Abstract
Climate change causes a substantial impact on wheat yield and heat stress at the end of the harvest is a major problem for wheat production in India. Therefore, a study was carried out to ascertain the effects of terminal heat stress on 40 genotypes of Indian wheat with respect to 7 physiological and 6 agronomic parameters. Using chemometric analysis, such as principal component analysis (PCA), agglomerative hierarchical cluster analysis (AHC), and heat maps, the genotypes were grouped. Based on AHC and heat maps, the genotypes were divided into three clusters: tolerant, moderate, and sensitive. Tolerant genotypes such as DBW14, RAJ 3765, WH730, NW1014, and HALNA exhibited improved physiological response in terms of membrane stability index (MSI), chlorophyll content (CHL), photosynthesis rate (PN), antioxidative performance, and yield attributes under heat stress conditions while genotypes like Chirya7, HW2004, and many others were found to be relatively heat sensitive. Physiological traits like MSI, CHL, Proline, catalase (CAT), and PN were found to be the main determinants of genotype group assignments and showed positive correlations with grain yield. The results indicated that Thousand-grain weight (TGW), Grain yield (GY), and MSI could be used for the identification and assortment of heat-tolerant genotypes. In conclusion,thousand-grain weight (TGW) can be employed as a final assessment of heat tolerance after harvest. The findings also suggest that tolerant genotypes such as DBW 14, RAJ 3765, WH730, NW1014 HALNA, HI1563, and WH730 can be employed to develop climate-resilient varieties for India or other countries experiencing high-temperature stresses (HTS) at their terminal stage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triticum aestivum L., also known as wheat, is a cereal crop that is grown and consumed as a staple food by people worldwide because it provides more protein and calories to the global diet than any other food crop (Shewry 2009). In terms of crop output, wheat is one of the cereals that is one of the most widely grown around the globe and contributes about 20% of the food resources in the world. Moreover, the demand for wheat has gradually increased with the expansion of the population. The projected increase in wheat grain output needed to feed the expanding global population is 2% annually (Al-Ashkar et al. 2020).
Over the past few decades, a domino effect of climate change has led to a rapid change in natural ecosystems and consequently affects agricultural output. It has been predicted that the alteration in climatic conditions led to the rise in the earth's temperature with heat waves at the global level (Fernie et al. 2022; IPCC 2022). In general, the wheat crop may flourish in a particular range of temperatures. Further, in the wheat-growing region, the cultivation of wheat is affected due to heat waves (Mondal et al. 2016; Xu et al. 2022). Wheat farming systems have started to see a slight decline in grain productivity as a result of heat stress. In the south-Asian scenario, a 3–4 ℃ rise in average temperatures could result in a 25–35% reduction in wheat yield. It is forecast that the global wheat yield will decrease by 6% for every degree Celsius as the average current temperature rises (Asseng et al. 2019; Djanaguiraman et al. 2020). In the Indian subcontinent scenario, the majority of developmental stages of native wheat crops, particularly blooming and grain filling, require temperatures between 12 and 22 ℃.
Heat stress, especially with brief periods of remarkably high temperatures, is one of the most important threats facing wheat farmers worldwide because it severely inhibits metabolic functions in all plant tissues throughout the developmental stages and consequently causes a negative impact on grain yield and quality (Al-Ashkar et al. 2020; Riaz et al. 2021; Fernie et al. 2022). Wheat is particularly vulnerable to high temperatures during the flowering and grain-filling stages (Asseng et al. 2015; Ullah et al. 2022). The production of carbohydrates is limited under conditions of heat stress. Heat stress damages the thylakoid membrane and consequently restricts photosynthetic electron transfer, and halts photochemical reactions (Djanaguiraman et al. 2020; Yadav et al. 2022). Heat stress during anther development causes pollen sterility limits embryo development and subsequently lowers the number of grains per spike (Al-Ashkar et al. 2020). Heat during the grain-filling stage shortens the grain-filling time and hastens leaf senescence (Rehman et al. 2021).
