Abstract
The interactions of two gemini surfactants (16–s–16, s = 5, 6) and their conventional counterpart cetyltrimethylammonium bromide (CTAB) with polyethylene glycols (PEG 3000 and PEG 35000) have been investigated using conductivity, steady state fluorescence, viscosity and TEM techniques. The results indicate that there is no interaction between the PEG 3000/CTAB complex at lower polymer concentrations. However, a very weak interaction is observed at higher concentrations (0.5 and 1.0 wt% PEG 3000), while PEG 3000 and PEG 35000 interact with the gemini surfactants. Both critical aggregation concentration (CAC) and critical micelle concentration (CMC) increases with polymer concentration but are independent of the polymer molecular weight. From steady state fluorescence it is found that the addition of PEG results in no drastic decrease in the aggregation number (N) for all surfactants. This suggests that the atmosphere surrounding the polyion-bound micelles, with respect to the influence on the forces acting at the micelle surface, is equivalent to the counterion/water atmosphere surrounding free micelles. The relative viscosity (η r) results show an enhancement in η r for all the surfactants. The increase in η r is quite significant with gemini surfactants. Polymer-surfactant interaction also depends on the polymer molecular weight. Also, the interaction seems to affect both inter polymer–polymer association as well as chain expansion. Additionally the surfactant induced changes in the polymer conformation depicted by TEM study at the micro structural level confirmed previously observed interactions determined by different analytical techniques.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is well understood that surfactant and polymer mixtures can be used in quite a few formulations and manufacturing processes to improve their characteristics and that these properties cannot be achieved by using polymer or surfactant alone [1, 2]. Due to this very important and useful behavior of polymer/surfactant systems have greatly stimulated the interest in such systems and now the understanding of polymer/surfactant interactions is considered to be the most important issue in surfactant science [3–10]. Though a lot of research on individual surfactants, polymers, and mixed polymer/surfactant materials has been performed, the interactions among surfactants and neutral polymers need detailed exploration. More so, the interaction of polymers with gemini surfactants is scarce [11–16]. The electrostatic force of interactions are very weak in neutral polymer/surfactant systems and other forces like hydrophobic forces are always considered to be present in such systems, and the hydrophobic forces may be the main interactive forces which make the system more convoluted. There have been extensive studies over the last decades on various aspects of how the association of uncharged polymers in dilute aqueous solution occurs [17–19]. To develop a systematic understanding of polymer-surfactant interactions from any of the above perspective, it is necessary to consider structural models of polymer-surfactant aggregates that may form in aqueous solutions.
Anionic surfactants have been shown in a number of studies to interact with neutral polymers to a greater extent than cationic surfactants. Anionic surfactants interact with both hydrophilic as well as hydrophobic polymers [20–23]. In many cases only a weak interaction (if any) is observed between cationic surfactants and hydrophilic polymers. The reason for this difference is not well understood; however, some authors have shown that the nature of both the head-group of the surfactant as well as the counterion may play an important role in determining whether or not a cationic surfactant will interact with a specific neutral polymer [24]. White et al. [25]. explained that weak interaction with cationic might be due to (1) the bulkiness of the cationic head group; (2) the electrostatic repulsion between polymer and surfactant due to the possible positive charge of polymer upon protonation; (3) the hydration shell of the polymer which favors interaction with anionic surfactant. However, an increase in hydrophobicity of a polymer can promote interaction with cationic surfactants [26].
In the present study, polymer-surfactant solution behavior was studied to investigate whether any interaction occurs in aqueous solution between the cationic gemini surfactants bis(hexadecyldimethylammonium)alkane dibromide (16–s–16, s = 5, 6) with neutral polymers polyethylene glycol (PEG) having two different molecular weights by electrical conductivity, fluorescence, viscosity and TEM studies. In addition, we also compared these parameters with those of a monomeric surfactant, cetyltrimethylammonium bromide (CTAB). This systematic study was carried out in order to gain a better understanding of how the cationic gemini surfactant/conventional surfactant interacts with neutral polymers. We have attempted to develop an empirical expression for the dependence of (1) CMC on polymer concentration, C P, and temperature, T, and (2) the solution viscosity in terms of surfactant solution concentration.
