Abstract
Gas condensate reservoirs are generally recovered using a pressure depletion drive. Gas can condensate into the liquid phase near the wellbore region when the reservoir pressure falls below the dew point pressure, which can kill gas deliverability. Wettability alteration is an effective means of overcoming this problem; core wettability can be altered from liquid-wet to gas-wet to alleviate the effect of condensate accumulation near the wellbore region. To establish the effect of fluoropolymer on wettability alteration in a gas-condensate reservoir, a gas-wetting alteration agent was synthesized by emulsion polymerization using different molar ratios of fluorine-containing monomers and acrylic monomers. FTIR and SEM were performed to analyze the structure of the gas-wetting agent. Contact angle measurements were used to assess surface alteration by the Owens two-liquid method. The effects of alteration agent concentration, salt concentration, pH and temperature on gas-wetting alteration were also evaluated. Results showed that the best molar ratio of fluoropolymer monomer to acrylic monomers was 1:2. The egg-like structure of the fluoropolymer latex on the core surface mainly contributes to gas-wetting alteration. The contact angles of brine and oil can be altered from 23° and 0° to 137° and 67° by 1 wt% FP-2 treatment, respectively. The surface free energy of the core was reduced from 67.52 to 1.66 mN/m. Moreover, the treated cores remain gas-wetting up to 100 g L−1 of salt solution, 120 °C and within the pH range of 5–7. This novel gas-wetting alteration agent can be used to solve the problem of liquid blocking effects in gas condensate reservoirs and improve gas recovery significantly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Reservoir wettability is a vital condition that dominates the flow and distribution of fluids in the porous media. It also has a significant effect on both the oil recovery and the relative permeability of the liquid and gas phases [1, 2]. Wettability is one of the indispensable physical parameters to evaluate and analyze a reservoir, which can also be applied to reduce the capillary force and mobilize the trapped oil in rock pores [3]. Surfactants have been used to change the wettability of reservoir rock with the goal of increasing oil recovery; however, more than two-thirds of the original oil is in place in a reservoir after primary and secondary production [4, 5].
When the pressure near the wellbore region drops below the dew point in gas-condensate reservoirs, gas can condense in the liquid phase near the wellbore region, resulting in a serious decline of gas productivity. This is known as the liquid-blocking effect [6], which is one of most severe damages of gas-condensate reservoirs. In 2000, Li and Firoozabadi [7] proposed the gas-wetting approach in which the wettability of core was altered from strong liquid-wetting to neutral gas-wetting by fluorosurfactant treatment. Recent studies have shown that the flow condition of a liquid in porous media would be significantly improved due to wettability alteration [8–11]. The influence of fluorosurfactants on wettability alteration at high temperatures has also been examined. Wettability results show that wettability alteration has a substantial effect on improving gas productivity at high reservoir temperatures [12, 13]. Stanly and Firoozabadi [14] revealed that wettability of Berea and low permeability reservoir rocks can be permanently altered from liquid-wetting to intermediate gas-wetting. Wang and Jin [15] investigated the wettability alteration of cores by a fluoropolymer. Their results showed that the wettability of core could be altered into the intermediate gas-wetting condition. The contact angle of water and n-hexadecane on the core surface was increased from 23° and 0° to 96.8° and 82° after 0.3 wt% fluoropolymer solution treatment, respectively.
Feng and Kong [16] investigated the influence of the organosilicon-acrylic on the wettability of porous media, and their results showed clearly that the emulsion could alter the wettability from a water-wetting to an intermediate gas-wetting regime and enhance water permeability in porous media. Recently, it was found that the strong liquid-wetting of the rock around the wellbore was altered into a gas-wetting condition by fluorosurfactant treatment, and the permeability of core and gas deliverability can be significantly enhanced after the treatment.
However, the fluoropolymers used in the previous studies cannot alter the wettability of core into the preferential gas-wetting condition. The objective of this study is to synthesize a gas-wetting agent to improve gas deliverability by altering the wettability near the wellbore region from a liquid-wetting to a gas-wetting regime in gas-condensate reservoirs. Fluoropolymer in this paper was synthesized by emulsion polymerization. Contact angle measurement in the gas (liquid)–liquid core system was conducted to evaluate the gas-wetting alteration of the core surface. The Owens two-liquid method was applied to determine the surface free energy of cores with pre- and post-treatment. Imbibition was used to investigate the effect of wettability alteration on water saturation of the core. The effects of concentration, inorganic salts, pH, and temperature on the gas-wetting core were also studied.