In India, especially in the Indo-Gangetic plane (IGP), the cultivation of long-duration rice varieties such as Swarna (150-155 days) and the late onset of monsoon are the reasons for the delayed sowing of wheat. This makes wheat suffer due to terminal heat stress at the reproductive stage during the February–March months at terminal stage (Pandey et al. 2015; Dwivedi et al. 2017). Therefore, delayed sowing of wheat led to the crop facing heat stress (Temperature ≥ 31 ℃) at the terminal stage and subsequently a reduction in the rate of grain filling and lower grain yield (Rehman et al. 2021; Shenoda et al. 2021).
The green color of leaves which proportionally reflects the chlorophyll concentrations significantly affects grain yield and its constituent parts (Lopes and Reynolds 2012). However, the green area diminishes during heat stress. By mobilizing the stored stem water-soluble carbohydrates, the plant is likely to make up for the detrimental effects of heat-induced green area loss on final grain size (Rehman et al. 2021). Moreover, certain wheat genotypes have traits that enable them to cool the canopy during grain filling, giving them access to subsurface moisture and improving photosynthetic maintenance and evaporation (Pinto et al. 2010). Furthermore, earlier research established the progressive link between low canopy temperature with longer grain yield production in many wheat genotypes (Pinto and Reynolds 2015). Apart from the aforementioned, high temperatures also led to the induction of oxidative stress in plants (Hasanuzzaman et al. 2013). Thus, ROS scavenging mechanism via higher levels and activity of antioxidants are required for the protection of cells from oxidative damage (Hasanuzzaman et al. 2013; Jahan et al. 2022).
Indirect selection can also be achieved by using physiological traits. Therefore, physiological traits must be investigated to choose wheat genotypes that yield well and can tolerate high temperatures. These characteristics could serve as selection criteria. An investigation into an assortment of physiological features is required to find wheat genotypes that yield well and show tolerance to high temperatures. Many previous studies that screened a large number of wheat genotypes mainly depended on a few agronomical and physiological traits. Further, the clustering of genotypes by use of PCA and heat map gave an idea about the resistance and susceptibility of genotypes as per their cluster in the group. Thus, the present study was chosen to identify relatively heat-tolerant genotypes using chemometric approaches and establish their level of tolerance in terms of higher content of pigment, better gas exchange activity, and improved ROS scavenging mechanism. The identification of germplasm that can withstand high temperatures will benefit India as well as the global wheat program.
Material and methods
Experimental layout and growth conditions
The trial was carried out during the dry or rabi (November to April) seasons at the experimental field of the ICAR Research Complex for Eastern Region, Patna, India (25.594°N, 85.1376°E; 53 m above sea level). The soil of the experimental site is categorized as Indo-Gangetic alluvium soil having the ratio of sand, silt, and clay is 2.3: 4: 3.7. The other physico-chemical soil characteristics of soil as follows: pH 7.5, EC 0.05 dSm–1, organic carbon 0.6%, available N, P, and K of 213, 19, and 416 kg ha–1, respectively. The Randomized Block Design (RBD) method was followed for the experimental layout having 6 rows. The size of each plot was 5.4 × 5.4 m and 23 cm of space between them. Prior to the trials, the experimental field was fully laser-labelled, and ideal moisture levels were kept throughout the seeding period. The highest standards of crop management were upheld by the judicious use of fertilizer, pesticides, and regular watering. Urea, DAP, and murate of potash (MOP) were used as fertilizers and delivered NPK in the ratios of 6:3:2 respectively. A full dose of P, K, and 50% N was supplied as a basal dose. The 25% N doses were delivered during the crown root initiation (CRI), whereas the rest 25% N was supplied at the maximum tillering stage. The trial was conducted for two consecutive years.
Plant materials and sowing time
Forty genotypes (n = 40) of wheat were used in the experiment. We acquired wheat genotypes from the Division of Genetics, Indian Agricultural Research Institute, New Delhi, India. Wheat genotypes were categorized into two groups: timely sown normal (TS)—seed was sown in November; Late sown heat stress (LS)—seed was sown in January to experience heat stress during their terminal phase.