Experimental Section
Materials
Polyethylene glycols, PEG (PEG 3000, mol wt ~2700–3300, Fluka, Germany and PEG 35000, mol wt 35000, Fluka, Germany) and CTAB (≥99.0%, Merck, Germany) were used without further purification. Gemini surfactants α,ω-bis(hexadecylammonium)alkane dibromides (16–s–16, s = 5, 6) were synthesized and purified as described elsewhere [27]. Their 1H-NMR spectra and C, H, N analysis data are given in Fig. S1 and S2 (SI) and are consistent with their assigned structures [28]. For synthesis, the materials used were: 1, 5-dibromopentane (≥99%, Himedia, India), 1,6-dibromohexane (≥98%, Fluka, Switzerland), N,N-dimethylhexadecylamine (≥95%, Fluka, Switzerland) and dry ethanol (99.9%, Changshu Yangyuan, China) [29]. The molecular structures of CTAB, gemini surfactants and polymer are shown in Fig. 1.
Conductivity Measurements
Conductivity of solutions was measured by a digital conductivity meter as per details given elsewhere [29]. The temperature within uncertainties of ±0.1 K was maintained in a bath, 25 ml of the polymer solution consisting of an appropriate amount of PEG in water was taken in the conductivity cell at the desired temperature. Then a known amount of stock solution of surfactants made with the same polymer solution was added. In this way, the precise conductance of PEG + surfactant mixture over the complete mole fraction range (0.05–1.0 wt%) in aqueous PEG [30, 31] was determined.
The plots of specific conductance (κ) versus [surfactant] were used to calculate the CAC and CMC of surfactants as explained earlier [14].
Fluorescence Measurements
Fluorescence measurements were taken on a Hitachi F-2500 Fluorescence spectrophotometer with excitation and emission slit width of 2.5 nm and scan speed 60 nm/min as per details given elsewhere [29]. The micellar aggregation numbers (N) of polymer and surfactant solutions were determined using steady-state fluorescence measurements [32].
A steady-state fluorescence quenching method had been used for estimating aggregation number (N) of various amphiphiles in different mixed media. By using the fluorescence quenching method at different weight percentages of PEG + surfactant mixtures, the aggregation number (N) was calculated using the following Eq.
where [Q] = quencher concentration, [S] = total surfactant concentration, [I 0] = fluorescence intensity in the absence of quencher, and [I] = fluorescence intensity in presence of quencher. The total surfactant concentration for CTAB + PEG was 4.0 mmol/dm3 and total concentration for gemini surfactant + PEG was 2.0 mmol/dm3. Equation (1) predicts a linear plot of ln(I 0/I) versus [Q] with a slope equal to N/{[S] − CMC}.
Viscosity Measurements
An Ubbelohde suspended level capillary viscometer was used to measure the viscosity—it was placed vertically in a thermostat with a temperature stability of ±0.1 K in the investigated region. The relative viscosity was calculated from the equation described elsewhere [14, 33, 34].
TEM Measurements
The samples were imaged under Jan EM-2100 electron microscope, Japan, in USIF, AMU, Aligarh. The JEM-2100 ultra high resolution analytical electron microscope is an advanced version of JEM-2010 with an accelerating voltage of 200 kV, having a maximum capability of ultra high resolution, high image quality, and stability of performance.
Results and Discussion
Conductivity Results
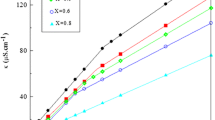
The specific conductivity (κ) profiles as a function of surfactant concentration are presented in Figs. 2 and 3. The intersection point of the two straight lines represents the usual CMC of surfactants and the corresponding values at four different temperatures for CTAB, 16–6–16 and 16–5–16 are presented in Table 1, which are in agreement with values reported in the literature [30]. In these Figs., some plots show two breaks in the presence of PEG in comparison to a single break observed in the absence of polymer. The two breaks in the presence of polymer are ascribed to the occurrence of two kinds of aggregation phenomena. The first break is called CAC where the interaction of polymer chain with surfactant starts. The second break point is called as CMC, where the polymer chain with surfactant and/or micelle‐like aggregates gets saturated, followed by formation of normal micelles on adding a greater amount of surfactant. Figures 2 and 3 show the effect of increasing concentration of PEG (PEG 3000 and PEG 35000) from 0.05 to 1.0 wt% on specific conductivity profiles of CTAB and gemini surfactants at 298.15 K. Similar plots were observed at other temperatures, i.e., at 303.15, 308.15 and 313.15 K, respectively.