Experimental
Materials
Dodecafluoroheptyl methacrylate (G06) was supplied by Harbin Xuejia Silicofluoride Chemical Reagent (China); azobisisobutyronitrile by Sinopharm; N,N-dimethylformamide (DMF), butyl acrylate (BA), and methacrylic acid (MAA) by Sinopharm; sodium dodecyl sulfonate (SDS) and NaHCO3 (chemical reagents) by Beijing Chemical Reagent (China); ethyl alcohol and ammonium persulfate (APS) (analytical reagents) by Sinopharm Chemical Reagent (China), and cores and brine were provided by SLOF (China). The distilled water used in this study was prepared in the laboratory.
Emulsion Copolymerization
The emulsion polymer was prepared according to the following procedures: 0.1 % SDS and 0.1 % NaHCO3 were added to 1 % DMF (50 ml) and mixed at high velocity for 30 min at 25 °C. The monomers mixture of BA, MAA, and G06 was added to the emulsifying mixture under the stirring condition. Then, the mixture was homogenized in a GS-3 Emulsion Shear Machine (Shanghai Hongsheng Mechanical and Electrical Technology) at 10-s intervals. The final emulsion was transferred to a 500-mL, four-necked, round-bottom flask fixed in an electric-heated thermostatic water bath. The flask was equipped with a reflux condenser, Teflon paddle stirrer, temperature sensor, and nitrogen gas inlet. Before the reaction started, the reactor was purged with nitrogen. Then, the APS dissolved in 10 mL water and 5 mL of the APS solution was added to the reactor when the temperature reached 65 °C, and another 5 mL was added to the reactor at 75 °C. The system was kept under the nitrogen atmosphere. The polymerization was carried out at 80 °C for 3 h to obtain a stable latex. The analysis and characterization of synthesized fluoropolymer were conducted by a Nexus Fourier transform infrared spectrograph (FITR) and Nova Nano Scanning electron microscope (SEM). The structures of the monomers and fluoropolymer can be seen in Fig. 1, in which m ranges from 15 to 20, and n and k range from 10 to 15. The difference between FP-1, FP-2 and FP-3 is the different molar ratio of BA, MAA, and G06, which are 1:1:1, 1:1:2, and 2:1:1, respectively. The latex particle size is approximately 50 nm, as shown in Fig. 5.
Contact Angle Measurements
Cores were polished by abrasive paper to obtain a horizontal smooth surface before treated by fluoropolymer solutions, and after aging for 24 h, the treated cores are dried at a temperature of 80 °C for 8 h. Fluoropolymer latex will preferentially absorb onto the core surface because of van der Waals force and electrostatic forces, forming a compact multi-adsorption layer on the core surface, with fewer adsorption sites left for DMF. Meanwhile, most of the DMF will evaporate at high temperature during long drying times. In any case, the amount of DMF in the dried polymer is negligible and can be neglected. The contact angles of brine and n-hexadecane on the core before and, after treatment in the gas–liquid-core system, were measured by a JC2000D Contact Angle Meter (Shanghai Zhongcheng Instruments) at 25 °C [17, 18]. Meanwhile, considering the liquid-blocking effect in the gas-condensate reservoir, contact angle measurement in the liquid–liquid core system was made using the arrangement shown in Fig. 2; the liquid used to immerse the core in the liquid–liquid–solid system was brine.
Owens Two-Liquid Method
There is an intimate relationship between the surface free energy and wettability of the core. To investigate the change of the wettability, the surface free energy of core surface before and after gas-wetting agent treatment were determined by the Owens two-liquid method [19–21]. The surface free energy is defined by the following equation:
where \( \gamma_{s} \) is the surface free energy of core surface, \( \gamma_{s}^{D} \) and \( \gamma_{s}^{P} \) are the dispersion and polar components of core surface energy, which can be determined from contact angles of two liquids against the solid. The two liquids used in this test were brine and n-hexadecane. If θ describes the contact angle of liquid on the core surface, the surface free energy can be calculated by the following equation:
If \( \theta_{1} \) is the contact angle of distilled water on the core surface, and \( \theta_{2} \) represents the contact angle of n-hexadecane, we have the following equations:
Surface free energy of core surface can be calculated by using Eqs. 3 and 4 as described in the next section.
