholipid and surfactant in the solution, respectively.
The phase behaviour of the binary mixture of QUAT 3d with varying mole fraction of DMPC (αL = 0 to 1) was determined by mixing the required volume of equimolar (0.01 M) QUAT 3d and DMPC. After mixing for 24 h, the phase behaviour was noted by visual observation and conductivity measurements as a function of αL.
Antibacterial Activity
The antibacterial activities were evaluated against Staphylococcus aureus (ATCC 25923), Staphylococcus epidermidis (ATCC 12228), Bacillus cereus (ATCC 11778, ATCC 10876), Klebsiella pneumoniae (ATCC 13883), Escherichia coli (ATCC 22922) and Salmonella typhimurium (ATCC 14028). The antibacterial activity was expressed as the minimum inhibitory concentrations (MICs) which was defined as the lowest concentration that inhibits the growth of bacteria. CTAB was used as a positive control. The antibacterial activities of the different SDS-QUAT 3d mixed micelle systems (αQUAT = 0, 0.2, 0.4, 0.6, 0.8) were determined starting from solutions with a total surfactant concentration (CT) of 0.01 M [(QUAT) + (SDS) = 0.01 M].
The antimicrobial susceptibility of the proline surfactants was determined by the Kirby–Bauer disc diffusion method [20]. Solutions of esters of free amine l-proline (1a–d) and their hydrochlorides (2a–d) in dimethyl sulfoxide (DMSO; 10 µL, 10 mg/mL) were pipetted onto discs on Mueller–Hinton Agar plates containing bacteria an allowed to incubate at 37 °C for 24 h. For each compound, the experiment was done in triplicate. The antibacterial activity was determined by measuring the average zone of inhibition in mm. The MIC was determined by the microdilution assay in a 96-well ELISA plate [21]. All wells were inoculated with 50 μL of a bacterial suspension adjusted to 0.5 McFarland in physiological solution. Microplates were covered and incubated for 24 h at 37 °C. The minimum inhibitory concentration (MIC) of the surfactants was detected following addition of 20 μl iodonitrotetrazolium chloride (0.4 mg/mL) and incubation at 37 °C for 30 min. MIC is defined as the lowest surfactant concentration that prevented the colour change from yellow to pink, due to inhibition of bacterial growth.
Foamability and Foam Stability Measurements
The surfactant solution (20 mL, 0.1 wt%) was shaken manually in a 100-mL glass cylinder for 10 s at 25 °C. The height of the foam (cm) was measured at different time intervals (5 min, 30 min, 1 h and 24 h). A 10-mL glass cylinder was used for the measurement of foamability of the different mixed micelle SDS-QUAT systems. 5-mL portions of 500 mM mixed micelle solution (αQUAT = 0, 0.2, 0.4, 0.6, 0.8, 1) were shaken vigorously for 10 s, and the initial heights of the foam were recorded. All the measurements were performed at 25 °C in triplicate and the results are reported as the mean value ± standard deviation.
Hemolytic Activity and Eye Irritation Potential of an SDS-QUAT Mixed Micelle System
Hemolytic activity of the SDS-QUAT mixed micelles was determined using the previously reported procedure [15]. The results were expressed by the concentration of the mixed micelle that induced 50 % hemolysis (HC50).
The eye irritation potential of the mixed micelle system was determined by the ratio of hemolysis to the protein denaturation index [23]. Protein denaturation was determined from the ratio Rx of absorbance at 575 and 540 nm in a UV–Vis spectrophotometer. R 1 was the ratio obtained with the distilled water lysis and R 2 was the ratio obtained with the denaturant SDS. The ratio obtained with each test material concentration was R i. The ratios were used to calculate the haemoglobin denaturation index (DI), where DI = R 1−R i/R 1−R 2. The relation between the hemolytic activity (HC50) and the denaturation index (DI) called the L/D ratio was used to classify the irritancy of the different surfactant mixtures as: non-irritant >100, weak irritant >10, moderate irritant >1, irritant >0.1 and very irritant <0.1.
