Abstract
Lung ultrasound (LUS) is a valid tool for the assessment of heart failure (HF) through the quantification of the B-lines. This study in HF patients aims to evaluate if LUS: (1) can accelerate the discharge time; (2) can efficiently drive diuretic therapy dosage; and (3) may have better performance compared to the amino-terminal portion of B type natriuretic peptide (NT-proBNP) levels in monitoring HF recovery. A consecutive sample of 120 HF patients was admitted from the Emergency Department (ED) to the Internal Medicine Department (Verona University Hospital). The Chest X-ray (CXR) group underwent standard CXR examination on admission and discharge. The LUS group underwent LUS on admission, 24, 48 and 72 h later, and on discharge. The Inferior Cava Vein Collapsibility Index, ICVCI, and the NT-proBNP were assessed. LUS discharge time was significantly shorter if compared to CXR group (p < 0.01). During hospitalization, the LUS group underwent an increased number of diuretic dosage modulations compared to the CXR group (p < 0.001). There was a stronger association between partial pressure of oxygen in arterial blood (PaO2) and B-lines compared to the association between PaO2 and NT-proBNP both on admission and on discharge (p < 0.001). The B-lines numbers were significantly higher on admission in patients with more severe HF, and the ICVCI was inversely associated with B-lines number (p < 0.001). The potential of LUS in tailoring diuretic therapy and accelerating the discharge time in HF patients is confirmed. Until the technique comes into common use in different departments, it is plausible that LUS will evolve with different facets.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, lung ultrasound (LUS) has emerged in different clinical settings [1,2,3,4,5] for the evaluation of patients with acute respiratory failure. In particular, the main fields of LUS applications are: pneumothorax (PNX), interstitial syndrome, lung consolidation and pleural effusion [6,7,8].
Heart failure (HF) is a clinical syndrome characterized by typical symptoms and signs caused by structural or functional cardiac abnormalities, resulting in a reduced cardiac output or elevated intra-cardiac pressures at rest or during stress [9].
The prevalence of HF is approximately 1–2% of the adult population in developed countries, rising to ≥10% among people >70 years of age, and it is the leading cause of hospitalization [10].
LUS has been demonstrated to be a valid tool for the assessment of pulmonary congestion [11,12,13] through the quantification of the B-lines. B-lines are defined as laser-like vertical hyperechoic reverberation artifacts that emerge from the pleural line (previously defined as “comet tails” or “ring-downs.”). Multiple B-lines are the sonographic sign of lung interstitial syndrome. Their number increases along with decreasing air content and increase in lung density [4, 14]. Clearing of B-lines significantly correlates with improved clinical symptoms and signs of HF. B-lines due to cardiogenic pulmonary edema are usually bilateral, and usually spread or recover symmetrically [4]. Their regular distribution allows differentiation between cardiogenic pulmonary edema, acute respiratory distress syndrome (ARDS) and pulmonary fibrosis [4, 14].
The plasma concentration of natriuretic peptides (NPs) can be used as an initial HF diagnostic test, especially in the setting of dyspnoea of unclear etiology. Elevated NPs help to establish an initial working diagnosis. They are considered predictors of prognosis of HF, and are used to dictate the intensity of the diuretic therapy [15, 16].
Our previous studies [17,18,19] strongly support the daily use of point of care ultrasound in Internal Medicine.
This study goes further.
The main objectives of this study are: (1) to test if LUS use can speed up the discharge time in HF patients; (2) to test if LUS can efficiently drive diuretic therapy dosage; (3) to compare the performance of LUS and of the amino-terminal portion of B type natriuretic peptide (NT-proBNP) levels in monitoring HF recovery.
Materials and methods
Ethics statement
The study was conducted in accordance with the ethical standards laid down in the Helsinki Declaration of 1975 and its late amendments. Informed consent was obtained from all individual participants included in the study.
Study setting and population
The study setting was the Internal Medicine department of the University Hospital of Verona, Italy, already certified as a first level ultrasound centre by the Società Italiana di Medicina Interna (SIMI).
