Abstract
The interaction between an ethoxylated alkylphenol polymer with formaldehyde (EAPPF) and a triblock polyEO–polyPO–polyEO copolymer (TBCP) in aqueous solutions has been investigated in detail by means of surface tension, steady-state fluorescence, dynamic light scattering (DLS) measurements and computer simulations. For comparison, the ethoxylated alkylphenol oligomer (EAPO), which is the monomer unit of EAPPF, and another nonionic water-soluble polymer PEG were also selected to get more information about the interaction between different types of surfactants and polymers. The surface properties of mixed systems at the air/water surface were evaluated from surface tension measurements. Information about the hydrophobic microenvironment and size of the aggregates was obtained from steady-state fluorescence using pyrene as a hydrophobic probe and DLS measurements, respectively. The dissipative particle dynamics simulation method was applied to simulate the interaction between EAPPF (or EAPO) and TBCP (or PEG) in aqueous solutions. The synergetic interaction between EAPPF (or EAPO) and TBCP in binary mixed solution enhanced the adsorption of surfactant molecules (EAPPF or EAPO) at the interface, while no obvious interaction between EAPPF (or EAPO) and PEG was observed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mixtures of surfactant and polymer have attracted wide attention for several decades both in practical applications and theoretical studies such as paints and coatings, detergents, cosmetics, and pharmaceuticals due to their improved performance in surface activity, wetting, adsorption, solubilization, emulsification, suspension, dispersion, and so forth, superior to that of single substances [1–3]. Mixed systems of conventional surfactants have been studied extensively to develop better functions or to make clear the interaction mechanism between them [4, 5]. Most of these studies have been focused on ionic surfactants and nonionic polymers where the interaction is generally described as a cooperative process. Among the polymers used, water soluble poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) triblock copolymers (PEO–PPO–PEO, commercially available in different proportions) are frequently selected [6]. These polymers have been widely used in detergency, pharmaceutics, and bioprocess because of their low toxicity and high biocompatibility. They are commercially available in a variety of molecular weights and PEO/PPO ratios and have high surface activities. The micelles of these copolymers in aqueous solution consist of a core containing the PO groups and an outer region containing the hydrated EO groups. In recent years, much attention has been focused on the interactions between triblock copolymer and surfactant [7, 8]. One of the fundamental requirements in understanding the behavior of these mixed systems is the knowledge of the interaction mechanism and binding mode, which are critical for solution properties and subsequent applications. The observed interaction modes, such as string-of-beads and coral-shape usually in the system of water soluble polymer and surfactant [9], may probably exist in the system of PEO–PPO–PEO copolymer and surfactant. We have studied the interaction of a star-like block polyether and linear PEO–PPO–PEO with sodium oleate (C17H33COONa), and the results show different interaction mechanisms between star-like block polyether/C17H33COONa and linear PEO–PPO–PEO/C17H33COONa [10]. It is recognized that the nonionic surfactant/polymer systems possess many properties superior to those of ionic ones, such as higher stability, better biological compatibility and lower toxicity, and they have been widely used in material synthesis and biology simulation [11].
In recent years, a new class of surfactants which can be broadly referred to as surfactant oligomers has attracted increasing interest [12, 13]. Surfactant oligomers are made up of two or more amphiphilic moieties connected at the level of, or very close to, the head groups by a spacer group, such as dimeric (Gemini), trimeric and tetrameric, etc. They have many intriguing physicochemical properties such as much lower CMC values and stronger surface tension lowering ability compared to that of corresponding conventional surfactants. These predominant properties have been mostly demonstrated for dimeric cationic [14, 15] and anionic [16, 17] surfactants. An ethoxylated alkylphenol polymer with formaldehyde (EAPPF) could be regarded as a polydisperse nonionic oligomeric surfactant, which was considered as an ethoxylated alkylphenol oligomer (EAPO), with a degree of polymerization below 7 and a single methylene group connecting two amphiphilic moieties at the level of the phenylene ring [18–20]. Studies on the combination of oligomeric surfactants are performed with the hope of observing synergism that would make the use of oligomeric surfactants in formulations more attractive. Recently, we have studied surface tension and dilational viscoelasticity properties of surfactants EAPPF (or EAPO) with cetyl trimethylammonium bromide (CTAB) at 25 °C [21]. The results show that there is synergistic behavior to enhance the adsorption of surfactant molecules at the interface in both the mixtures at higher mole fractions of nonionic surfactant. However, few studies on surfactant oligomers have been reported and relatively few studies concerning the interaction between nonionic oligomers and nonionic water-soluble polymer have been reported. Our another recent results on the interaction between an ethoxy-modified trisiloxane and triblock polyEO–polyPO–polyEO copolymer (TBCP) show that the surface activity and aggregation behavior of an ethoxy-modified trisiloxane are affected by TBCP in aqueous solutions and the hydrophobic association and twist of the TBCP coil contribute to the formation of different aggregates of an ethoxy-modified trisiloxane and TBCP [22, 23].
Thus, in view of our current interest in surfactant oligomers of EAPPF and surfactant-polymer interactions, we decided to investigate the interaction between EAPPF and TBCP in aqueous solutions and to compare this with its monomer, EAPO. Moreover, we also selected another nonionic water-soluble polymer PEG to compare with TBCP. For this purpose, the surface tension, steady-state fluorescence, dynamic light scattering (DLS) measurements and computer simulation were explored to investigate the interaction between them. The results reveal important differences between the properties of different mixed systems.