Results and Discussions
Esters of l-proline-free amine (chain lengths C8–C14) 1a–d were synthesized by the reaction of proline with the corresponding alcohols. The cationic surfactants were obtained either by protonation of the amino group or by reacting the esters with excess MeI yielding the hydrochlorides 2a–d and the quaternary ammonium derivatives (QUATS) of proline 3a–d (Fig. 1). Quaternisation was confirmed by the presence of two singlets corresponding to (2 × 3H) at δ 3.21–3.74 ppm. Compared to the l-proline ester hydrochloride series 2a–d, the l-proline QUATS have a permanent positive charge on the head group, irrespective of the pH.
The purity of the proline surfactants were confirmed by spectral and analytical data. The 1H and 13C NMR of the different proline derivatives are concordant with the expected structures.
Critical Micelle Concentration
The CMCs of the cationic surfactant derivatives of proline, namely the ester hydrochlorides (2a–d) and the QUATS (3a–d), are summarised in Table 1.
The CMCs of the ester hydrochlorides (2a–d) were found to be higher compared to the QUAT derivatives (3a–d), implying that the QUATS have a stronger tendency to form micelles than the ester hydrochlorides.
Mixed surfactant systems are known to have physical properties different from that of the individual components and these systems are encountered in several applications [24, 25]. The electrostatic interactions between cationic and anionic surfactants are known to enhance their surface activities [26]. In this study, the QUAT C14 derivative of proline 3d displayed the highest micelle-forming ability. In view of providing superior properties, the use of the QUAT 3d in mixed micelle solutions with the anionic surfactant SDS have been investigated. To study the effect of the counterions on the mixed micelle solutions, the CMCs of the mixed systems were also determined in a swamping excess of the counterions; i.e., 5 mM NaI solution. The CMC values of the different binary combinations of SDS and QUAT 3d are presented in Table 2.
The CMC values of the SDS-QUAT C14 surfactant was lower compared to the CMC of the pure SDS solution, suggesting that micellar formation is more favoured in the mixed micellar system. This is mainly due to the electrostatic attraction between the anionic SDS molecules and the cationic QUAT molecules, which result in a better packing in the mixed micelle system. The lower CMCs of the different SDS-QUAT C14 mixed systems in NaI compared to those observed in deionised water indicate that the presence of NaI facilitates micellar formation of the different mixed systems.
The ideal CMC of the binary mixture can be predicted by the Clint equation [27] (Eq. 1):
where αQUAT is the mole fraction of the QUAT in the mixture, cmcQUAT and cmcSDS correspond to the CMCs of pure components of QUAT and SDS, respectively, and cmc* is the value under ideal mixing.
The micellar molar fraction of the QUAT in the ideal state was evaluated according to Eq. 2, assuming binary ideal mixture.
The experimental value and theoretical value predicted by the Clint equation for the CMC of each of the surfactant binary mixture is shown in Fig. 2.
In both deionised and NaI solution, the CMC values obtained for the SDS-QUAT binary system were slightly higher than those predicted by the Clint model. The larger CMC values than those predicted by the Clint model are generally due to unfavourable interactions between the two surfactants, which is highly unusual in the SDS-QUAT system due to the oppositely charged surfactants. Similar behaviour of cationic and anionic surfactant binary mixtures has also been reported by Bakshi et al. [28]. Based on this, it was assumed that the higher CMC values of the mixed SDS-QUAT compared to the ideal values obtained from the Clint equation was due to the precipitation of the neutral catanionic complex formed between equimolar anionic SDS and cationic QUAT. For the different ratios, the excess charges cause destabilisation of the precipitate, leading to vesicle formation [29]. This causes the concentration of the SDS and QUAT to decrease and, hence, fewer monomers would be available for the mixed micelle formation process. Thus, the CMC is reached later and becomes greater than the predicted CMC.