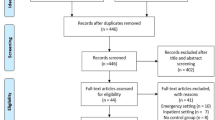
The authors studied a consecutive sample of 120 patients (aged 70–94). They were admitted from the ED to the Internal Medicine Department of the University Hospital of Verona with the clinical diagnosis of HF.
Exclusion criteria were: concomitant acute coronary syndrome, pneumonia, chronic obstructive pulmonary disease, lung cancer or metastases, lung fibrosis, previous pneumonectomy or lobectomy, breast prothesis, obesity, also in order to avoid the detection of B-lines other than due to HF.
The study was conducted in winter 2016–2017 and spring 2017.
Patients were classified according to the New York Heart Association (NYHA) classes [20].The NYHA functional classification was used to describe the severity of symptoms and exercise intolerance at admission. HF was classified according to the recent guidelines of the European Society of Cardiology [9]: patients with normal left ventricular ejection fraction (LVEF) (≥50%, HF with preserved EF, HFpEF), patients with reduced LVEF (<40%, HF with reduced EF, HFrEF), patients with an LVEF in the range of 40–49% (HFmrEF).
Description of the study protocol
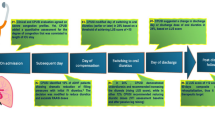
Patients were randomly allocated into two groups: the Chest X-ray group (CXR) and the LUS group.
The CXR group underwent a standard CXR examination on admission and on discharge.
The LUS group underwent LUS on admission, 24, 48 and 72 h later, and on discharge.
Trans-thoracic echocardiography at rest was performed in all patients in order to classify the LVEF on admission.
Trans-thoracic echocardiography was performed by an Internal Medicine specialist (CM) and a colleague (MDDP) (they were certified by the Societa` Italiana di Ecografia Cardiovascolare, SIEC). LUS examinations were performed by CM, who was certified by the Società Italiana di Ultrasonologia in Medicina e Biologia (SIUMB). All LUS examinations were performed by a single operator (CM) in order to reduce the interobserver variability in the B-lines counting.
All ultrasound examinations were performed using an EnVisor C HD Philips. The sector probe (2.5–3.5 MHz) was the first choice of use for LUS examination (the convex and the linear ones were also available). LUS examinations were performed with patients in the supine or near-supine position for the anterior scanning, and in the sitting position for the dorsal scanning. A B-lines score, defined as the total number of the detectable B-lines, was determined, according to the approach proposed by Gargani and Volpicelli [4, 21].
Inferior cava vein (ICV) maximum and minimum diameter and its collapsibility index (Inferior Cava Vein Collapsibility Index, ICVCI) were measured in subcostal view in M-mode, 2 cm from the right atrial junction in the LUS group. ICVCI was calculated according to the formula [(ICVmax − ICVmin)/ICVmax] × 100. These measurements were obtained using a convex transducer. The ICVCI % cutoffs were: >75 (hypovolemia), ≥40 and ≤75 (euvolemia) and <40 (hypervolemia).
NT-proBNP dosage was obtained from peripheral venous blood samples (Immunochemistry Analyzer, COBAS 6000) in the LUS group on admission and on discharge. Urine output and diuretic dosage were carefully reported daily. Arterial blood samples were collected on admission and on discharge in both groups to test the partial pressure of oxygen (PaO2) as indicator of HF severity (on admission) and recovery (on discharge).
Statistical analysis
Categorical and continuous characteristics at admission are summarized as percentages and means with standard deviations, respectively. Differences in the distribution of the baseline characteristics between CXR and LUS groups were tested using Chi squared test, Student’s T test, or Mann–Whitney’s U test, as appropriate.
A quantile regression model was adopted to test the difference in the number of days of hospitalizations from admission to discharge in CXR group vs LUS group, using a stepwise forward approach (with p < 0.20 for entry and p ≥ 0.25 for removal) for selecting the variables to include in the multivariate model.
The correlations of number of the B-lines and NT-pro-BNP, opportunely log-transformed to achieve normal distribution, with PaO2 and ICVCI were evaluated using linear regression models using the data on admission and on discharge, taking into account intra-subject variability using cluster-robust standard errors.
The velocity of clearance of log(number of the B-lines) at 0, 24, 48, and 72 h after admission according to HF severity was evaluated using an two-way ANOVA model, with time-dependent repeated measures.