Experimental Section
Materials
The EAPO brand name is Triton X-100 and the EAPPF is Tyloxapol, a copolymer of the previous oligomer with formaldehyde, with 7 oligomer units. The triblock TBCP trade name is Pluronics F127 with an average composition EO97PO69EO97. All samples were purchased from Sigma Chemicals and were used without further purification. Polyethylene glycol (PEG, M W = 10,000) was purchased from Shanghai Chemicals and used as received. The water used in the experiments was triply distilled by a quartz water purification system. The formulas of these materials are shown in Fig. 1.
Methods
Surface Tension Measurements
Surface tension measurements of aqueous solutions of single and mixed surfactants at various concentrations were carried out on the K12 processor tensiometer (Krüss, Germany) with the ring device. The value of surface tension was the average of readings from three separate measurements.
Surface Dilational Viscoelasticity Measurements
The interfacial dilational viscoelasticity meter JMP2000A (Powereach Ltd., Shanghai, China) was used to measure the parameters of dilational viscoelasticity of systems with various components. The construction of the JMP2000A apparatus and the method of measurement have been described previously [24].
Steady-State Fluorescence Measurements
Steady-state fluorescence measurements were performed on a Perkin Elmer LS-50 fluorescence spectrophotometer with an excitation and emission slit openings of 10 and 2.5 nm, respectively. Each sample solution containing pyrene was excited at a wavelength of 335 nm and the emission spectrum between 350 and 550 nm was recorded. The intensity ratio of the first and third vibronic peaks (I 1/I 3) in the emission spectrum of pyrene was then obtained and analyzed.
Dynamic Light Scattering (DLS) Measurements
DLS measurements were carried out on a Brookhaven Laser Light Scattering spectrometer at a scattering angle of 90°. The light source is a power adjustable argon ion laser with a maximum power of 100 mW and a wavelength of 488 nm, respectively. All samples were filtered carefully with 0.45-μm filters before characterization.
All experiments were performed at (25.0 ± 0.1) °C.
Dissipative Particle Dynamics (DPD) Simulation
The DPD simulation method was applied to simulate the interaction between EAPPF (or EAPO) and TBCP (or PEG) in aqueous solutions.
In the DPD simulation, the beads are needed with the same volumes, therefore, we select the EAPO hydrophobic tail (T) for reference and the number of monomers contained in other beads can be determined by calculating the fraction of the volume of monomer to it [25, 26]. Thus, PPO, PEO, and water were represented by DPD beads P, E, and W as shown in Fig. 2. Through the calculation, each monomer volume and correspondingly each bead containing numbers of monomer are shown in Table 1. One bead E represented four ethylene oxide molecules, one bead P represented three propylene oxide molecules, and one bead W represented nine water molecules for convenience in Blend module of Cerius 2.
The spring constant was chosen as 4 according to [24]. In the simulation, a 3D box of size 10 × 10 × 10 with periodic boundary conditions was adopted. The dissipative parameter γ was set to a value of 4.5 kT. For each system, 20,000 time steps per simulation were carried out.
Through DPD simulation, the solubility parameters δ of monomers were obtained (Table 1) and then according to the formula (1), the interaction parameter between the beads (Flory–Huggins parameter χ) was determined.
According to the formula (2), the relationship between simulation parameters of DPD (α) and χ is:
The values for the interaction parameters between various beads are given in Table 1.
Take the diffusion of beads of EAPPF with the simulation steps increasing at X EAPPF = 0.4 for example; the results show that equilibrium was reached before 6,000 time steps, so 10,000 time steps per simulation was sufficient for the simulation. It also can be concluded from the result that the diffusibility of bead E was better than that of bead T (Table 2).
Results and Discussion
Aggregation of EAPPF/TBCP at the Air/Water Surface
Surface Activities of Different Mixed Systems
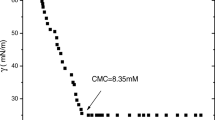
Surface tension measurement is a traditional and simple method of studying the interaction between polymer and surfactant [27, 28]. Figure 3a gives the variation of surface tension (γ) with EAPPF or TBCP concentration. For single EAPPF or TBCP solutions, the surface tension shows a substantial decrease even at very low concentrations, reflecting the high surface activity. A first break is observed in the surface tension curve at a characteristic concentration, after which the surface tension values continued to decrease until a second break was reached. After that, the surface tension remained constant with further increase of EAPPF or TBCP concentration, indicating the formation of EAPPF or TBCP micelles; it is reported that the structure of TBCP micelle was well described by the core-corona model, in which a spherical core composed of PPO is surrounded by a corona composed of Gaussian chains of strongly hydrated PEO [9]. The reason that surface tension curve of EAPPF or TBCP has two breaks may be mainly ascribed to the following two points: (1) the molecular weight of EAPPF or TBCP is not a single value, but a distribution range, (2) in addition to adsorb at the air/water interface, EAPPF or TBCP molecule may also undergo structural evolvement or the formation of unimolecular micelles or oligomers before CMC [29, 30]. In other words, the concentration corresponded to first break point is not the “real” CMC value. Therefore, the second break in surface tension curve corresponds to the CMC of EAPPF or TBCP, which is 2.79 × 10−5 mol L−1 for EAPPF and 1.29 × 10−4 mol L−1 (1,625 mg L−1) for TBCP, respectively. And the surface tensions of EAPPF and TBCP at CMC (γCMC) are 36.5 and 37.1 mN m−1, respectively. That is, both the efficiency and effectiveness of reducing the surface tension of EAPPF is higher than that of TBCP.