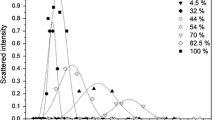
SDS exists mainly in the form of micelles at a concentration of 20 mM which is well above its CMC (8.3 mM). The phase transitions of the SDS-QUAT binary system were investigated. Addition of QUAT 3d (αQUAT = 0–0.3) to an aqueous solution of SDS caused an increase in the electrical conductivity values due to the increase in number of ions (Fig. 3). A further increase in mole fraction of QUAT 3d above 0.3 caused the solution to change from clear to turbid, leading to the formation of a white precipitate at αQUAT = 0.5–0.6 (Fig. 4). At αQUAT = 0.3–0.6, the conductivity values were found to vary slightly due the formation of vesicles in the solution. An increase in αQUAT to 0.8 caused the solution to become clear and highly viscous, together with a drastic decrease in conductivity values. This is attributed to the adsorption of the counterions in the vesicles that are being formed, hence, decreasing the number of conducting ions. Similar observations were made by Ghosh et al. with the binary system of cetyltrimethyl ammonium bromide and the anionic surfactant 1-butyl-3-methylimidazolium octyl sulfate [30]. Based on these observations, it can be inferred that the SDS-QUAT mixtures above αQUAT = 0.6 exist predominantly as vesicles.
The extent of non-ideality of surfactant interactions is usually evaluated using the regular solution theory (RST) which includes an interaction parameter, β12, to characterise the interactions between the two components within the mixed micelles. This parameter is related to the activity coefficients, f, of the surfactants within the micelle, according to:
where χQUAT, the molar fraction of QUAT 3d in the mixed micelle, can be obtained solving the following equation iteratively:
The interaction parameter β12 can then be evaluated from
A negative β12 value accounts for synergism while a positive value indicates antagonism behaviour for the mixed micelle formation. In the SDS-QUAT system, positive β12 (2.5–40.9) values were obtained for the different mole ratios studied. Positive β12 values were also observed in the presence of 5 mM NaI. Similar results were observed by Bakshi et al. [28] and based on this, we assume that the positive β values are not a result of antagonistic behaviour, but due to the higher value of the CMC of the mixed micelle compared to the those predicted by the Clint model which arises from the dimerisation of the oppositely charged SDS and QUAT monomers.
Antibacterial Activity of Proline Surfactants in Single and Mixed Surfactant Systems
The esters of free amine l-proline (1a–d), their hydrochlorides (2a–d) as well as the QUATS (3a–d) were screened for their antibacterial activity. They were found to exhibit considerable activity towards gram-positive bacteria and were less active with respect to gram-negative strains (Fig. 5). In the case of the free amine ester series (1a–d), the highest activity was observed at C12 for gram-positive bacteria. For the ester hydrochloride series (2a–d), the highest activity was observed at C12 with respect to S.aureus and B.cereus, while in the case of S.epidermidis, the activity was found to increase up to C14. For gram-negative bacteria, the activity was found to decrease from C10 to C14 for the free amine esters, while in the case of the ester hydrochlorides, the cut-off point was observed at C12.
Based on the data obtained on the average zone of inhibitions with respect to the different bacteria tested, the C12 and C14 derivatives of the free amine esters and ester hydrochlorides showed promising antibacterial activity. Therefore, the MIC values of only the C12 and C14 derivatives of the free amine ester (1c and 1d) and ester hydrochlorides (2c and 2d) were evaluated. The MIC of the QUATS 3a–d were also evaluated since the presence of a quaternary ammonium moiety has been known to enhance antibacterial activity [15]. The results are summarised in Table 3.
In general, the compounds showed moderate to good antibacterial activities with respect to the different bacterial strains tested. Lower MIC values were observed in the case of gram-positive bacteria, showing that the proline derivatives exhibited better activity with respect to these bacterial strains. The QUAT 3d, with a chain length of C14 , was found to be the most active among the series, displaying the lowest MIC values with respect to all the bacteria tested. The QUAT 3d even showed better antibacterial effect compared to the positive control CTAB.
The antibacterial activities of the proline esters are attributed to the surfactant structure of these compounds which allows them to interact with the bacterial membrane, leading to cell lysis and eventually cell death [31].
Quaternisation of the amino group was found to enhance the antibacterial activity of the QUATS. This might be due to their relatively larger head group that causes greater membrane disruption, leading to a larger free volume in the bacterial membrane, hence facilitating the destruction of the microorganisms [15].