For lower concentrations of PEG 3000 (0.05, 0.1 and 0.2 wt%), a single break is observed at all temperatures under consideration and the trend of variation of electrical conductivity with the (CTAB) is similar to that in the absence of polymer. The CMC value slightly increases with polymer concentration (Table 1); Hence, no interaction takes place at lower PEG concentrations (≤0.2 wt%). However, in the presence of higher concentrations of PEG 3000 (0.5 and 1.0 wt%) with CTAB, two break points in the conductivity curves appear, being represented as CAC and CMC, hence showing interaction with CTAB only at concentrations above 0.2 wt% PEG 3000. However, in case of gemini surfactants (16–s–16, s = 5, 6), two break points in the conductivity curves appear at all concentrations of PEG 3000 (Figs. 2, 3b, c). These break points can be attributed to the occurrence of two kinds of aggregation processes in the presence of PEG 3000 and gemini surfactants. For PEG 35000 and CTAB/geminis (16–s–16, s = 5, 6), the conductivity curves are again represented by three linear relations with two breakpoints (Fig. 3). The second breakpoint, attributed to the polymer bound micelles, is about 2–3 times higher than the first one. The CAC and CMC values obtained from these breakpoints are listed in Table 2. Hence we can say that gemini surfactants interact strongly as compared to the conventional counterpart as explained earlier [35].
The above facts indicate that the amount of polymer and polymer hydrophobicity play important roles in the interaction between CTAB and PEG [22]. Gemini surfactants, on the other hand, due to two polar head groups and two alkyl chains, can interact even with lower concentrations of low molecular weight PEG 3000, showing better properties of these surfactants as compared to their monomeric counterpart.
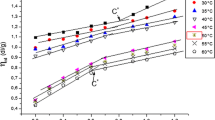
From Tables 1 and 2 it can be seen that both CAC and CMC values increase with an increase in the amount of PEG as explained earlier [11, 19]. The CMC values for all surfactants with both PEG 3000 and PEG 35000 increased as the concentration of PEG increases and are shown in Figs. 4 and 5 respectively. Both CAC and CMC values increase with temperature for all concentrations of polymers as per details given in Tables 1 and 2, respectively. Obviously, the process of aggregation/micellization is less favored at higher temperatures.
For both PEG 3000 and 35000 the dependency of CMC on polymer concentration, C P, and on temperature, T, was obtained by regression using a power law model
The values of constant k and indices n1 and n2 are given Table 3. For all the systems, the maximum value for the mean absolute deviation (MAD) was found to be less than 8.2 and 3.4%, respectively, for PEG 3000 and PEG 35000.
The degree of ionization (g) of the micelle can be calculated using Fig. 3. In a simple approach (g) is taken as the ratio of the slopes of the conductivity curve below slope 1 and above slope 2 the critical micelle concentration [1]. The degree of ionization of the surfactant-PEG complex (g 1) calculated from slopes, is lower than the degree of ionization for ordinary micelles in polymer containing solutions (g 2), indicating less ionized micelles at the CAC than at the CMC. This can be explained on the basis of the reduced interfacial polarity values obtained in the presence of PEG and due to the structure transitions [39]. The g values thus obtained in presence of PEG 35000 at 298.15 K for CTAB, 16–6–16 and 16–5–16 are shown in Fig. 6. A nonlinear variation was observed in g values due to micelle-polymer interactions in this concentration range of the mixture. Similar trend was observed for PEG 3000. It can be concluded that PEG may bind Na+ as observed earlier [36]. This interaction limits the mobility of Na+ and lead to a reduction in g values at higher polymer concentration.