The surface tension isotherms of EAPPF/TBCP mixed systems are also shown in Fig. 3a. At lower concentrations of single TBCP solution, TBCP unimers are the main state in solution, the relatively hydrophobic block PPO of TBCP usually winds to prevent direct contact with water. When a small amount of TBCP is initially added to EAPPF solution, the random coil of TBCP unimer swells [31], and the stretched polymer chains partly insert into the hydrophilic layer of EAPPF micelles or wrap around them forming a TBCP/EAPPF complex. That is, the hydrophobic interaction between the PO blocks and EAPPF micelles drives TBCP unimers to aggregate on EAPPF micelles forming a TBCP/EAPPF complex. At higher concentration of TBCP, TBCP exists predominantly in the form of micelles. Thus, in EAPPF/TBCP mixed systems, when the concentration of EAPPF is low, small amounts of EAPPF may penetrate into the TBCP micelles forming rich-in-TBCP complex. The penetration of EAPPF may possibly extract some water from the hydrophobic core, because it is composed of a PO block and contains a certain amount of water [32]. Thus, the unshielding effect of water on PO protons is weakened. When the concentration of EAPPF reached a certain excessive amount, TBCP aggregations start to disassociate because of the increased EAPPF “spacer effect” [33], and the structure of complex transfers from rich-in-TBCP to rich-in-EAPPF. It should be noted that, the breakup of the complex composed of copolymer and ionic surfactant has also been observed by Jansson et al. [33] and it is mainly attributed to the electrostatic repulsion between copolymer micelles and ionic surfactant. And the “spacer effect” of surfactant plays an important role in the breakdown of copolymer micelles in the system of nonionic copolymer and nonionic surfactant.
From the above results, it can be seen that any difference in surface tension of EAPPF between TBCP-free and TBCP-containing systems can only be ascribed to surfactant-polymer interactions. After addition of TBCP, the EAPPF/TBCP mixed micelles are formed and there are still two transition points appearing in EAPPF/TBCP mixed systems and the surface tension isotherms of mixed systems are below the individual component at any concentration of TBCP in our experiment range. It is indicated that there is synergetic interaction between nonionic surfactant EAPPF and TBCP which enhances the adsorption of surfactant molecules (EAPPF or EAPO) at the interface; probably the hydrophobic parts of EAPPF interact with the PPO parts of TBCP to form hydrophobic cores of mixed micelles and the PEO parts of EAPPF and TBCP interact with each other to form the hydrophilic layers of mixed micelles.
Figure 3b shows the surface tension isotherms of EAPPF/PEG mixed systems. The surface tension of the aqueous PEG solution remains as 62 mN m−1, indicating that PEG has low surface activity due to the absence of PPO blocks compared to TBCP. When PEG was added into the EAPPF solution, the surface tension isotherms of mixed systems are between the single surface tension isotherms of EAPPF and PEG at any concentration of PEG in our experiment range. It is reported [9] that the presence of PEG leads to the separation of donor and acceptor in the EAPPF micelle and some of the PEG segments penetrate into EAPPF micelles making the microstructure of PEG-bound EAPPF aggregates looser than that of free micelles; this is the main factor for the microstructure change of EAPPF micelles. Besides, PEG would be wrapped around the EAPPF micelles and interact with numbers of them and form coral-shaped clusters.
Because EAPPF is an oligomer of the EAPO, in order to compare the different properties between EAPPF and EAPO, we also studied the interaction between EAPO and TBCP (or PEG), as shown in Fig. 4a, b. For single EAPO, there is only one break in the surface tension curve corresponding to the CMC of EAPO which is 2.40 × 10−4 mol L−1; this value is even larger than seven times the CMC of EAPPF (2.79 × 10−5 × 7 = 1.95 × 10−4 mol L−1), indicating that the surface activity of EAPPF is much higher than that of EAPO because of its oligomeric character.
In the presence of 10 mg L−1 TBCP, when the EAPO concentration is below 1.7 × 10−5 mol L−1, the surface tension remains constant at 48.3 mN m−1, this value corresponds to that of the pure 10 mg L−1 TBCP solution. When the EAPO concentration is between 1.7 × 10−5 and 1.5 × 10−4 mol L−1, the surface tension of EAPO/TBCP mixed solution decreases gently, but it is still lower than that of single EAPO, indicating that the formation of a TBCP and EAPO mixed layer. As EAPO concentration is increased to around 2.0 × 10−4 mol L−1, the two curves of EAPO and EAPO/TBCP converge. The surface tension of the mixed solutions coincides with that of single EAPO, which suggests that the surface is completely dominated by the EAPO molecules. In this region, free EAPO micelles may coexist with larger EAPO/TBCP complexes.
For EAPO/PEG mixtures, when the EAPO is below 2.6 × 10−6 mol L−1, the surface tension of EAPO in the presence of 10 mg L−1 PEG remains constant at 62 mN m−1 (Fig. 4b), which corresponds to that of the single PEG solution. This means that PEG molecules adsorb at the surface together with small amount of EAPO molecules making the surface tension low. With increasing EAPO concentration in bulk solution, more EAPO molecules adsorb at the surface so the surface tension value is close to that of single EAPO and most of PEG molecules are displaced from the surface. It can be seen from the surface tension isotherm that the addition of both TBCP and PEG have no obvious effect on the CMC of EAPO in the investigated concentration range.
Surface Dilational Viscoelasticity
The measurement of dilational rheology provides important information on the properties of adsorption layers and the study of the dilational viscoelastic behavior of air/liquid interfaces is also of fundamental importance for understanding relaxation mechanisms [34]. In order to investigate further the interaction between surfactants (EAPPF or EAPO) and polymers (TBCP or PEG) at the air/water surface, the surface dilational viscoelasticity of the system was measured. The dynamic surface dilational moduli of single EAPPF and EAPO are shown in Fig. 5a, b. It can be seen that the dilational moduli of both EAPPF and EAPO pass through the maximum values with increasing concentration. The maximum values of the dilational modulus occur at c EAPPF = 1 × 10−6 mol L−1 and c EAPO = 1.2 × 10−6 mol L−1 when the time is at 8,000 s, respectively (from the inset of Fig. 5). Comparing Fig. 5a with b, in EAPO system, the dilational modulus (13 mN m−1) is found to be very low in comparison with EAPPF system (50 mN m−1), suggesting that the strength against perturbation of surface layer of EAPPF is higher than that of EAPO.