Subsequently, the antibacterial activity of the binary mixture of the surfactant, 3d, with the conventional surfactant SDS was evaluated against 2 g of gram-positive and 1 g of gram-negative bacteria. The results are shown in Table 4.
SDS exhibits some antimicrobial properties against the bacteria tested. An increase in activity was observed upon increasing the amount of the QUAT 3d. The binary mixture αQUAT = 0.4 and 0.6 exhibited better antimicrobial activity. From Fig. 6, it can be seen that the antimicrobial activity of the binary mixture as a function of the αQUAT do not show a linear trend. Comparing the MIC values with the CMC obtained for the different binary mixtures of SDS-3d, it was found that the antibacterial activities of these surfactants are observed below their CMC, showing that the mixed monomers influence their activity.
Pure SDS showed moderate antibacterial activity which might be due to the absence of a positive charge, which plays an essential role in electrostatic interaction with the negatively charged bacterial membrane. Addition of the QUAT to the SDS enhances the antibacterial effect due to the involvement of the positively charged head group of the QUAT in the electrostatic interaction with the negatively charged bacterial membrane, followed by hydrophobic interaction with the bacterial membrane giving rise to cell lysis and cell death.
Surfactant-DMPC Mixed Micelle Formation
The biological membrane is crucial for bacterial survival and serves as a permeability barrier for transport of molecules in and out of the cell. Phospholipid bilayers in membranes play a key role in the regulation of in vivo barriers. Phospholipids having long hydrocarbon chains are major components of cell membranes. In aqueous solutions, these phospholipids form closed spherical liposomes, responsible for cellular partitioning which is crucial for biological activity.
Phosphatidyl choline is the most abundant membrane phospholipid in cells and can be found in significant amounts in bacterial membranes. DMPC has been used as membrane models to study the antibacterial properties of diacyl glycerol arginine-based surfactants [32]. DMPC has also been used to study the mixed micelle formation with the anionic Gemini surfactants derived from cysteine, leading to the formation of lipid-surfactant systems [16].
In this study, the mixed micelle formation between the cationic proline surfactants, namely the ester hydrochloride and the QUAT derivatives with the zwitterionic phospholipid DMPC (1,2-dimyristoyl-sn-glycero-3-phosphocholine) were studied, in order to investigate the possible mode of action of these compounds based on their effectiveness in solubilising bacterial membranes. The effect of the head group structures on the formation of the mixed micellar aggregates was also compared.
The mixed micelle formation process of the proline ester hydrochloride 2d with a varying mole fraction of DMPC is shown in Fig. 7.
The CMC values obtained for the different binary mixtures of the surfactant and DMPC were lower than the CMC of the pure surfactant, a consequence of attractive interaction between the surfactant and the phospholipid. These two factors lead to a net decrease in the hydrophilicity and, therefore, leading to lower CMC values.
Normalized CMCs (CMC/CMC0, where CMC0 is the CMC of pure surfactant) for the DMPC-surfactant mixtures studied, are shown in Fig. 8. An increase in the mole fraction of DMPC leads to a substantial decrease of the CMC, and further increase above αDMPC of 0.6. From Fig. 8, it was observed that the decrease in CMC/CMC0 was more pronounced in the case of the QUAT derivative than the ester hydrochloride, showing that the QUATS-DMPC mixed micelle formation is more favoured compared to the ester hydrochloride-DMPC mixed micelle. This might be the reason for the relatively enhanced antibacterial activity of the QUATS over the proline ester hydrochloride derivatives.