Statistical analyses were performed using STATA 14.2.
Results
The baseline demographic and clinical characteristics of the patients on admission are reported in Table 1. All patients were classified in NYHA IV class.
Drug therapy was similar in the CXR and LUS groups (angiotensin-converting enzyme inhibitors, β-blockers, statins, anticoagulant/anti-platelets drugs) except for diuretics (more frequent in the LUS group). The subjects included in the LUS group had lower PaO2 at admission compared to those included in the CXR group (mean: 62.9 vs 70.3, p < 0.001); the calculated ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2 ratio) was, respectively, 299.52 vs 334.76, FiO2 0.21 (21%, without oxygen supply).
The average time required to acquire and interpret LUS was 7 ± 1 min.
Figure 1 shows the discharge time (measured as hospitalization days) for the CXR and the LUS groups.
LUS discharge time was significantly shorter if compared to the CXR group: median (range) CXR: 8 (4–17); LUS 7 (3–10), p value for difference: p < 0.001.
In the stepwise multivariate regression model, LUS discharge time was significantly shorter if compared to CXR group (coefficient: −1.812, 95% CI −2.719, −0.906; p < 0.001). The PaO2 on admission was also significantly associated with discharge time, with higher PaO2 levels associated with shorter hospital stay (coefficient: −0.063; 95% CI −0.107, −0.018; p = 0.006). The use of diuretics was also included in the final stepwise model with the above two variables, but its association with time to discharge did not reach statistical significance (coefficient: 0.688; 95% CI −0.166, 1.541; p = 0.113).
During hospitalization, the LUS group underwent an increased number of diuretic dosage modulations compared to the CXR group (p < 0.01). In the CXR group, 33% (20 out of 60) of the patients, had a single diuretic modulation, while 67% (40 out of 60) had two; on the other hand, none of the patients in the LUS group had only one diuretic modulation, 12% had two modulations (7 out of 60), and 88% (53 out of 60) had three.
Figure 2 shows the associations of PaO2 and the B-lines on admission and discharge and PaO2 and NT-proBNP on admission and discharge (both expressed in log10). The B-lines are strongly associated with the levels of PaO2, as log10 (B-lines) predicted 33% of the variability of PaO2 (r2 = 0.331; p < 0.001). On the other hand, the association between NT-proBNP and PaO2 is weaker, with log10 (B-lines) predicting only 1% of the PaO2 variability (r2 = 0.012; p = 0.034).
The associations between PaO2 and B-lines on admission and discharge (on the left), and PaO2 and NT-pro-BNP on admission and discharge (on the right) in the LUS group. PaO 2 partial pressure of oxygen in arterial blood (mmHg), NT-pro-BNP N-terminal fragment brain natriuretic peptides, LUS lung ultrasound, black dots admission; white dots discharge; p < 0.001
The number of the B-lines was significantly higher on admission in patients with more severe HF.(p < 0.01) (Fig. 3). Overall the B-lines diminished of −23, −39, and −50% after 24, 48, and 72 h, respectively, compared to the number of the B-lines on admission. There was no interaction between time and severity of HF (p = 0.866). This indicates that, despite the velocity of clearance in absolute numbers was quicker in the groups with higher B-lines on admission, the relative velocity in B-lines clearance was similar across the three groups of HF severity.
The ICVCI was measured on admission and discharge in the LUS group. There is an inverse significant association between this index and the number of B-lines (p < 0.01), as shown in Fig. 4.
PaO2 (mmHg) on discharge was 98 ± 1 for both groups without oxygen supply.
Discussion
LUS is becoming a standard tool in critical care for the early diagnosis of acute respiratory failure [21]. The “decision tree” used to guide this diagnosis is the well-known bedside lung ultrasound evaluation (BLUE) protocol [22]. The advantage of this protocol is its rapidity.
Moreover, LUS is not only a simple approach to discriminating among its main fields of application [6, 7]; LUS can be also used as a monitoring tool in HF patients.