The dilational viscoelasticities of EAPPF/TBCP, EAPPF/PEG, EAPO/TBCP and EAPO/PEG solutions are plotted as a function of EAPPF or EAPO concentration at 8,000 s in Fig. 6. Both of the concentrations of TBCP and PEG are fixed at 10 mg L−1. It can be seen that all of the dilational moduli of three systems pass through the maximum values at c EAPPF = 1 × 10−6 mol L−1 and c EAPO = 1.2 × 10−6 mol L−1, and then decrease with increasing concentration of EAPPF or EAPO. Moreover, the addition of TBCP in the mixed systems makes the maximum value of the dilational modulus of EAPPF/TBCP system lower and that of the EAPO/TBCP system higher, but the addition of PEG in the mixed systems has no obvious effect on the maximum value of the dilational modulus, demonstrating that TBCP has a higher mole fraction in the mixed monolayer compared with PEG which nearly has no surface activity.
It is also worth noting that the maximum values of the dilational modulus of EAPPF/TBCP and EAPPF/PEG mixtures are always higher than that of EAPO/TBCP and EAPO/PEG mixtures. This could be explained according to the mole fractions of nonionic surfactant in the mixed monolayer. The results suggest that the mixed monolayer is mainly dominated by the nonionic surfactant (EAPPF and EAPO).
Aggregation of the EAPPF/TBCP System in Aqueous Solutions
Micropolarity of Micelle
The ratio of the first vibrational peak located at 373 nm to the third one at 384 nm (I 1/I 3) in the fluorescence spectrum of pyrene is very sensitive to the polarity of the microenvironment [35, 36]. It is well-established that in aqueous solutions of amphiphilic polymers, pyrene is solubilized into hydrophobic domains, showing a decrease in I 1/I 3. Thus, pyrene is a useful fluorescence probe for the characterization of molecular assemblies of associating polymers, and for the evaluation of the micropolarity of the local environment [37].
Figure 7a, b shows the change of I 1/I 3 as a function of EAPPF or EAPO concentrations for EAPPF/TBCP and EAPPF/PEG mixed aqueous solutions, respectively. It can be seen that when the concentration of EAPPF is low, at the lower TBCP (10 and 100 mg L−1) or all of PEG concentrations, I 1/I 3 is around 1.67, which is practically the same as that for pyrene in water. As the EAPPF concentration is increased, I 1/I 3 begins to decrease significantly at a certain EAPPF concentration. For EAPPF/TBCP system, It can be seen that I 1/I 3 of the mixed micelles is lower than that of single EAPPF or TBCP solutions. And I 1/I 3 continues to decrease with the increase of TBCP concentration added to the system, indicating a decrease in the polarity of the microenvironment at the site of pyrene molecules. Because both EAPPF and TBCP have high surface active and can form micelles in aqueous solution, the TBCP molecules can be incorporated into EAPPF micelles, leading to the formation of more closely-packed EAPPF/TBCP mixed micelles.
However, for an EAPPF/PEG system, the addition of PEG to EAPPF solution makes I 1/I 3 always higher than that of single EAPPF solution and I 1/I 3 continues to increase with the increase in PEG concentration added to the system, indicating the increase in the hydrophobic microenvironment. This is because PEG is a hydrophilic polymer and it cannot form micelles, when EAPPF and PEG are mixed together, maybe only small amounts of PEG molecules insert themselves into the hydrophilic region of EAPPF micelles and form EAPPF/PEG mixed micelles, making the EAPPF micelles looser and also leading to the formation of less closely-packed EAPPF/PEG mixed micelles. Most of other PEG molecules adsorb on the surfaces to wrap around EAPPF micelles and play the role of connection.
Compared with EAPPF/TBCP and EAPPF/PEG mixed systems, it can be seen that because of different molecular structures and properties of TBCP and PEG, they have different effects on EAPPF solution. TBCP can make the mixed system form more closely-packed EAPPF/TBCP mixed micelles while PEG can make it looser.
The Hydrodynamic Radii of Aggregates
DLS is a powerful technique for characterizing nanoscale particles. The hydrodynamic radii (R h) of the aggregates were obtained from DLS measurements and are shown in Fig. 8. Considering the CMC of EAPPF and EAPO, 2 × 10−4 mol L−1 EAPPF and 2 × 10−3 mol L−1 EAPO were selected, respectively. It can be seen that only one type of aggregate exists in EAPPF and EAPO solutions, whose R h is 3.38 and 3.39 nm, respectively. These results are consistent with the size of EAPPF and EAPO micelles obtained from Cryo-TEM as reported by Regev and Zana [19].