The larger values of g 1 and g 2 for the complex micelle indicates enhanced degree of ionic dissociation due to polymer-surfactant interaction [37]. The values of free energies of aggregation, ∆G agg, and micellization, ∆G mic, were calculated as describe elsewhere [14, 38, 39]. The negative values of ∆G t, as shown in Tables 1 and 2, confirm the feasibility of interaction between the surfactants and polymers. Again, the values are larger in case of gemini surfactants, which confirms that the gemini surfactants show better properties as compared to their monomeric counterpart.
Fluorescence Results
Figures 7 and 8 show the changes of N and I 1/I 3 for the PEG 3000 and PEG 35000 with different surfactants systems. We can observe that there is no significant change in aggregation number (N) for all the three surfactants with addition of PEG as compared to corresponding free micelles, suggesting that the atmosphere surrounding the polyion bound micelles, with respect to the influence on the forces acting at the micelle surface, is equivalent to the counterion/water atmosphere surrounding free micelles, as explained earlier also [40].
The above results can further be explained on the basis of quenching. The strength of the hydrophobic environment can be evaluated by determining the first order quenching rate constant, the so-called Stern–Volmer binding constant, K SV using the relation
K SV is the product of the rate of the quenching process and lifetime of the probe in the absence of bimolecular quenching and gives an idea about bimolecular quenching and unimolecular decay [41, 42]. The ratio of intensity of first and third vibronic peaks, i.e., I 1/I 3, of pyrene fluorescence emission spectrum in the presence of surfactant is considered to be the index of micropolarity of the system. A value less than 1 generally indicates that pyrene is in nonpolar environment while values greater than 1 mean pyrene having polar surrounding [40, 43]. Characteristic values of I 1/I 3 of pyrene in some solvents are: 0.6 in cyclohexane; 1.04 in toluene; 1.23 in ethanol; 1.33 in methanol and 1.84 in water [44, 45]. The average values of I 1/I 3 ratio at different quencher concentration are given in Tables 4 and 5. The values of I 1/I 3 for all the systems are close to that of I 1/I 3 value for water which result in an increase in polarity of the system [43]. The apparent dielectric constant (D) values for both PEG 3000 and PEG 35000 can be estimated by Eq. 4 and are presented in Tables 4 and 5.
We can see that there is no definite trend in D values, they lie in between 60–86 for PEG 3000 and 43–72 for PEG 35000. These values are close to the D value for water [40].
Viscosity Results
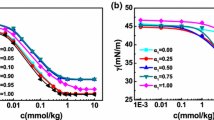
Figure 9 shows the variation of polymer viscosity with polymer concentration. We can observe that the viscosity of the PEG solution increases with polymer concentration as well as molecular weight of polymer due to the additional amount of polymer chains available for hydrolysis. Higher viscosity is obtained in the case of PEG 35000 due to more resistance to flow as a result of entanglement among polymer chains.
Figures 10 and 11 show the comparative plot of solution viscosities for all the surfactants and for all concentrations of PEG 3000 and PEG 35000 along with solution viscosities of pure surfactants in water. The relative viscosity increases with polymer concentration. For PEG 3000 + CTAB system, there is a slight increase in relative viscosity. The relative viscosities of CTAB solutions in pure water and in 1.0 wt% PEG 3000 at 50 mmol/dm3 are 1.143 and 1.36. This is due to the very weak interaction between the macromolecule and micelles, as was evident by conductivity data. The PEG 3000/16–6–16/water systems show significant viscosity increment only at high concentration of surfactant (>80 mmol/dm3); the relative viscosity of 16–6–16 solution in pure water and in 1.0 wt% PEG 3000 at 100 mmol/dm3 of 16–6–16 are 7.10 and 11.32 showing interaction between PEG 3000 and 16–6–16. While PEG 3000 + 16–5–16 system show decrease in relative viscosity at all concentrations of polymer and surfactant in comparison to pure 16–5–16 viscosity in water. The decrease in relative viscosity in the presence of polymers can be explained in terms of a mixed micelle formation between the surfactant and the amphiphilic polymer. In order to have cross-linking and thus viscosity effect, there must be a sufficiently high number of polymer hydrophobes per micelle. Due to loss in cross-linking effects the viscosity decreases. Also, relative viscosity slightly increases with increase in PEG 3000 concentration for all the surfactants.