Sampling time and analysis parameters
The decimal code developed by Zadoks et al. (1974) was used to evaluate crop phenology. As per the Zadoks code, the 50% anthesis (Z65) and the completion of the anthesis (Z69) were documented. The plant samples at the Z69 stage were taken for physiological and biochemical analyses. The experiment included a heat tolerance screening of 40 wheat genotypes using phenological data [Days taken to 50% anthesis (DTA), days to physiological maturity (DTPM), grain filling durations (GFD)], physiological traits [(membrane stability index (MSI), relative water content (RWC), chlorophyll content (CHL), proline content, and catalase activity)], yield attributes [ear length (EL), thousand grains weight (TGW), and grain yield (GY)], and chemometric analysis [principal component analysis (PCA), agglomerative hierarchical cluster analysis (AHC), and heat map)].
Analysis of physicochemical parameters
Relative water content
The topmost fully expanded fresh leaves were collected, washed, and cut into 4 mm small discs. The fresh, turgid, and dry weight of 10 leaf discs were observed following the procedure outlined by Weatherley (1950). The RWC is computed by the equation mentioned below.
Membrane stability index (MSI)
The procedure outlined by Sairam et al. (1997) was used to calculate the leaf MSI. The MSI estimation was carried out using two sets of test tubes (C1 and C2), each containing 100 mg of leaf material and 10 mL of double-distilled water. In a water bath, set C1 was heated at 40 °C for 30 min, and set C2 was boiled at 100 °C for 10 min. Thereafter, the electrical conductivity (EC) of leaf samples was assessed on a conductivity bridge using a TDS analyzer (Elico, CM183EC-TDS analyzer, India). The MSI was computed as:
Total chlorophyll content
The total chlorophyll content (CHL) was extracted in 80% chilled acetone following the procedure outlined by Arnon (1949). The UV–Vis double beam spectrophotometer (UNICAM 500, Thermo, USA) was to measure the absorbance of extracted samples at 663 nm and 645 nm. The CHL was expressed as mg g−1 fresh weight and computed by following the equation
where A645 is the absorbance at 645 nm; A663 is the absorbance at 663 nm; W is the mass of the sample (in mg); and V is the volume of the solvent used (in mL).
Photosynthesis rate
The photosynthetic rate (PN) for fully grown flag leaves was determined between 10:00 and 11.30 AM by a portable infrared gas analyzer (LI-6400 Model, LICOR, USA) using an artificial light source with a power of 1000 mol (photon) m–2 s–1. The PN was expressed as μmol (CO2) m–2 s–1.
Analysis of catalase and proline
The catalase activity in pulverized plant leaf tissue was assessed following the methods described by Hameed et al. (2011). Catalase activity was calculated by recording the lysis of hydrogen peroxide (µmoles) per minute gram of fresh weight. The proline content was assessed spectrophotometrically (UNICAM 500, Thermo, USA) by examining the absorbance of leaves tissue extract at 240 nm. The leaves tissue extract formed by grinding 100 mg fresh tissue in 1.5 mL sulfosalicylic acid (3% w/v) following the outline prescribed by Bates et al. (1973). The calculated value was expressed in µmol g–1 FW.
Chemometric analysis
To evaluate the impact of heat stress on the observed traits of various wheat genotypes and identify the better wheat genotype under heat stress conditions, two chemometric analyses, namely PCA and AHC, were performed. Prior to the application of chemometrics, all the variables were autoscaled.
Statistical analysis and graph preparation
The experiments were carried out in triplicate and ASSISTAT 7.6 β-version software was employed to analyze the observed data for statistical significance. Duncan’s Multiple Range Test (DMRT) was used to compare one-way analysis of variance (ANOVA) and mean separations at the p ≤ 0.05 significant level. The standard error (SE) of the mean was calculated using MS Excel 2007. The correlation study was carried out using the SPSS16 program. The PCA and AHC were performed using JMP13 software.
Results
Forty wheat genotypes were taken under different sowing windows (timely (N) and late sown (HS) conditions) to assess their performances in terms of the occurrence of phenological stages physiological changes and grain yield. The study revealed that on an average days taken to 50% anthesis were 83.10 and 70.44 days under timely and late sown heat stress conditions respectively. Late sown genotypes take less time (13 days less than timely sown) to attain 50% anthesis. Further days taken to 75% physiological maturity was 117.6 days under TS and 93.95 days under late sown conditions. The difference between timely and late sown conditions was 23.65 days. The finding showed that under late sown heat stress conditions crop cycle was shortened and led to early maturity. Further grain filling duration was also shortened under late sown conditions, providing a shorter time for grain development. The average grain filling duration under timely sown condition was 34.5 while under late sown it was 23.51 days indicating 10.99 days less time to fill the developing grain at the crucial reproductive phase (Table 1).