Spontaneous imbibition tests
The schematic of the apparatus used in our study for the measurement of liquid spontaneous imbibition is shown in Fig. 3. The core sample was kept vertical. When the bottom of the rock touched the liquid surface, the liquid was spontaneously imbibed into the core. The balance then recorded the change in the weight of the liquid with the imbibition time. After the imbibition test, the core sample is weighed, and the total amount of liquid in the core can be obtained from the weights of the core before and after the measurements. The experimental imbibition data recorded by the balance is a function of imbibition time. The readability of the balance was 0.01 g.
Ionic Strength
The ionic strength of a solution is a measure of the concentration of ions in a solution, (first introduced by Lewis and Randall [22]) by the following equation:
where \( c_{i} \) is the molar concentration of ion i (M, mol L−1), \( z_{i} \) is the charge number of that ion, and the sum is taken over all ions in the solution. The ionic strength of the solutions used in this study is shown in Table 1.
Results and Discussion
Characterization of Fluoropolymer
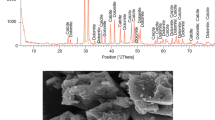
The characterizations of synthesized fluoropolymer were conducted with FTIR and SEM, and Fig. 4 illustrates the FTIR spectrum of the fluoropolymer. After copolymerization, the weak absorption at 3450 cm−1 corresponds to the hydroxyl group. The well-known absorption lines of long carbon alkane at 2930 and 1740 cm−1 correspond to the –CH3 and C=O functional groups, respectively. The absorption of the C=C bond disappears at 1680–1640 cm−1, confirming that the monomers used in the synthesis were fully reacted. The peak of the stretching C-F takes place at 1240 cm−1, which plays a vital role in gas-wetting alteration. The characteristic absorption by the O–C=O group at 1190 cm−1 appears, revealing that fluoropolymer has been successfully synthesized by the addition reaction; the molecular structure of fluoropolymer can be seen in Fig. 1. In the fingerprint region, the stretching vibration absorption of C–H in the long carbon alkane are at 980 and 675 cm−1, respectively.
Figure 5 presents a series of SEM images of core surface before and after fluoropolymer treatment. It can be clearly observed that the surface structure of the untreated core is mainly composed of a smooth surface, crevice, and irregular particles with sharp edges, as shown in Fig. 5a. Figure 5b exhibits a fluoropolymer adsorption layer with an egg-like structure coated on the core surface, which is rougher than the surface structure of the untreated core, increasing the surface roughness. Figure 5c shows the partially enlarged view of Fig. 5b, as enlarged to 500 nm, and it can be seen that the core surface is covered by the compact multi-adsorption layer, with neither crevice nor irregular particles exposed. Generally, the gas-wetting surface is based on two principles: a low surface energy of core surface and an increased surface roughness, and the egg-like microstructure formed by fluoropolymer adsorption can effectively alter the smooth surface to a rough surface, which contributes to the gas-wetting alteration on the core surface.
Contact Angle Measurement
The contact angle measurement in the gas–liquid–solid system was conducted to evaluate gas-wetting of the core. The results of the contact angle measurements are summarized in Table 2 and also visualized in Fig. 6. For the untreated cores, it was observed that the contact angles of water and hexadecane were 23° and 0°, respectively, and droplets of water and hexadecane spread immediately when placed on the core surface. Therefore, the wettability of untreated core is strong liquid-wetting. After treatment, the contact angles of water and hexadecane significantly increased, the shape of water droplets was nearly spherical, and hexadecane stayed on the treated core, while the contact angle of the water and hexadecane increased to 137° and 67°, respectively, after treatment with 1 % FP-2 solution.
These results may be explained by the change in the wettability of the core. The fluoropolymer is composed of polar and nonpolar segments. The polar segments of the fluoropolymer can absorb on the charged core surface by electrostatic attraction, and the nonpolar segments with the characteristics of hydrophobicity and oleophobicity are exposed on the surface. Therefore, the contact angles of water and hexadecane increase significantly because of the wettability alteration of the core surface, which results in a decrease in the contact area of the water and hexadecane.
To further illustrate the gas-wetting alteration for the liquid-blocking effect, the contact angles measurement in the liquid–liquid–solid system was performed and analyzed. The results can be seen in Fig. 7. For the untreated core, the contact angles of CH4 and hexadecane are 109° and 106°, respectively. After treatment with fluoropolymer solution, the results show that the contact angles of CH4 and hexadecane increase significantly, especially when treated with 1 % FP-2. The contact angles of CH4 and hexadecane increase to 152° and 151°, respectively. Moreover, the shape of the CH4 and hexadecane droplets was nearly spherical, which helps the flow of CH4 and hexadecane in the porous media. The contact angles measurement was conducted in the CH4 (hexadecane)–brine–solid system, both CH4 and hexadecane were injected into the brine solution by syringe. Methane is insoluble in brine and forms an inverted gas bubble on the core surface.