In case of PEG 35000 (Fig. 11), the viscosity increment is quite significant, and sufficiently high viscosities were observed, especially for gemini surfactants. For CTAB, the increase in relative viscosity is around two fold in the presence of higher concentrations, i.e., 1.0 wt% PEG 35000 as compared to pure CTAB in water. The viscosities of the mixtures (100 mmol/dm3 16–6–16 + 1.0 wt% PEG 35000 + water) were around 3.5 times larger than those in water alone. Also, 16–5–16 gives a slight viscosity increment with PEG 35000 at all concentrations of polymer.
Addition of surfactants to polymer solution results in an increase in relative viscosity and this increase in relative viscosity is more with the addition of gemini surfactants as compared to conventional surfactant CTAB as shown clearly in Figs. 10 and 11, respectively as explained earlier [14, 41]. η r values at 1.0 wt% PEG 35000 at 50 mmol/dm3 of surfactant are 1.82, 2.15 and 3.12 for CTAB, 16–6–16 and 16–5–16, respectively, higher viscosities observed for 16–5–16 for the same (surfactant) show the ability of gemini surfactant of shorter spacer to give rise to rod-shaped micelles at fairly low surfactant concentration [43].
An empirical correlation between surfactant concentration (c) and relative viscosity (η r) was developed in the following form:
where the values of k 0, k 1 and k 2 are given in Table 6. The values of relative viscosity are plotted in Fig. 12 and we can see that more than 95% of the data points lie close to the diagonal line. The maximum value for the mean absolute deviation (MAD) was found to be less than 12.1%.
TEM Study
Figure 13 shows typical TEM micrograph images of PEG 3000-CTAB, PEG 35000-CTAB, PEG 3000–16–6–16, PEG 35000–16–6–16, PEG 3000–16–5–16 and PEG 35000–16–5–16 system. Figure 13a shows the surface morphology for PEG 3000-CTAB system, here the surface is somewhat smooth and due to lower molecular weight of polymer very weak/no interaction is seen, while in Fig. 13b, we can easily see lumps or aggregates showing interaction between PEG 35000-CTAB system and support conductivity and viscosity data. From Fig. 13c, d, we can clearly see that in PEG-16–6–16 system the surfactant was homogeneously dispersed into the polymer and cross-linking takes place in case of higher molecular weight polymer. Similar trend is observed in case of PEG-16–5–16 system.
Conclusions
A conductometric, fluorescence and viscometric study of interaction between two molecular weights of PEG (PEG 3000 and PEG 35000) and gemini surfactants (16–s–16, s = 5, 6) and their conventional counterpart (CTAB) was carried out. The results show that there is no PEG 3000/CTAB complex formation at lower concentrations of PEG 3000, while for PEG 35000 interaction with CTAB occurs. But both PEG 3000 and PEG 35000 interact with the cationic gemini surfactants, indicating the role of polymer hydrophobicity. Also, gemini surfactants interact strongly with both PEGs as compared to conventional counterpart. Steady state fluorescence reveals that the addition of PEG results in a no significant change in the aggregation number for all surfactants in the aggregates present in all the systems. The viscosity results show that the interactions between the surfactant and the polymer affect both inter polymer–polymer association as well as chain expansion. From the TEM micrographs, we can concludes that in PEG—gemini system the surfactant was homogeneously dispersed into the polymer and cross-linking takes place in case of higher molecular weight polymer.
References
Hansson P, Lindman B. Surfactant–polymer interactions. Curr Opin Colloid Interface Sci. 1996;1:604–13.
Goddard ED, Ananthapadamanabhan KP. Interactions of surfactants with polymers and proteins. Boca Raton: CRC Press; 1993.
Robb ID (1981) Anionic surfactants–physical chemistry of surfactant action. In: Lucassen–Reynders EH (ed) surfactant science series, vol 11. New York: Marcel Dekker; 1981.
Goddard ED. Polymer–surfactant interaction Part I. uncharged water-soluble polymers and charged surfactants. Colloids Surf. 1986;19:255–300.
Saito S. In: Nonionic surfactants: physical chemistry, Schick MJ (ed) Surfactant science series, vol 23. New York: Marcel Dekker; 1987.