The addition of phospholipid to the micellar solution of proline surfactants leads to a decrease in CMC values. Similar observation has been observed by Faustino et al. [16]. Based on this, the decrease in CMC might be assumed to be due to the incorporation of DMPC molecules into the proline micelles, causing less ionic head group repulsions between the similar proline surfactants and, hence, causing the DMPC-proline surfactant mixed micelles to be more stable. However, an increase in the CMC/CMC0 is observed at higher concentrations of DMPC. DMPC normally forms vesicles rather than micelles in aqueous solutions [16]. Therefore, a higher concentration of DMPC in the mixed-system can cause a transition from mixed micelle to the formation of vesicles and, hence, causing an increase in the CMC values. The phase behaviour of the mixture of QUAT 3d with varying mole ratio of DMPC was studied. An increase in αDMPC was found to increase the turbidity of the solutions together with a decrease in conductivity (Fig. 9). The decrease in conductivity confirmed the formation of vesicles, which absorbs the ions present in the mixtures, hence making the solution less conducting.
Foamability and Foam Stability of the Proline Surfactants
Foaming is a property inherent to all surfactant solutions and this is widely used in detergents and cosmetics. Foam is an important aspect in detergency and, hence, the design of a product is often focused upon foaming abilities, rendering investigation of foaming an active field of research. Due to their high level of interfacial free energy, foams are thermodynamically unstable, and, therefore, the control of foam stability is important in many applications.
The foaming property of the proline QUAT derivatives, which exhibited good antibacterial activity, was studied in view of investigating the use of these surfactants as potential ingredients in detergent-like formulations. The foam stability of the QUATS was evaluated by monitoring the changes in foam height (h) as a function of time (t).
Figure 10 shows the foam height of aqueous solution of the QUATS at different times (0 min, 5 min, 30 min, 1 h, 24 h). The foaming ability of the QUATS was found to increase with the hydrocarbon chain length. QUATS with longer alkyl chains tend to form more stable monolayers compared to those with shorter chains, giving rise to stable foams.
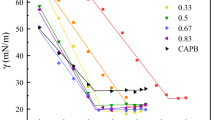
Comparing the effect of the head group on the foaming abilities of the different proline derivatives (1d, 2d and 3d) of the same chain length, it was found that the foamability increases in the order of QUATS > free amine ester > ester hydrochloride. A lower foaming ability was observed for the ester hydrochloride derivative compared to the free amine ester derivative. This might be attributed to the molecular arrangement of the surfactants in water. In the case of the proline ester hydrochloride, the presence of the positively charged headgroup causes electrostatic repulsion between the molecules, which hinders the formation of foams. However, quaternising the nitrogen center caused an increase in foaming ability which might be due to an increase in hydrophobic character of the surfactant and the screening of the positively charged nitrogen by the methyl groups, hence decreasing the electrostatic repulsion between the molecules. From the biological studies, it was found that the SDS-QUAT binary mixture showed enhanced properties over the individual surfactants. In view of their potential applications as cosmetics or detergents, the foaming properties of the different binary mixtures were studied (Fig. 11).
Foamability of the mixed system was found to decrease with increasing the mole fraction of 3d up to a mole fraction of 0.6, and then increases with further addition of the QUAT. The decrease in foaming ability may be due to the dimer formation between the anionic SDS and QUAT 3d as observed in the mixed micelle system, causing a decrease in the concentration of the monomers (SDS and QUAT 3d) that account for the good foamability.
Hemolytic Activity
The in vitro red blood cell method (RBC) is used to evaluate the eye irritation potential of detergents and surfactants. This assay determines hemolysis (HC50), denaturation index (DI) and the ratio of both parameters (L/D ratio), which is used to characterize in vitro effects of test substances. Red blood cell assays provide reliable results, reduces and even avoids testing on animals [33].
The results obtained via hemolysis and denaturation tests of the different mixed micelle solutions as well as their in vitro classification are shown in Table 5.
The results showed that pure SDS (αQUAT = 0) is an irritant, and upon increasing the mole fraction of the QUAT 3d, the mixed micelle system changes from an irritant to a moderate irritant, showing that the presence of the QUAT decreases the irritancy of SDS. The mixed surfactant mixture consisting of αQUAT = 0.4 and 0.6 showed the least irritation index among the mixed surfactant system studied.