Imbibition
Figure 8 shows the gas recovery by spontaneous imbibition as a function of time. The imbibition tests were conducted with the cores before and after treatment. The water saturation and oil saturation were 72.6 and 76 %, respectively. The gas recovery of the untreated cores in water and oil increased with time, and oil was more pronounced than that of water, which tends to stabilize when the imbibition time reached approximately 2 h. After treatment with 1 wt% FP-2 solution for 24 h, the imbibition of the core to water was almost close to zero, and that to oil increased slowly at first and then stabilized at 45.7 % as time reached approximately 2.5 h. The possible explanation for this phenomenon is the wettability alteration of the core. It is believed that gas recovery of the core by spontaneous imbibition can decrease significantly if the wettability is altered from liquid-wet to gas-wet. Moreover, the liquid wetting is stronger, and the gas recovery by spontaneous imbibition is higher, and the degree of reduction in gas recovery to water is more significant than that in oil. The result of spontaneous imbibition is consistent with the contact angle measurements.
The Surface Free Energy of Cores Before and After Treatment
The Owens two-liquid method was used to determine the surface free energy of cores before and after treatment, as shown in Fig. 9. The surface free energy of untreated core was approximately 70 mN/m, which decreased sharply to 15 mN/m after FP solution treatment. As the concentration of FP solution reached 1 %, the surface free energy of cores reaches to a minimum. After exceeding 1 %, the surface free energy gradually increased to approximately 20mN m−1. Moreover, the surface free energy of core treated by FP-2 is the least, reaching 1.66 mN m−1. One possible explanation is that when the polar segments of the fluoropolymer absorb on the core surface by electrostatic attraction, the nonpolar segments of the fluoropolymer are exposed on the surface, resulting in a change in the core surface wettability. As FP molecules in solution fully adsorbed on the core surface, the wettability of the core changed to strong gas wetting. With increasing FP concentration, free molecules in the solution may intertwine with the polymer chain absorbed on the core surface so that the nonpolar segments may be blocked in the intertwined molecule chains. Therefore, the gas-wetting of the core could decrease when the concentration of FP solution exceeds 1 %.
The Effect of Inorganic Cation on the Gas-Wetting Core
In studying the effect of inorganic cations on the gas-wetting core, some inorganic salts were added into 1 % FP-2 solutions. The solution mixtures were composed of all inorganic salt and 1 % FP-2. As can be seen from Table 3, Na+, Mg2+, and Ca2+ have almost no effect on the gas-wetting of the core, and the contact angles of water and hexadecane are approximately 120° and 60°, respectively. However, Al3+ and Fe3+ have a significant effect on the gas-wetting of the core. The contact angle of water did not change, but the contact angle of hexadecane decreased from 60° to approximately 31°. The result illustrates that gas-wetting core can maintain good hydrophobicity in the presence of inorganic cations, and the oleophobicity of the gas-wetting core may become weak. A reasonable explanation for the phenomenon is that, as the size of fluorine atoms is much larger than that of the hydrogen atoms, and the resulting chain is no longer flat, the fluoropolymer is forced into a spiral structure with the fluorine atoms packed tightly around the central C–C bonds [23], which provides excellent protection for the fluoropolymer from Na+, Mg2+, and Ca2+. However, the ionic strengths of Fe3+ and Al3+ are obviously more than those of Na+, Mg2+, and Ca2+ at the equivalent concentration solution, as shown in Table 1, which means that more fluoropolymer can adsorb on the core surface in the presence of Fe3+ and Al3+, and the resulting hydrophobicity increases slightly. However, due to the similar structure between the fluoropolymer and hexadecane, an excess adsorption of the fluoropolymer on the core surface decreases the oleophobicity of the gas-wetting core [24, 25].