Hayakawa K, Kwak JCT. In: Cationic surfactants: physical chemistry, Rubingh DN, Holland PM (ed) Surfactant science series, vol 37. New York: Marcel Dekker; 1991.
Lindman B, Thalberg K. In: Interactions of surfactants with polymers and proteins, Goddard E, Ananthapadmanabhan KP (ed), Boca Raton: CRC Press; 1993.
Suurkuusk J, Wadso I. A multichannel microcalorimetry system. Chem Scr. 1982;20:155–63.
Kwak JCT. Polymer-surfactant systems, surfactant science series, vol. 77. New York: Marcel Dekker; 1998.
Jonsson B, Lindman B, Holmberg K, Kronberg B. Surfactants and polymers in aqueous solution. London: Wiley; 1998.
Shah DO. Surface phenomena in enhanced oil recovery. New York: Plenum Press; 1981.
Cahn A, Lynn J. Encyclopedia of technical technology. New York: Wiley-Interscience; 1983.
Ali MS, Suhail M, Ghosh G, Kamil M, Kabir-ud-Din. Interactions between cationic gemini/conventional surfactants with polyvinylpyrrolidone: specific conductivity and dynamic light scattering studies. Colloids Surf A Physicochem Eng Asp. 2009;350:51–6.
Sardar N, Kamil M, Kabir-ud-Din. Interaction between nonionic polymer (hydroxypropyl)methyl cellulose and cationic gemini/conventional surfactants. Ind Eng Chem Res. 2012;51:1227–35.
Sardar N, Kamil M, Kabir-ud-Din. Solution behavior of anionic polymer sodium carboxymethyl cellulose (NaCMC) in presence of cationic gemini/conventional surfactants. Colloids Surf A. 2012;415:413–20.
Sardar N, Kamil M, Kabir-ud-Din. Interactions between polyvinylpyrrolidone and cationic gemini/conventional surfactants. Chem Eng Commn. 2013;200:1–18.
Banipal TS, Sood AK, Singh K. Micellization behavior of the 14–2–14 gemini surfactant with some conventional surfactants at different temperatures. J Surfact Deterg. 2011;14:235–44.
Mirgorodskaya AB, Yatskevich EI, Zakharova L, Konovalov AI. Gemini surfactant-nonionic polymer mixed micellar systems. Colloid J. 2012;74:91–8.
Kumar B, Tikariha D, Ghosh KK, Barbero N, Quagliotto P. Effect of polymers and temperature on critical micelle concentration of some gemini and monomeric surfactants. J Chem Thermodyn. 2013;62:178–85.
Zen Sheu Ji. Ph.D. Thesis, The Pennsylvania State University, 1983.
Piculell L, Lindman B. Association and segregation in aqueous polymer/polymer, polymer/surfactant, and surfactant/surfactant mixtures: similarities and differences. Adv Colloid Interface Sci. 1992;41:149–78.
Brackman JC, Engberts JBFN. Polymer micelle interactions: physical organic aspects. Chem Soc Rev. 1993;22:85–92.
Brackman JC, Engberts JBFN. Effect of surfactant charge on polymer–micelle interactions: n-dodecyldimethylamine oxide. Langmuir. 1994;8:424–8.
Brackman JC, Engberts JBFN. Influence of polymers on the micellization of cetyltrimethylammonium salts. Langmuir. 1991;7:2097–102.
Witte FM, Engberts JBFN. Perturbation of SDS and CTAB micelles by complexation with poly(ethylene oxide) and poly(propylene oxide). J Org Chem. 1987;52:4767–72.
Nagarajan R. Thermodynamics of nonionic polymer-micelle association. Colloids Surf. 1985;1:1–17.
Kabir-ud-Din, Siddiqui US, Kumar S, Dar AA. Micellization of monomeric and Dimeric (Gemini) Surfactants in Polar Nonaqueous-Water-Mixed solvents. Colloid Polym Sci. 2006;284:807–12.
Kabir-ud-Din, Fatma W, Khan ZA, Dar AA, (1)H NMR and viscometric studies on cationic gemini surfactants in presence of aromatic acids and salts. J Phys Chem B. 2007;111:8860–8867.