The concentration dependences of R h distribution in the TBCP solution are shown in Fig. 8a. Only one main peak (R h = 1.74 nm) was observed at c TBCP = 10 mg/L, which was assigned to TBCP free chains in the solution. With an increase in TBCP concentration from c TBCP = 10 mg L−1 to c TBCP = 100 mg L−1, there is also only one main peak but a little larger R h (R h = 3.11 nm) of TBCP free chains in the solution. Interestingly, with further increases in the sample concentration (up to c TBCP = 104 mg L−1), two main peaks appeared in the solution. These two size distributions are probably related to the free chains along with the triblock copolymer micelles in solution since above the CMC, the micelles are in equilibrium with its unimers. That is, the smaller one was assigned to TBCP free chains (R h = 3.15 nm), whereas the larger peak should be attributed to single TBCP micelles (R h = 7.97 nm) because c TBCP is higher than its CMC (1,625 mg L−1). When the concentration of TBCP reached to 5 × 104 mg L−1, the smaller R h value becomes 2.69 nm and the larger R h value becomes 17.1 nm, respectively. The smaller one was still assigned to TBCP free chains and the larger R h values indicated the TBCP micelles were able to associate when they were close enough to each other. The results suggested that the TBCP conformation in the solution can be altered by changing concentrations, and a higher concentration led to aggregations as a result of the association of the TBCP micelles. But for the PEG solution (Fig. 8b), when the concentration of PEG was 10 mg L−1, no peak was observed. When the concentrations of PEG were increased to 100, 104 and 5 × 104 mg L−1, only one main peak (R h = 3 nm) was observed, indicating that there were only random coils in the PEG solutions (comparable to the same size of the TBCP free chains).
When TBCP (c TBCP = 10 mg L−1 or c TBCP = 100 mg L−1) were added to the 2 × 10−4 mol L−1 EAPPF solutions (R h = 3.41 and 3.37 nm), it can be seen that no obvious shift of the peak appeared, suggesting that the main aggregates were EAPPF micelles, TBCP free chains and EAPPF/TBCP aggregates and the interaction of EAPPF and TBCP did not change the conformation and morphology of mixed micelles greatly. When more TBCP was added (c TBCP = 104 mg L−1), a shift of the peak to higher R h values appeared (R h = 7.58 nm), indicating that larger sizes of aggregates appeared. This can be attributed to EAPPF micelles, TBCP micelles and EAPPF/TBCP aggregates coexist in the solutions. With further increasing of TBCP concentration (c TBCP = 5 × 104 mg L−1), two main peaks appeared in the solution. The smaller one was assigned to TBCP free chains or EAPPF micelle (R h = 2.72 nm), whereas the larger peak should be attributed to the aggregates of TBCP micelles or EAPPF/TBCP aggregates. Compared with the concentration of 104 mg L−1 TBCP, the R h became larger (from 7.58 to 18.9 nm) and are the main component in the solutions, these results suggested that more TBCP molecules were incorporated into EAPPF micelles, leading to the formation of more closely-packed EAPPF/TBCP mixed micelles and more TBCP micelles aggregated together. Great changes of R h values when the concentration of TBCP was above 104 mg L−1 compared with 10 and 100 mg L−1 TBCP for EAPPF/TBCP system obtained from DLS results are consistent with the surface tension and steady-state fluorescence measurements.
When EAPPF was changed to EAPO, nearly the same phenomenon occurred in the EAPO/TBCP mixed system (Fig. 8c). When TBCP was changed to PEG, only when the concentration of PEG reached 5 × 104 mg L−1, did two main peaks appear in the solution, the smaller one was single EAPO micelle and random coils of PEG (R h = 2.63 nm), whereas the larger peak should be the EAPO/PEG aggregates (R h = 18.8 nm) and the larger aggregates were the main components in the solutions. However, in the EAPO/PEG system (Fig. 8d), although there were some larger sizes of aggregates existing, the smaller aggregates were the main components of the solutions. The detailed information of R h in all the systems we observed is shown in Table 3.
The Morphology of Aggregates
Besides experimental techniques, computer simulation has also been extensively utilized to study surfactant/polymer mixtures in recent years and is able to provide more microcosmic information. The DPD simulation technique can be used to investigate systems that contain millions of atoms. The parameters used for carrying out DPD simulation can be obtained from Flory–Huggins theory [38]. The elementary units in DPD method are soft beads. A bead contains at least several molecules or molecular groups, but is still macroscopically small. In our previous works, we had investigated aggregation behavior [39], phase diagrams [40] of surfactant systems and the interaction between surfactant and polymer [for example, the interaction between tetradecyl dimethyl betaine (C14BE) and sodium carboxymethyl cellulose (Na–CMC)] [41, 42] and calculated the change of the end-to-end distance of macromolecule to reflect the aggregation process of systems using the DPD method, which can give the three-dimensional graphics to depict the aggregation morphology of the macromolecule in surfactant solutions [43–46].
Here, we studied some mesoscopic properties for mixed systems using DPD techniques. Figure 9 shows the morphology of the aggregate of these mixed systems at different molar fractions. For the EAPPF/TBCP system, when X EAPPF = 0.01 and X TBCP = 0.01 (Fig. 9a) in the solution, there no interaction occurred which is observed from the simulation result. When X EAPPF = 0.1 and X TBCP = 0.01 (Fig. 9b), it can be seen that some EAPPF molecules have been absorbed around the polymer TBCP. It means that the interaction between TBCP and EAPPF is stronger than that between EAPPF and water molecules. These phenomena indicate that in the present of TBCP, EAPPF molecules prefer to aggregate around the TBCP coil, not in the solution when the concentration of EAPPF is around the CMC, and also prove that the interaction between EAPPF and TBCP is essential to form aggregates. When X EAPPF = 0.1 and X TBCP = 0.3 (Fig. 9c), there are more TBCP molecules than EAPPF, most of EAPPF molecules were absorbed around the TBCP cluster. When X EAPPF = 0.3 and X TBCP = 0.1 (Fig. 9d), EAPPF molecules was sufficient, thus one part of them formed EAPPF/TBCP aggregates and another part formed single EAPPF micelles. When X EAPPF = 0.3 and X TBCP = 0.3 (Fig. 9e), it can be seen that the sizes of EAPPF/TBCP aggregates become larger.