Different studies [13, 23,24,25] have been designed in different settings other than Emergency settings. They have been designed to define the performance of LUS compared to clinical assessment, to NPs levels and to traditional CXR in HF patients, both outpatients and inpatients. These studies have confirmed that the B-lines are prognostic markers for hospital re-admission or death in HF patients. Moreover, the prognostic value of LUS is confirmed independently of the method used to evaluate the B-lines burden (semi-quantitative, 8 zones, 28 zones, 72 zones), allowing a prognostic risk stratification on discharge. They provide an important step forward for the implementation of LUS in the clinical evaluation of HF patients.
It is well known that the costs associated with HF hospitalization are consistent in Internal Medicine, with increased hospital stay days [26, 27]. Moreover, these costs are compounded by a high rate of re-admission.
In this context, the main finding of this study is that LUS speeds up the discharge time in HF patients, and that congestive failure is one of the most common admission diagnosis in the Internal Medicine Department. This result may be due to different reasons. The LUS operator is not blinded to the patients’ clinical conditions (while the Radiologist usually has only an information summary about them). Furthermore, the possibility of performing LUS bedside at any moment allows for an easier management of the therapy. In fact, in this study, the LUS group underwent an increased number of diuretic dosage modulations compared to the CXR group. Remarkably, the LUS group discharge time was shorter although with a lower PaO2 on admission. We have no precise explanation about this point because patients were randomly allocated to the LUS or to the CXR group. This fact reinforces the ability of LUS in speeding up the discharge time also in patients with more severe conditions at baseline.
The second important result of this study concerns the B-lines and their role in monitoring HF recovery. The results of this study show a stronger association between PaO2 and B-lines compared to the association between PaO2 and NT-proBNP both on admission and on discharge.
The B-lines are not mentioned in HF ESC guidelines [9]. In this study, the reduction of the B-lines does not occur in accordance with the NT-proBNP levels, suggesting that serum NT-proBNP may not reliably indicate pulmonary congestion having been resolved. These results lead us to consider this molecule a useful marker for the discrimination of the possible origin of respiratory failure, but it is not so precise in monitoring HF recovery. NT-pro-BNP has proved to be effective only in excluding or confirming congestive HF. These data support the use of this molecule in the Critical care setting rather than in the Internal Medicine Department. Moreover, it has been established that NPs levels are affected by age, as known, [28] or by other conditions such as body mass index [29], myocardial ischemia and hypoxia even in the absence of left ventricular dysfunction [30], hormonal dysfunctions [31], renal failure and diabetes [32]. In this study, age is probably the major confounder in the evaluation of NT-pro-BNP levels.
The B-lines clearance time is longer in patients with HFrEF compared to those with HFpEF and HfmrEF. This result underlines the importance of a combined approach (ecocardiography and LUS). This fact is confirmed also by the importance of the ICVCI evaluation. In fact, the ICVCI was computed on admission and on discharge in the LUS group and a significant association between this index and the number of the B-lines is found.
Nevertheless, this study has several limitations: the sample is composed of elderly people so it is arguable that the acoustic window could be affected by the patient positioning (effort of maintaining the correct position both the supine and the sitting one).
Also the method of quantification of the B-lines could be a source of disagreement. Scan techniques can be broadly divided into two groups: the scanning modality by zones or the scanning modality by fixed points, as described in Methods section. The authors counted the total number of the detectable B-lines in anterolateral and posterior scanning sites. This protocol is well established [4, 21]; nevertheless, this evaluation could be even excessively accurate, taking too much time.
The context makes the difference. The Internal Medicine Department can spend more time on the patients’ clinical examination compared to the emergency one.
It has to be recognized that the LUS technique in the B-lines identification and counting is not fully standardized and many issues against LUS in HF exist, in particular because of the low specificity of the B-lines in interpreting the acoustic interactions. In fact, in this study, precise exclusion criteria have been adopted in order to avoid the detection of B-lines other than due to HF.
Some authors prefer a subjective overview of the B-lines, not a precise counting, and the choice of the probe can also be controversial. By now, these limitations have been deeply investigated [33, 34]. The integration of LUS with a comprehensive multi-organ ultrasound evaluation is mandatory to avoid common pitfalls and misdiagnosis, as recently reviewed by Blanco and Volpicelli [35].