Conclusion
The proline surfactants showed good foaming and antibacterial properties with the QUAT C14 derivative displaying the best activity among the series. Studies on the surfactant-DMPC mixed system revealed that the presence of the quaternary ammonium moiety enhances the antibacterial activity due to the formation of a more favourable mixed micelle with the phospholipids. The SDS-QUAT C14 mixed system exhibited interesting physicochemical properties whereby positive β values were observed in both deionised water and NaI solution. This is assumed to be due to dimerization of the SDS and QUAT, causing a decrease in the concentration of these monomers and, hence, increasing the CMC of the mixed system higher than the ideal values. The SDS- QUAT C14 mixed system displayed good antibacterial activities with an optimum activity observed for the αQUAT = 0.4 and 0.6. These surfactant mixtures also showed moderate irritancy compared to the pure SDS. Overall, evidence from this study shows that the SDS-QUAT C14 mixtures with αQUAT = 0.4 and 0.6 can be employed as potential ingredients in detergent-like formulations due to their good antibacterial property and relatively lower irritancy.
References
Scheibel JJ. The evolution of anionic surfactant technology to meet the requirements of the laundry detergent industry. J Surfact Deterg. 2004;7:319–28.
Yu Y, Zhao J, Andrew EB. Development of surfactants and builders in detergent formulations. Chin J Chem Eng. 2008;16:517–27.
Rakutani K, Onda Y, Inaoka T. Surfactants derived from secondary alcohols. In: Karsa DR, editor. Industrial applications of surfactants IV. Cambridge: The Royal Society of Chemistry; 1999.
Johnson JR, Chirash W. Softener, bleach and anti-cling composition. US Pat 4203852 A. 1980.
Tamura T, Iihara T, Nishida S, Ohta S. Cleaning performance and foaming properties of lauroylamidopropylbetaine/non-ionic mixed systems. J Surfact Deterg. 1999;2:207–11.
Infante MR, Perez L, Pinazo A, Clapes P, Moran MC, Angelet M, Garcia MT, Vinardell MP. Amino acid-based surfactants. Comptes Rendues Chimi. 2004;250:583–92.
Holmberg K. Preparation, application and biodegradability, 2nd edition, surfactant science series. New York: Marcel Dekker; 2004.
Pinazo A, Pons R, Perez L, Infante MR. Amino acids as raw material for biocompatible surfactants. Ind Eng Chem Res. 2011;50:4805–17.
Chandra N, Tyagi VK. Synthesis, properties and applications of amino acid based surfactants: a review. J Dispers Sci Technol. 2013;34:800–8.
Moran MC, Pinazo A, Perez L, Clapes P, Angelet M, Garcia MT, Vinardell MP, Infante MR. Green amino acid-based surfactants. Green Chem. 2004;6:233–40.
Castillo JA, Pinazo A, Carilla J, Infante MR, Alsina MA, Haro I, Clapes. Interaction of antimicrobial arginine-based cationic surfactants with liposomes and lipid monolayers. Langmuir. 2004;20:3379–87.
Lukac M, Lacko I, Bukovsky M, Kyselova Z, Karlovsta J, Horvath B, Devinsky F. Synthesis and antimicrobial activity of a series of optically active quaternary ammonium salts derived from phenylalanine. Cent Eur J Chem. 2010;8:194–201.
Perez L, Pinazo A, Garcia MT, Lozano M, Manresa A, Angelet M, Vinardell MP, Mitjans M, Pons R, Infante MR. Cationic surfactants from lysine: synthesis, micellization and biological evaluation. Eur J Med Chem. 2009;44:1884–92.
Joondan N, Jhaumeer-Laulloo S, Caumul P. A study of the antibacterial activity of l-Phenylalanine and l-Tyrosine esters in relation to their CMCs and their interactions with 1,2-dipalmitoyl-sn-glycero-3-phosphocholine, DPPC as model membrane. Microbiol Res. 2014;169:675–85.
Joondan N, Caumul P, Akerman M, Jhaumeer-Laulloo S. Synthesis, micellisation and interaction of novel quaternary ammonium compounds derived from l-Phenylalanine with 1,2-dipalmitoyl-sn-glycero-3-phosphocholine as model membrane in relation to their antibacterial activity, and their selectivity over human red blood cells. Bioorg Chem. 2015;58:117–29.