Snapshots of typical aggregates from simulation for EAPPF/TBCP mixed systems at different molar fractions. a X EAPPF = 0.01, X TBCP = 0.01, b X EAPPF = 0.1, X TBCP = 0.01, c X EAPPF = 0.1, X TBCP = 0.3, d X EAPPF = 0.3, X TBCP = 0.1, right omitting the hydrophilic beads, e X EAPPF = 0.3, X TBCP = 0.3; right omitting the hydrophilic beads. The red color represents the hydrophobic part of EAPPF, the purple color represents the hydrophobic part of TBCP and the green color represents the hydrophilic part of EAPPF and TBCP (PEO groups) (color figure online)
If we changed EAPPF to EAPO as shown in Fig. 10a–d, EAPO/TBCP aggregates are also formed in the system and interaction can occur when molar fractions of them in the solution are low (X EAPO = 0.01 and X TBCP = 0.01, Fig. 10a). And even when X EAPO = 0.3 and X TBCP = 0.1 (Fig. 10d), no single EAPO micelles exist but only EAPO/TBCP aggregates comparable with the EAPPF/TBCP system (X EAPPF = 0.3 and X TBCP = 0.1), suggesting that more EAPO molecules are needed to adsorbed on the TBCP clusters to form EAPO/TBCP aggregates than EAPPF because of the small molecular structure of EAPO.
Snapshots of typical aggregates from simulation for EAPO/TBCP mixed systems at different molar fractions. a X EAPO = 0.01, X TBCP = 0.01, b X EAPO = 0.1, X TBCP = 0.01, c X EAPO = 0.1, X TBCP = 0.3, d X EAPO = 0.3, X TBCP = 0.1; omitting the hydrophilic beads. The red color represents the hydrophobic part of EAPO, the purple color represents the hydrophobic part of TBCP and the green color represents the hydrophilic part of EAPO and TBCP (PEO groups) (color figure online)
For EAPPF/PEG (Fig. 11a, b) and EAPO/PEG (Fig. 12a, b) systems, because PEG is a hydrophilic polymer, the PEG molecules cannot form micelles by themselves but only act as hydrophilic molecules to participate in the formation of EAPPF/PEG or EAPO/PEG mixed micelles.
Snapshots of typical aggregates from simulation for EAPPF/PEG mixed systems at different molar fractions. a X EAPPF = 0.1, X PEG = 0.01, b X EAPPF = 0.1, X PEG = 0.3. The yellow color represents the hydrophobic part of EAPPF, the green color represents the hydrophilic part of EAPPF and PEG (PEO groups) (color figure online)
Snapshots of typical aggregates from simulation for EAPPF/PEG mixed systems at different molar fractions. a X EAPO = 0.1, X PEG = 0.01, b X EAPO = 0.1, X PEG = 0.3. The yellow color represents the hydrophobic part of EAPO, the green color represents the hydrophilic part of EAPO and PEG (PEO groups) (color figure online)
From the simulations above, it is concluded that the molecular simulation is an adjunct to experiments and can provide much inaccessible or not so easily accessible microscopic information through experiments.
Mechanism of the Interaction Between Surfactant and Polymer
On the basis of several observations and the detailed analysis mentioned above, the mechanism of the interaction between surfactant and polymer when the amount of surfactant is in excess can be rationalized as shown in Scheme 1. For EAPPF/TBCP and EAPO/TBCP systems, when the concentration of TBCP is 10 mg L−1, TBCP unimer and EAPPF or EAPO micelles coexist in solution as shown in Scheme 1a. At c TBCP = 104 mg L−1, the formation of clusters of EAPPF/TBCP or EAPO/TBCP complexes with EAPPF or EAPO micelles as a skeleton and single EAPPF or EAPO or TBCP micelles as shown in Scheme 1b. However, when c TBCP = 5 × 104 mg L−1, more and more TBCP molecules participate in the formation of the EAPPF/TBCP or EAPO/TBCP complexes. It is possible that the PPO parts of TBCP insert into the hydrophobic parts of EAPPF or EAPO micelles to form hydrophobic cores of mixed micelles and the PEO parts of EAPPF or EAPO and TBCP interact with each other to form the hydrophilic parts of the mixed micelles (Scheme 1c).
For EAPPF/PEG and EAPO/PEG systems, at low PEG (10 mg L−1) concentration, isolated PEG molecular and EAPPF or EAPO micelles coexist in solution and PEG cannot change the surface activity of the solution so much, as shown in Scheme 1d, suggesting that the effect of PEG on the aggregation of EAPPF or EAPO is negligible. When the PEG concentration is 5 × 104 mg L−1, EAPPF/PEG or EAPO/PEG complexes by the penetration of PEG into surfactant micelle are formed (Scheme 1e) and Ge et al. [47] have also demonstrated this structure using fluorescence resonance energy transfer (FRET) and 1H-NMR measurement.
From our experiments, it can be seen that all the results obtained for EAPO/TBCP or EAPO/PEG mixed systems are similar to EAPPF/TBCP or EAPPF/PEG mixed systems. However, there are different and similar molecular structures between EAPPF and EAPO, thus the surface activity of EAPPF is much higher than that of EAPO because of EAPPF’s oligomeric character, inducing the interaction between EAPPF and TBCP (or PEG) is also stronger than that of EAPO and TBCP (or PEG). It can be said that the similarities and differences on the behavior of EAPPF/TBCP and EAPO/TBCP have been intensely studied throughout this work.