Sardar N. Studies on polymers and gemini surfactants interaction, PhD Thesis, AMU, Aligarh, 2014
Zana R, Benrraou M, Rueff R. Alkanediyl-α,ω-Bis (Dimethylalkylammonium bromide) surfactant 1.effect of the spacer chain length on the critical micellar concentration and micelle ionization degree. Langmuir. 1991;7:1072–5.
Zana R. Dimeric and oligomeric surfactants. behavior at interfaces and in aqueous solution: a review. Adv Colloid Interface Sci. 2002;97: 205–253.
Hunter TF. The distribution of solubilisate molecules in micellar assemblies. Chem Phys Lett. 1980;75:152–5.
Kabir-ud-Din, Kumar S, Aswal VK, Goyal PS. Effect of the addition of n-alkylamines on the growth of sodium decyl sulfate micelles. J Chem Soc Faraday Trans. 1996;92:2413–5.
Ozeki S, Ikeda S. The viscosity behavior of NaCl solutions of dodecyldimethylammonium chloride and the flexibility of its rod-like micelles. J Colloid Interface Sci. 1980;77:219–26.
Zana R. Dimeric (Gemini) Surfactants: effect of the spacer group on the association behavior in aqueous solution. J Colloid Interface Sci. 2002;248:203–20.
Dubin PL, Gruber JH, Xia J, Zhang H. The effect of cations on the interaction between dedecylsulfate micelles and poly(ethyleneoxide). J Colloid Interface Sci. 1992;148:35–41.
Zana R, Lang J, Lianos P. Fluorescence probe studies of the aggregation state of sodium dodecylsulfate in aqueous solutions of polyoxyethylene glycol and poly-N-vinylpyrrolidone, In microdomains in polymer solutions. Polym Prepr Am Chem Soc Div Polym. 1982;23:39–40.
Lu JR, Marrocco A, Su TJ. Adsorption of dodecyl sulfate surfactants with monovalent metal counterions at the air-water interface studied by neutron reflection and surface tension. J Colloid Interface Sci. 1993;158:303–16.
Wang SC, Wei TC, Chen WB, Tsao HK. Effects of surfactant micelles on viscosity and conductivity of poly(ethylene glycol) solutions. J Chem Eng Phys. 2004;120:4980–8.
Azum N, Naqvi AZ, Akram M, Kabir-ud-Din. Studies of mixed micelle formation between cationic gemini and cationic conventional surfactants. J Colloid Interface Sci. 2008;328:429–35.
Oda R, Huc I, Homo JC, Beinrich B, Schmutz M, Candau S. Elongated aggregates formed by cationic gemini surfactants. Langmuir. 1999;15:2384–90.
Turro NJ, Yekta. Luminescent probes for detergent solutions. A simple procedure for determination of the mean aggregation number of micelles. J Am Chem Soc. 1978;100:5951.
Fang L, Ganzuo X, Hanqing Guiying W. Formation and aggregation number of sodium dodecyl sulphonate-polyvinylpyrrolidone aggregates. Chin Sci Bull. 1997;42:181.
Kalyanasundram K, Thomas JK. Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J Am Chem Soc. 1977;99:2039.
Weast RC. CRC handbook of chemistry and physics. West Palm Beach: CRC Press; 1978.
Kabir-ud-Din Rub MA, Naqvi AZ. mixed micelle formation between amphiphilic drug amitriptyline. Hydrochloride and surfactants (conventional and Gemini) at 293.15–308.15 K. J Phys Chem B. 2010;114:6354–64.
Behera K, Om H, Pandey S. Modifying properties of aqueous cetyltrimethylammonium bromide with external additives: ionic liquid 1-hexyl-3-methylimidazolium bromide versus cosurfactant n-hexyltrimethylammonium bromide. J Phys Chem B. 2009;113:786–93.
Azum N. Studies on surfactant-additive systems. Thesis: Department of Chemistry, Aligarh Muslim University; 2009.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Sardar, N., Kamil, M. & Kabir-ud-Din Studies on Solution Behavior of Aqueous Mixtures of Nonionic Polymer in Presence of Cationic Surfactants. J Surfact Deterg 20, 631–645 (2017). https://doi.org/10.1007/s11743-017-1932-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-017-1932-1