Faustino CMC, Calado ART, Garcia-Rio L. Mixed micelle formation between amino-acid based surfactants and phospholipids. J Colloid Interface Sci. 2011;329:493–8.
Ohta A, Toda K, Morimoto Y, Asakawa T, Miyagishi S. Effect of the side chain of N-acyl amino acid surfactants on micelle formation: an isothermal titration calorimetry study. Colloid Surf A Physicochem Eng Asp. 2008;317:316–22.
Jadhav V, Maiti S, Dasgupta A, Das PK, Dias RS, Miguel MG, Lindman B. Effect of head group geometry of amino acid based cationic surfactants on interaction with plasmid DNA. Biomacromolecules. 2008;9:1852–9.
Dasgupta A, Das PK, Dias RS, Miguel MG, Lindman B, Jadhav VM, Gnanamani M, Maiti S. Effect of headgroup on DNA-cationic surfactant interactions. J Phys Chem B. 2007;111:8502–8.
Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–6.
Fait ME, Garrote GL, Clapes P, Tanco S, Lorenzo J, Morcelle SR. Biocatalytical synthesis, antimicrobial properties and toxicity studies of arginine derivative surfactants. Amino Acids. 2015;47:1465–77.
Li H, Yu C, Chen R, Li J, Li J. Novel inic liquid-type Gemini surfactants: synthesis, surface active property and antimicrobialo activity. Colloid Surf A Physicochem Eng Asp. 2012;395:116–24.
Yang Y, Yang X, Xue J, Curren R, Huang J, Tan X, Xie X, Xiong X, et al. Altex Proceedings, 1/12, Proceedings of WC8, 139–143
Holland PM, Rubingh DN. Mixed surfactant systems—an overview. ACS Symp Ser. 1992;501:2–30.
Ong CP, Ng CL, Lee HK, Li SFY. The use of mixed surfactants in micellar electrokinetic chromatography. Electrophoresis. 1994;15:1273–5.
Chen LG, Bermudez H. Charge screening between anionic and cationic surfactants in ionic liquids. Langmuir. 2013;29:2805–8.
Clint JH. Surfactant aggregation. New York: Chapman and Hall; 1992.
Bakshi MS, Sachar S, Mahajan N, Kaur I, Kaur G, Singh N, Sehgal P, Doe H. Mixed-micelle formation by strongly interacting surfactant binary mixtures: effect of head-group modification. Colloid Polym Sci. 2002;280:990–1000.
Silva BFB, Marques EF. Thermotropic behavior of asymmetric chain length catanionic surfactants: the influence of the polar head group. J Colloid Interface Sci. 2005;290:267–74.
Ghosh S, Ghatak C, Banerjee C, Mandal S, Kuchlyan J, Sarkar N. Spontaneous transition of micelle-vesicle-micelle in a mixture of cationic surfactants and anionic surfactant-like ionic liquid: a pure nonlipid small unilamellar vesicular template used for solvent and rotational relaxation study. Langmuir. 2013;29:10066–76.
Jing-Liang L, Bing-Hung C. Surfactant-mediated biodegradation of polycyclic aromatic hydrocarbons. Materials. 2009;2:76–94.
Lozano N, Perez L, Pons R, Luque-Ortega JR, Fernandez-Reyes M, Rivas L, Pinazo A. Interaction studies of diacyl glycerol arginine-based surfactants with DPPC and DMPC monolayers, relation with antimicrobial activity. Colloid Surf A Physicochem Eng Asp. 2008;319:196–203.
Pape WJW, Hopper U. Standardization of an in vitro red blood cell test for evaluating the acute cytotoxic potential of tensides. Drug Res. 1990;4:498–502.
Acknowledgements
One of the authors is thankful to Tertiary Education Commission (TEC) of Mauritius for the grant of a scholarship.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Joondan, N., Caumul, P. & Jhaumeer-Laulloo, S. Investigation of the physicochemical and biological properties of proline-based surfactants in single and mixed surfactant systems. J Surfact Deterg 20, 103–115 (2017). https://doi.org/10.1007/s11743-016-1895-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-016-1895-7