Conclusion
From the experiment results, it can be concluded that the interaction between EAPO and TBCP is similar to that of EAPPF and TBCP, but there is no obvious interaction between EAPPF (or EAPO) and PEG. The interaction mechanism between EAPPF (or EAPO) and TBCP (or PEG) strongly depends on the molecular structures of these materials. The hydrophobic parts of TBCP molecules can insert themselves into the EAPPF (or EAPO) micelles while PEG does not thread through EAPPF (or EAPO) micelles but is absorbed on the surface or penetrates into the hydrophilic parts of EAPPF (or EAPO) micelles. Comparing EAPPF (or EAPO)/TBCP and EAPPF (or EAPO)/PEG mixed systems, it can be seen that because of different molecular structures and properties of TBCP and PEG, they have different effects on EAPPF (or EAPO) solution. TBCP can make the mixed system form more closely-packed EAPPF (or EAPO)/TBCP mixed micelles while PEG can make them looser.
References
Langevin D (2009) Complexation of oppositely charged polyelectrolytes and surfactants in aqueous solutions. Adv Colloid Interface Sci 147–148:170–177
Bain CD, Claesson PM, Langevin D, Meszaros R, Nylander T, Stubenrauch C, Titmuss S, Klitzing RV (2010) Complexes of surfactants with oppositely charged polymers at surfaces and in bulk. Adv Colloid Interface Sci 155:32–49
Trabelsi S, Langevin D (2007) Co-adsorption of carboxymethyl-cellulose and cationic surfactants at the air-water interface. Langmuir 23:1248–1252
Dar AA, Rather GM, Das AR (2007) Mixed micelle formation and solubilization behavior toward polycyclic aromatic hydrocarbons of binary and ternary cationic–nonionic surfactant mixtures. J Phys Chem B 111:3122–3132
Tejwant SK, Srinivasa R, Arvind K (2012) Effect of ethylene glycol and its derivatives on the aggregation behavior of an ionic liquid 1-butyl-3-methyl imidazolium octylsulfate in aqueous medium. J Phys Chem B 116:1612–1622
Löf D, Schillén K, Torres MF, Müller AJ (2007) Rheological study of the shape transition of block copolymer-nonionic surfactant mixed micelles. Langmuir 23:11000–11006
Ganguly R, Aswal VK, Hassan PA, Gopalakrishnan IK, Kulshreshtha SK (2006) Effect of SDS on the self-assembly behavior of the PEO–PPO–PEO triblock copolymer (EO)20(PO)70(EO)20. J Phys Chem B 110:9843–9849
Ganguly R, Kuperkar K, Parekh P, Aswal VK, Bahadur P (2012) Phenol solubilization in aqueous pluronic solutions: investigating the micellar growth and interaction as a function of pluronic composition. J Colloid Interface Sci 378:118–124
Ge LL, Guo R, Zhang XH (2008) Formation and microstructure transition of F127/TX-100 complex. J Phys Chem B 112:14566–14577
Xin X, Xu GY, Wang YJ, Mao HZ, Zhang ZQ (2008) Interaction between star-like block copolymer and sodium oleate in aqueous solutions. Eur Polym J 44:3246–3255
Carnali JO, Shah P (2008) Correlation of surfactant/polymer phase behavior with adsorption on target surfaces. J Phys Chem B 112:7171–7182
Zhou LM, Jiang XH, Li YT, Chen Z, Hu XQ (2007) Synthesis and properties of a novel class of Gemini pyridinium surfactants. Langmuir 23:11404–11408
Sharma R, Shaheen A, Mahajan RK (2011) Cyclic voltammetry and viscosity measurements of aggregated assemblies of anionic surfactants with nonionic surfactants and triblock copolymers. Colloid Polym Sci 289:43–51
Wu D, Xu GY, Sun YH, Zhang HX, Mao HZ (2007) Interaction between proteins and cationic Gemini surfactant. Biomacromolecules 8:708–712
Chen YJ, Xu GY, Yuan SL, Sun HY (2005) Dynamic study of Gemini surfactant and single-chain surfactant at air/water interface. Chin Chem Lett 16:688–690
Acharya DP, Kunieda H, Shiba Y, Aratani K (2004) Phase and rheological behavior of novel Gemini-type surfactant systems. J Phys Chem B 108:1790–1797
Acharya DP, Gutiérrez JM, Aramaki K, Aratani K, Kunieda H (2005) Interfacial properties and foam stability effect of novel Gemini type surfactants in aqueous solutions. J Colloid Interface Sci 291:236–243
Westensen K, Bunjes H, Koch MH (1995) Phase behaviour of Tyloxapol/TX-100/water mixtures. J Pharm Sci 84:544–550
Regev O, Zana R (1999) Aggregation behavior of Tyloxapol, a nonionic surfactant oligomer, in aqueous solution. J Colloid Interface Sci 210:8–17
Chang CH, Tseng SC, Chuang TK (2000) Inhibitory effects of Tyloxapol on the surface activity of albumin at the air/liquid interface. Colloids Surf A 164:287–295
Zhu YY, Xu GY, Xin X, Zhang HX, Shi XF (2009) Surface tension and dilational viscoelasticity of water in the presence of surfactants Tyloxapol and Triton X-100 with cetyl trimethylammonium bromide at 25 °C. J Chem Eng Data 54:989–995
Pang JY, Xu GY, Tan YB (2012) Experimental research and DPD simulation on the interaction between an ethoxy-modified trisiloxane and F127. Colloid Polym Sci 290:953–964
Pang JY, Xu GY, Tan YB, He F (2010) Water-dispersible carbon nanotubes from a mixture of an ethoxy-modified trisiloxane and pluronic block copolymer F127. Colloid Polym Sci 288:1665–1675