The Effect of pH on Gas-Wetting Core
The effect of pH on the gas-wetting core was also studied. FP solutions were prepared with pH ranging from 1 to 13 and used to treat the cores. It can be seen from Fig. 10, the wettability of core can maintain neutral gas wetting at pH ranges from 5 to 7. Strong acid and base conditions evidently have a negative effect on the gas-wetting of the core. When the pH of FP solution was less than 5, the contact angles of the water and hexadecane decreased from 120° and 64° to 94° and 28°, respectively. When the pH of the FP solution was higher than 7, the contact angle of the water decreased sharply to approximately 60°. Therefore, when the pH of FP solution ranges from 5 to 7, the core can remain as neutral gas-wetting. The spiral structure of the fluoropolymer can protect it from acid or base in a certain range, then the gas-wetting of the core can remain stable. When the pH of the solution exceeds the optimal range, the spiral structure of the fluoropolymer becomes loose, showing that gas-wetting decreases under the strong acid or base condition.
The Effect of Temperature on the Gas-Wetting Core
Figure 11 illustrates the effect of temperature on the gas-wetting cores. The cores were aged with 1 % of FP-2 for 24 h and dried at temperatures of 20, 40, 60, 80, 100 and 120 °C for 4 h, respectively. When the treatment temperature reached 120 °C, the wettability of the core remains gas-wetting. The contact angles of the brine and hexadecane changed insignificantly because the gas-wetting alteration agent has excellent temperature resistance. Since the C–F bond of the fluoropolymer plays a major role in the gas-wetting alteration, it appears that these functional groups are more exposed at high temperatures. If anything, the gas-wettability of the core surface gradually becomes stronger as the temperature increases.
The Mechanism of Gas-Wetting Alteration
Figure 12 displays the interaction between the fluoropolymer and the core surface. The fluoropolymer latex with an egg-like structure is surrounded by SDS molecules. There are three stages in the process of the gas-wetting alteration. In the first stage, when the fluoropolymer concentration is low, the core surface is partly covered by a few latex particles due to the van der Waals force and the electrostatic forces and the core remains liquid-wet [26–28]. In the second stage, more latex particles absorb until the core surface is fully covered, creating a tight gas-wetting adsorption layer. In general, the adsorption process on the core surface is faster than the desorption process. In stage 3, fluoropolymer latex particles randomly aggregate into egg-like multilayers, as shown in Fig. 5. The gas-wetting efficiency in this last stage is less efficient than in stage 2.
Conclusions
In summary, a gas-wetting alteration agent was synthesized by an emulsion copolymerization method. FTIR and SEM were employed to characterize the gas-wetting agent. The egg-like adsorption layer of the gas-wetting agent on the core surface plays a vital role in gas-wetting alteration. The contact angle measurement was conducted to evaluate the wettability of the core before and after treatment. The results show that the wettability of cores can be altered from a liquid-wetting to a gas-wetting regime using 1 wt% FP-2 treatment. The surface free energy of cores of the core surface decreased sharply from 70.01 to 1.66 Mn m−1 as the core wettability was altered from preferential liquid-wetting to gas-wetting. Also, the gas-wetting alteration agent can be applied under the condition of high temperature and high salinity without the loss of gas-wetting efficiency.
References
Danesh A, Henderson GD, Peden JM (1991) Experimental investigation of critical condensate saturation and its dependence on interstitial water saturation in water-wet rocks. SPE Reservoir Eng 6:336–342
Afidick D, Kaczorowski NJ, Bette S (1994). Production performance of a retrograde gas reservoir: a case study of the Arun Field. SPE 28749, SPE Asia Pacific Oil and Gas Conference, Melbourne, Australia, 7–10 November
Jin LC, Mahesh B (2016) Predicting microemulsion phase behavior for surfactant flooding. SPE 179701, SPE Improved Oil Recovery Conference, Tulsa, Oklahoma, 11–13 April
Delshad M, Najafabadi NF, Anderson GA (2006) Modeling wettability alteration in naturally fractured reservoirs. SPE 100081, SPE/DOE Symposium on Improved Oil Recovery, Tulsa, Oklahoma, 22–26 April
Najafabadi NF, Delshad M, Sepehrnoori K (2008) Chemical flooding of fractured carbonates using wettability modifiers. SPE 113369, SPE Symposium on Improved Oil Recovery, Tulsa, Oklahoma, 20-23 April