Conclusions
The results of this study confirm the potential of LUS in tailoring diuretic therapy and speeding up the discharge time in HF hospitalization. The study stresses the real need for appropriate timing and modality of LUS in Internal Medicine. Until the technique comes into common use in different departments, it is plausible that LUS will evolve with different facets and needs accordingly.
Abbreviations
- CXR:
-
Chest X-ray
- HF:
-
Heart failure
- HFpEF:
-
Heart failure with preserved ejection fraction
- HFmrEF:
-
Heart failure with mid-range ejection fraction
- HFrEF:
-
Heart failure with reduced ejection fraction
- ICVCI:
-
Inferior Cave Vein Collassability index
- LUS:
-
Lung ultrasound
- NT-pro BNP:
-
Circulating N-terminal pro-hormone of brain natriuretic peptide
- NYHA:
-
New York Heart Association
References
Bouhemad B, Zhang M, Lu Q, Rouby JJ (2007) Clinical review: bedside lung ultrasound in critical care practice. Crit Care 11(1):205–213
Via G, Storti E, Gulati G, Neri L, Mojoli F, Braschi A (2012) Lung ultrasound in the ICU: from diagnostic instrument to respiratory monitoring tool. Minerva Anestesiol 78:1282–1296
Reissig A, Copetti R, Kroegel C (2011) Current role of emergency ultrasound of the chest. Crit Care Med 39(4):839–845
Gargani L (2011) Lung ultrasound: a new tool for the cardiologist. Cardiovasc Ultrasound 9:6–12
Filopei J, Siedenburg H, Rattner P, Fukaya E, Kory P (2014) Impact of pocket ultrasound use by internal medicine house staff in the diagnosis of dyspnoea. J Hosp Med 9(9):594–597
Lichtenstein D (2014) Lung ultrasound in the critically ill. Curr Opin Crit Care 20:315–322
Lichtenstein D (2014) Lung ultrasound in the critically ill. Ann Intensive Care 4:1–12
Volpicelli G (2013) Lung sonography. J Ultrasound Med 32:165–171
The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) (2016) 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 37:2129–2200
Maggioni AP (2010) EUR observational Research Programme. The heart failure pilot survey (ESH-HF Pilot). Eur J Heart Fail 12(10):1076–1084
Glöckner E, Christ M, Geier F, Otte P, ThiemU Neubauer S et al (2016) Accuracy of point-of-care B-line lung ultrasound in comparison to NT-ProBNP for screening acute heart failure. Ultrasound Int Open 2:E90–E92
Martindale JL, Noble VE, Liteplo A (2013) Diagnosing pulmonary edema: lung ultrasound versus chest radiography. Eur J Emerg Med 20(5):356–360
Ang SH, Andrus P (2012) Lung ultrasound in the management of acute decompensated heart failure. Curr Cardiol Rev 8:123–136
Volpicelli G, The International Liaison Committee on Lung Ultrasound (ILC-LUS) for the International Consensus Conference on Lung Ultrasound (ICC-LUS) International (2012) Evidence-based recommendation for point of care lung ultrasound. Intensive Care Med 38:577–591
Roberts E, Ludman AJ, Dworzynski K, Al-Mohammad A, Cowie MR, McMurray JJV et al (2015) The diagnostic accuracy of the natriuretic peptides in heart failure: systematic review and diagnostic meta-analysis in the acute care setting. BMJ 350:h910
Maisel A, Mueller C, Adams K, Anker SD, Aspromonte N, Cleland JGF et al (2008) State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail 10:824–839
Mozzini C, Fratta Pasini AM, Garbin U, Cominacini L (2016) Lung ultrasound in internal medicine: training and clinical practice. Crit Ultrasound J 8(1):10. doi:10.1186/s13089-016-0048-6
Mozzini C, Garbin U, Fratta Pasini AM, Cominacini L (2015) Short training in focused cardiac ultrasound in an Internal Medicine department: what realistic skill targets could be achieved? Intern Emerg Med 10(1):73–80
Ticinesi A, Lauretani F, Nouvenne A, Mori G, Chiussi G, Maggio M, Meschi T (2016) Lung ultrasound and chest X-ray for detecting pneumonia in an acute geriatric ward. Med (Baltim) 95(27):e4153