Principal component analysis (PCA)
To identify any potential clusters within the examined samples, PCA was used for 40 different genotypes of wheat in the current study. The results of PCA produced a new set of 13 orthogonal variables (PCs). With an eigenvalue of 7.00, the first principal component represented 53.85% of the overall variability in the data set. The eigenvalues of the second and third principle components (PCs) were 1.28 and 1.02, respectively, accounting for 9.84% and 7.87% of the variance in the data. The last ten PCs (PC 4 to PC 13), which had progressively decreasing eigenvalues, cumulative represented less than 29% of the data set variability. To simplify the data interpretation, Varimax orthogonal rotation was applied solely to the first four PCs, which accounted for approximately 78.37% of the overall variability. Of the entire variability, the first rotating factor (PC1) explained 50.84%, and the second one (PC2) accounted for 10.49% variability. Whereas, 8.81% and 8.22% of the overall variability were explained by the third (PC3) and fourth PC (PC4), respectively. The most significant Varimax rotated PCs produced from the 13 variables in the current investigation, along with their statistical loading, are displayed in Table 2.
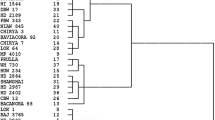
The genotype under investigation was also subjected to agglomerative hierarchical clustering (AHC). Based on shared characteristics, the genotypes were categorized. The outcomes acquired using AHC are shown in a heat map (Fig. 1) and are consistent with the PCA's results. AHC showed a clear clustering of genotypes under heat stress and normal conditions. Nevertheless, the distance between the genotype grown under normal and heat-stressed conditions was more pronounced. The first cluster comprised all the genotypes growing under heat stress conditions due to their lower value of DTA, DTPM, GFD, PN, TGW, GY, RWC, MSI, EL, CHL and intermediate to higher values of proline and CAT. Similarly, the second cluster consists of all the genotypes grown under normal conditions due to their higher value of DTA, DTPM, GFD, PN, TGW, GY, RWC, MSI, EL, CHL and lower values of proline and CAT. Two clusters were further subdivided into four sub-cluster. Subclusters 3 and 4 of cluster 1 were found to be the closest to the genotype grown under normal conditions due to their intermediate values of GFD, PN, TGW, GY, RWC, MSI, and EL.
Agglomerative hierarchical cluster analysis (AHC) and heat map of physiological properties of wheat genotype under normal and stress conditions. Comparative variable levels correspond to the color temperature. The color temperature scheme indicates relative variable levels ranging from minimum (green) to maximum (red) contents of the respective variable. DTA days taken to 50% anthesis, DTPM days to physiological maturity, GFD grain filling durations, PN photosynthesis rate, TGW thousand grains weight, GY grain yield, RWC relative water content, MSI membrane stability index, EL (Ears length), CHL chlorophyll content, CAT catalase activity, TR tiller per plant. Prefix N and S before the variety name represents timely sown/normal and late-sown/heat stress, respectively
Physiological and biochemical changes
Physiological and biochemical traits were significantly influenced under heat stress conditions (Table 1; Fig. 1). The study revealed that the mean RWC, MSI, Chl, and PN rate declined while the activity of catalase and proline content was improved under heat stress conditions. The average RWC under timely sown condition was 71.06 while under late sown it was 60.54%. The RWC showed a 14.8% reduction as compared to the normal sown condition. Moreover, the stability of the membrane is also compromised under heat stress conditions. The mean MSI was 65.45 and 56.61% under timely and late sown conditions, respectively and the average reduction was 13.5% as compared to normal conditions. The photosynthetic pigment content was also measured and findings revealed that heat stress has a significantly negative impact on the total chlorophyll content. The mean chlorophyll content was 4.01 and 3.45% under timely and late sown conditions, respectively. The average reduction from timely sown to late sown conditions was 13.9%. Further, the genotypes Halna, DBW 14, Raj3765, GW273, and NW1014 were able to maintain high RWC (%) and MSI (%) as compared to other genotypes while Babax, HW5210, and HI1531 were found susceptible. Further, the heat stress has a significant negative effect on gas exchange parameters (Photosynthesis; PN and transpiration rate; E) also. The average PN rate is 23.98 and 16.32 under normal and heat stress conditions, respectively. Further genotypes DBW14, Halna, Raj3765, NW1014, and GW273 were able to maintain the rate of PN and showed lower reduction under heat stress conditions as compared to other genotypes studied. Transpiration rate was also studied vis a vis under both sets of conditions. The study showed that the mean transpiration rate was lesser (4.95) under heat stress conditions as compared to normal conditions (5.12) across the genotypes studied.