Wu D, Xu GY, Feng YJ, Li YM (2007) Aggregation behaviors of gelatin with cationic Gemini surfactant at air/water interface. Intern J Biol Macromol 40:345–350
Lam YM, Goldbeck-Wood G, Boothroyd C (2004) Mesoscale simulation and cryo-TEM of nanoscale drug delivery systems. Mol Simul 30:239–247
Li YY, Hou TJ, Guo SL, Wang KX, Xu XJ (2002) The mesodyn simulation of pluronic water mixtures using the ‘equivalent chain’ method. Phys Chem Chem Phys 2:2749–2753
Zhang J, Xie ZL, Hill AJ (2012) Structure retention in cross-linked poly(ethylene glycol) diacrylate hydrogel templated from a hexagonal lyotropic liquid crystal by controlling the surface tension. Soft Matter 8:2087–2094
Taylor DJF, Thomas RK, Penfold J (2007) Polymer/surfactant interactions at the air/water interface. Adv Colloid Interface Sci 132:69–110
Kriz J (2012) Interaction of premicellar states of a PEO–PPO–PEO triblock copolymer with partially hydrophobic substances: NMR study. J Phys Chem B 116:4386–4393
De Lisi R, Milioto S (2000) Poly(ethylene oxide)13–poly(propylene oxide)30–poly(ethylene oxide)13 electrolyte interactions in aqueous solutions at some temperatures. Langmuir 16:5579–5583
Yuan S, Xu G, Luan Y, Liu C (2005) The interaction between polymer and AOT or NaDEHP in aqueous solution: mesoscopic simulation study and surface tension measurement. Colloids Surf A 256:43–50
Pandit N, Trygstad T, Croy S, Bohorquez M, Koch C (2000) Effect of salts on the micellization, clouding, and solubilization behavior of pluronic F127 solutions. J Colloid Interface Sci 222:213–220
Jansson J, Schillen K, Olofsson G, da Silva RC, Loh W (2004) The interaction between PEO–PPO–PEO triblock copolymers and ionic surfactants in aqueous solution studied using light scattering and calorimetry. J Phys Chem B 108:82–92
Xin X, Xu GY, Wu D, Gong HJ, Zhang HX, Wang YJ (2008) Effects of sodium halide on the interaction between polyvinylpyrrolidone and sodium oleate: surface tension and oscillating barrier studies. Colloids Surf A 322:54–60
Panmai S, Prudhomme RK, Peiffer DG, Jockusch S, Turro NJ (2002) Interactions between hydrophobically modified polymers and surfactants: a fluorescence study. Langmuir 18:3860–3864
Xin X, Xu GY, Zhang ZQ, Chen YJ, Wang F (2007) Aggregation behavior of star-like PEO–PPO–PEO block copolymer in aqueous solution. Eur Polym 44:3106–3111
Zhu CS, Pang SP, Xu JP (2011) Aggregation-induced emission of tetraphenylethene derivative as a fluorescence method for probing the assembling/disassembling of amphiphilic molecules. Analyst 136:3343–3348
Shillcock JC, Lipowsky R (2002) Equilibrium structure and lateral stress distribution of amphiphilic bilayers from dissipative particle dynamics simulations. J Chem Phys 117:5048–5061
Yuan SL, Cai ZT, Xu GY (2003) Mesoscopic simulation of aggregates in surfactant/oil/water systems. Chin J Chem 21:112
Yuan SL, Cai ZT, Xu GY, Jiang YS (2002) Mesoscopic simulation study on phase diagram of the system oil/water/aerosol OT. Chem Phys Lett 365:347–353
Yuan SL, Cai ZT, Xu GY, Jiang YS (2003) Mesoscopic simulation study on the interaction between polymer and C12NBr or C(9)phNBr in aqueous solution. Colloid Polym Sci 281:1069–1075
Li YM, Xu GY, Luan YX, Yuan SL, Zhang ZQ (2005) Studies on the interaction between tetradecyl dimethyl betaine and sodium carboxymethyl cellulose by DPD simulations. Colloids Surf A 257–258:385–390
Sun HY, Xu GY, Li YM, Chen YJ (2006) Mesoscopic simulation of the aggregation behavior of fluorinated surfactant in aqueous solution. J Fluorine Chem 127:187–192
Yuan SL, Cai ZT, Xu GY (2002) Dynamic simulation of aggregation morphology in surfactant solution. Acta Chim Sinica 60:241–245
Cao XR, Xu GY, Li YM, Zhang ZQ (2005) Aggregation of poly(ethylene oxide)–poly(propylene oxide) block copolymers in aqueous solution: DPD simulation study. J Phys Chem A 109:10418–10423
Yuan SL, Cai ZT, Xu GY, Jiang YS (2003) Molecular simulation studies on the interaction between different polymers in aqueous solution. Colloid Polym Sci 281:66–72
Ge LL, Zhang XH, Guo R (2007) Microstructure of Triton X-100/poly (ethylene glycol) complex investigated by fluorescence resonance energy transfer. Polymer 48:2681–2691
Acknowledgments
We gratefully acknowledge financial support from the Special Program for Major Research of the Science and Technology, China (Grant Nos. 2011ZX05024-004-08) and the National Natural Science Foundation of Shandong province, China (ZR2010BM002).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Xin, X., Zhu, Y., Cao, X. et al. Interaction Between an Ethoxylated Alkylphenol Polymer with Formaldehyde and Triblock polyEO–polyPO–polyEO Copolymer in Aqueous Solutions. J Surfact Deterg 17, 71–83 (2014). https://doi.org/10.1007/s11743-013-1447-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-013-1447-3