El-Banbi AH, McCain WD (2000) Investigation of well productivity in gas-condensate reservoirs. SPE 59773, SPE/CERI Gas Technology Symposium, Calgary Alberta, Canada, 3–5 April
Li K, Firoozabadi A (2000) Experimental Study of wettability alteration to preferential gas-wetting in porous media and its effects. SPE Reservoir Eval Eng 3:139–147
Barnum RS, Brinkman FP, Richardson TW (1995) Gas condensate reservoir behaviour: productivity and recovery reduction due to condensation. SPE 30767, SPE Annual Technical Conference and Exhibition, Dallas, Texas, 22–25 October
Tadros TF (1980) Thermodynamics of micellization of fluorocarbon surfactants. J Colloid Interf Sci 74:196–200
Li K, Firoozabadi A (2000) Phenomenological modeling of critical condensate saturation and relative permeabilities in gas/condensate systems. SPE J 5:1–10
Munkerud PK, Torsaeter O (1995) The effects of interfacial tension and spreading on relative permeability in gas condensate systems, European Symposium on Improved Oil Recovery, Vienna, Austria, 15–17 May
Fahes MM, Firoozabadi A (2007) Wettability alteration to intermediate gas-wetting in gas-condensate reservoirs at high temperatures. SPE J 12:397–407
Noh MH, Firoozabadi A (2008) Wettability alteration in gas-condensate reservoirs to mitigate well deliverability loss by water blocking. SPE Reservoir Eval Eng 11:676–685
Wu S, Firoozabadi A (2010) Permanent alteration of porous media wettability from liquid-wetting to intermediate gas-wetting. Transport Porous Med 85:189–213
Wang YL, Jin JF, Ma L (2015) Influence of wettability alteration to preferential gas-wetting on displacement efficiency at elevated temperatures. J Disper Sci Technol 36:1274–1281
Feng CY, Kong Y, Jiang GC (2012) Effect of organosilicon-acrylic emulsion treatment on wettability of porous media. Transport Porous Med 92:619–631
Tang GQ, Firoozabadi A (2000) Relative permeability modification in gas-liquid systems through wettability alteration to intermediate gas-wetting. SPE 81195, SPE Annual Technical Conference and Exhibition, Dallas, Texas, 1–4 October
Li Y, Jiang G, Xu W (2015) The effects of gas-wetting on the electrical properties of condensate gas reservoir cores. Energy Sources Part A 37:1766–1773
Wu S, Firoozabadi A (2009) Effect of salinity on wettability alteration of porous media from liquid wetting to intermediate gas wetting. J Petrol Technol 61:121–129
Kwok DY, Neumann AW (1999) Contact angle measurement and contact angle interpretation. Adv Colloid Interface 81:167–249
Jiang G, Li Y, Zhang M (2013) Evaluation of gas wettability and its effects on fluid distribution and fluid flow in porous media. Petrol Sci 10:515–527
Lewis GN, Randall M (1921) The activity coefficient of strong electrolytes. J Am Chem Soc 43:1112–1154
Kulkarni PV, Chapkhhane NK (2012) Development and testing of PTFE based composite bearing material for turbine pump. Int J Eng Adv Technol 1:15–20
Jin LC, Jamili A, Li Z (2015) Physics based HLD–NAC phase behavior model for surfactant/crude oil/brine systems. J Petrol Sci Eng 136:68–77
Jin LC, Jamilia A (2015) Modeling and interpretation of single well chemical tracer tests (SWCTT) for pre and post chemical EOR in two high salinity reservoirs. SPE 173618, SPE Production and Operations Symposium, Oklahoma City, Oklahoma, 1–5 March
Xie X, Liu Y, Sharma M (2009) Wettability alteration to increase deliverability of gas production wells. J Nat Gas Sci Eng 1:39–45
Huang PY, Chao YC, Liao YT (2004) Preparation of fluoroacrylate nanocopolymer by miniemulsion polymerization used in textile finishing. J Appl Polym Sci 94:1466–1472
Su C, Guo P, Li SL (2002) Investigation of the microvisual flow and relative permeability law of condensate oil and gas. Nat Gas Ind 22:61–64
Acknowledgments
The authors wish to thank the financial support and technical assistance from the Natural Foundation for Outstanding Youth of China (50925414), Fundamental Research Funds for the Central Universities (15CX06034A), China Scholarship Council (CSC) and the National Key Basic Research Program of China (2015CB250904), and I am also grateful to Dr. Bogdan Donose for fruitful discussions. All the funding agencies had no conflicts of interest in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
About this article
Cite this article
Jin, J., Wang, Y., Ren, J. et al. The Effect of Fluoropolymer on Wettability Alteration of Sandstone at Elevated Temperatures. J Surfact Deterg 19, 1241–1250 (2016). https://doi.org/10.1007/s11743-016-1866-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-016-1866-z