The study showed that the level of osmolyte i.e. proline was significantly increased under heat stress conditions. The average proline content was 27.77 and 31.05 under timely and late-sown conditions, respectively. Further, at the genotypic level, the increase in proline content was maximum in HI1563 followed by GW273 and then HD2987. However, maximum proline content under late sown heat stress conditions was observed in Sonalika followed by HD2733 then in Raj3765. Apart from osmolyte, the activity of anti-oxidant (CAT) was also increased to combat the ROS activity. The finding of the study showed that the activity of antioxidant CAT was significantly increased under late-sown heat stress conditions. The mean CAT activity was 2.78 and 5.11 under normal and heat stress conditions, respectively. Further, at the genotypic level, the activity of CAT varies significantly among genotypes. It was observed that the increase in CAT activity was maximum in PBW273 followed by WH730 and then Raj3765 as compared to normal timely sown conditions. Further, the maximum CAT activity under late sown heat stress conditions was also observed in PBW273 followed by WH730 and then Raj3765.
Discussion
Heat stress at the terminal stage of crops severely affects the grain-filling of wheat leading to dramatic yield loss. Thus exploration of wheat genotypes for heat tolerance under extreme heat environments is the utmost requirement for sustainable productivity. Further, the mechanism of heat tolerance involves a series of morpho-physiological and molecular changes that often vary among wheat genotypes (Dhyani et al. 2013). The crop varieties can be more adapted to the predicted future climate by knowing how crops react to high temperatures and how to make crops more heat-tolerant (Halford 2009; Hossain et al. 2021). In the past several researchers (Viswanathan and Khanna-Chopra 2001; Sharma et al. 2013) adopted the method of stress susceptibility indices of yield for screening of the wheat genotypes for heat tolerance. Moreover, very little information is available on the screening of wheat genotypes for heat tolerance using various physiological and biochemical traits. Various physiological and biochemical parameters were employed in the current study to screen 40 wheat genotypes for heat stress resistance. The PCA is a variable reduction technique that identifies a smaller set of artificial variables, or principal components, that together explain the majority of the variation in the observed variables. The correlation between the original variable and the component (or rotated factor) obtained via PCA is known as factor loading. These figures indicate that the physiological parameter significantly contributes to the overall variability. The most crucial variables and characteristics in each dimension, or PCs, were found using significant factor loading values greater than or equal to 0.7, in accordance with the theoretical justifications of PCA presented by Hair et al. (2005). PC1 was positively contributed by DTA, DTPM, GFD, EL, GY, TGW, MSI, RWC, and PN whereas PC2 was positively contributed by negatively by CHL and positively by CAT. PC3 positively contributed by proline and PC4 contributed by TR (Table 2). The majority of heat-tolerant genotypes in the current investigation, including DBW 14, HALNA, WH730, and NW1014, were found in cluster I. The genotype DBW 14 has also been observed to be heat tolerant in the North Western/Eastern Plain Zone of India (Chatrath 2004). A total of four cultivars—RAJ 3765, HALNA, DBW 14, and WH730—that belong to the tolerant group were primarily grown in significantly warmer regions of India including western, eastern, and central areas. Further, the GY, TGW, CHL, MSI, and PN were determined to be the primary factors influencing the genotype clustering. These conclusions are supported by the correlation analysis. In the correlation study (Table 3), a positive association between grain yield and MSI, Chl, TGW, GFD, and PN were observed. Moreover, a significant positive correlation of PN with CHL value, and MSI emphasized the importance of these physiological traits as key factors for heat stress tolerance in wheat (Gupta et al. 2015).