The criteria committee of the New York Heart Association (1994) Nomenclature and criteria for diagnosis of diseases of the heart and great vessels, 9th edn. Little, Brown & Co, Boston, Mass, pp 253–256
Gargani L, Volpicelli G (2014) How i do it: lung ultrasound. Cardiovasc Ultrasound 12:25–35
Lichtenstein DA, Mezière G (2008) Relevance of lung ultrasound in the diagnosis of acute respiratory failure. The BLUE protocol. Chest 134:117–125
Cogliati C, Casazza G, Ceriani E, Torzillo D, Furlotti S, Bossi I et al (2016) Lung ultrasound and short-term prognosis in heart failure patients. Int J Cardiol 218:104–108
Miglioranza MH, Gargani L, Tofani R, Rover MM, Martins VM, Mantovani A et al (2013) Lung ultrasound for the evaluation of pulmonary congestion in outpatients. JACC Cardiovasc Imaging 6(11):1141–1151
Platz E, Lewis EF, Uno H, Peck J, Pivetta E, Merz AA et al (2016) Detection and prognostic value of pulmonary congestion by lung ultrasound in ambulatory heart failure patients. Eur Heart J 36:1244–1251
Kilgore M, Patel KH, Kielhorn A, Maya FJ, Sharma P (2017) Economic burden of hospitalizations of Medicare beneficiaries with heart failure. Risk Manag Healthc Policy 10:63–70
Joynt KE, Jha AK (2011) Who has higher readmission rates for heart failure, and why? Implications for efforts to improve care using financial incentives. Circ Cardiovasc Qual Outcomes 4(1):53–59
Moroni F, Pinelli M, Bindi M, Moruzzo D, Castiglioni M, Pentimone F (2008) NT-proBNP in elderly patients: an useful marker for detecting heart failure? Recenti Prog Med 99(9):451–457
Huang F, Wang H, Huang B, Liu W, Peng Y, Zhang C et al (2016) The influence of body composition on the N-terminal pro-B-type natriuretic peptide level and its prognostic performance in patients with acute coronary syndrome: a cohort study. Cardiovasc Diabetol 15:58
Sabatine MS, Morrow DA, de Lemos JA, Omland T, Desai MY, Tanasijevic M et al (2004) Acute changes in circulating natriuretic peptide levels in relation to myocardial ischemia. J Am Coll Cardiol 44:1988–1995
Pinelli M, Bindi M, Cassetti G, Moroni F, Pandolfo C, Rosada J et al (2007) Relationship between low T3 syndrome and NT-proBNP levels in non-cardiac patients. Acta Cardiol 62(1):19–24
Burke MA, Cotts WG (2007) Interpretation of B-type natriuretic peptide in cardiac disease and other comorbid conditions. Heart Fail Rev 12:23–36
Trovato GM, Sperandeo M (2013) Sounds, ultrasounds and artifacts: which clinical role for lung imaging. Am J Respir Crit Care Med 187:780–781
Sperandeo M, Rotondo A, Guglielmi G, Catalano D, Feragalli B, Trovato GM (2014) Transthoracic ultrasound in the assessment of pleural and pulmonary diseases: use and limitations. Radiol Med 119:729–740
Blanco P, Volpicelli G (2016) Common pitfalls in point-of-care ultrasound: a practical guide for emergency and critical care physicians. Crit Ultrasound J 8:15–27
Author information
Authors and Affiliations
Contributions
CM and MDDP conceived the study and performed the ultrasound examinations; GP statistically analyzed the data, AT,AN, TM, AF, UG revised the data; LC, AC and MS revised the paper, CM wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Statement of human and animal rights
The study was conducted in accordance with the ethical standards laid down in the Helsinki Declaration of 1975 and its late amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Mozzini, C., Di Dio Perna, M., Pesce, G. et al. Lung ultrasound in internal medicine efficiently drives the management of patients with heart failure and speeds up the discharge time. Intern Emerg Med 13, 27–33 (2018). https://doi.org/10.1007/s11739-017-1738-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-017-1738-1