The physiological functions of wheat and its phenological stages attainment differ significantly under heat stress conditions (Gupta et al. 2015). Further, genotypic differences play a key role in determining the tolerance level of wheat in terms of morpho-physiological responses (Almeselmani et al. 2012). In this study the grain yield decline under late shown heat stress conditions that might be due to lesser time for grain filling (GFD) (Table 1). Moreover, heat stress shortens the duration between anthesis and physiological maturity of the crop (Warrington et al.1977), which in turn reduces the grain weight (Warrington et al.1977; Shpiler and Blum 1991). Due to the high temperature at the terminal stage, the loss of an ample amount of water leads to lesser relative water content in leaves (Dwivedi et al. 2017). Heat stress led to disruptions in the chlorophyll pigment in terms of structure and function, and its content thus lower photosynthesis (Xu et al.1995). Heat stress severely altered the rate of photosynthesis and transpiration (Ahmad et al. 2021). Furthermore, damage to cellular membranes also resulted in the inhibition of chlorophyll biosynthesis and interruption in photosynthetic performance, thereby reducing the productivity of plants (Camejo et al. 2005). In another study, Savicka and Škute (2010) accounted that heat stress triggers the production and accumulation of ROS inside the plant cells. As a result, the lipid membrane of cell organelles like chloroplast is damaged and consequently, photosynthesis and overall growth of the plant are hampered. A similar observation was also observed by other workers (Hasanuzzaman et al. 2020; Dubey et al. 2020). Moreover, the recent finding revealed that heat stress decreases CO2 assimilation rate which was attributed to the reduction in stomatal conductance and intercellular CO2 (Kumari et al. 2021; Hussain et al. 2021). Furthermore, photosystem II (PSII) has more sensitivity towards heat stress (Bibi et al. 2008; Brestic et al. 2012); thus, impairment of PSII results in an interruption in electron transport and ATP synthesis during the photosynthetic function of pigment (Wang et al. 2018). Chovancek et al. (2019) demonstrated that the recovery mechanism following heat stress was provided by the effective control of linear electron transport and the avoidance of over-reduction in the acceptor side of photosystem I. Further, heat stress affects more promptly on water status of the plant by modulating osmotic adjustment due to the deprived photosynthetic ability, which subsequently causes sinking sugar content, decreasing the osmotic potential, and increasing transpiration rate (Hemantaranjan et al. 2018). In the study, wheat genotypes WH 730, Halna, HI1563, and NW 1014 maintained the PN rate under LS condition thereby validating its thermo-tolerance. Furthermore, scavenging of ROS by enhanced activity of the antioxidant enzyme in flag leaves under high-temperature conditions helped in the mitigation of oxidative damage (Zhao et al. 2018). In the current study also wheat genotypes RAJ 3765, GW 273, and HI1563 showed enhanced CAT activity and thus have the ability to detoxify the ROS and attenuated heat stress-induced oxidative membrane damage, and grain filling (Zhao et al. 2018). Osmoprotectant accumulation is expected to be another tactic employed by plants to withstand heat stress (Nahar et al. 2016). This study demonstrated a significant increase in proline content in RAJ2765, GW273, and HI1563, which is consistent with previous findings. Proline has also been reported to perform as a ROS scavenger and to protect cells from stress-induced damage (Hasanuzzaman et al. 2014).
Conclusion
This study demonstrated significant genotype-to-genotype heterogeneity in wheat's resistance to terminal heat stress. It would be preferable for the genotype screening process to combine physiological variables with agronomic, morphological, and yield traits. Our study clearly showed that the physic-chemical parameters found to be from cultivar to cultivar and even also varied with climate change played a crucial role in providing tolerance from the heat stress. In conclusion, 7 cultivars viz Raj3765, NW1014, Halna, HI1563, WH730, GW273, and DBW14 were found to be relatively more heat tolerant. These are the promising cultivars that can be employed in breeding programs for the further development of heat-tolerant new wheat varieties.
Availability of data and materials
The data used in this manuscript will be available upon reasonable request.
Abbreviations
- CAT:
-
Catalase
- TGW:
-
Thousand-grain weight
- MSI:
-
Membrane stability index
- PN:
-
Photosynthesis rate
- GY:
-
Grain yield
- HTS:
-
High-temperature stresses
References
Ahmad M, Waraich EA, Skalicky M, Hussain S, Zulfiqar U, Anjum MZ, Habib Ur Rahman M, Brestic M, Ratnasekera D, Lamilla-Tamayo L, Al-Ashkar I, El Sabagh A (2021) Adaptation strategies to improve the resistance of oilseed crops to heat stress under a changing climate: an overview. Front Plant Sci. https://doi.org/10.3389/fpls.2021.767150
Al-Ashkar I, Alotaibi M, Refay Y, Ghazy A, Zakri A, Al-Doss A (2020) Selection criteria for high-yielding and early-flowering bread wheat hybrids under heat stress. PLoS ONE 15(8):e0236351. https://doi.org/10.1371/journal.pone.0236351
Almeselmani M, Deshmukh PS, Chinnusamy V (2012) Effect of prolonged high temperature stress on respiration, photosynthesis and gene expression in wheat (Triticum aestivum L.) varieties differing in their thermotolerance. Plant Stress 6:25–32
Arnon DI (1949) Copper enzymes in isolated chloroplasts poly polyphenol oxidase Beta Vulgaris. Plant Physiol 24(1):1–15. https://doi.org/10.1104/pp.24.1.1
Asseng S, Ewert F, Martre P, Rotter RP et al (2015) Rising temperatures reduce global wheat production. Nat Clim Change 5:143–147. https://doi.org/10.1038/nclimate2470
Asseng S, Martre P, Maiorano A, Rötter RP, O’Leary GJ et al (2019) Climate change impact and adaptation for wheat protein. Glob Chang Biol 25(1):155–173. https://doi.org/10.1111/gcb.14481
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Bibi A, Oosterhuis D, Gonias E (2008) Photosynthesis, quantum yield of photosystem II and membrane leakage as affected by high temperatures in cotton genotypes. J Cotton Sci 12:150–159
Brestic M, Zivcak M, Kalaji HM, Carpentier R, Allakhverdiev SI (2012) Photosystem II thermostability in situ: environmentally induced acclimation and genotype-specific reactions in Triticum aestivum L. Plant Physiol Biochem 57:93–105. https://doi.org/10.1016/j.plaphy.2012.05.012
Camejo D, Rodrıguez P, Moralesb MA, Amicoa JMD, Torrecillas A, Alarcon JJ (2005) High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J Plant Physiol 162:281–289. https://doi.org/10.1016/j.jplph.2004.07.014
Chatrath R (2004) Breeding strategies for developing wheat varieties targeted for rice-wheat cropping system of Indo-Gangetic plains of Eastern India. In: Joshi AK, Chand R, Arun B, Singh G (eds) A compendium of the training program on wheat improvement in eastern and warmer regions of India: conventional and nonconventional approaches, 26–30 Dec 2003. NATP project, (ICAR), BHU, Varanasi, India.
Chovancek E, Zivcak M, Botyanszka L, Hauptvogel P, Yang X, Misheva S, Hussain S, Brestic M (2019) Transient heat waves may affect the photosynthetic capacity of susceptible wheat genotypes due to insufficient photosystem I photoprotection. Plants 8:282. https://doi.org/10.3390/plants8080282
Dhyani K, Ansari MW, Rama-Rao Y, Verma RS, Shukla A, Tuteja N (2013) Comparative physiological response of wheat genotypes under heat stress. Plant Signal Behav 8(6):e24564. https://doi.org/10.4161/psb.24564
Djanaguiraman M, Narayanan S, Erdayani E, Prasad PVV (2020) Effects of high temperature stress during anthesis and grain filling periods on photosynthesis, lipids and grain yield in wheat. BMC Plant Biol 20(1):268. https://doi.org/10.1186/s12870-020-02479-0
Dubey R, Pathak H, Chakrabarti B, Singh S, Gupta DK, Harit R (2020) Impact of terminal heat stress on wheat yield in India and options for adaptation. Agric Syst 181:102826. https://doi.org/10.1016/j.agsy.2020.102826
Dwivedi SK, Basu S, Kumar S, Kumar G, Prakash V, Kumar S, Mishra JS, Bhatt BP, Malviya N, Singh GP, Arora A (2017) Heat stress induced impairment of starch mobilisation regulates pollen viability and grain yield in wheat: study in Eastern Indo-Gangetic Plains. Field Crops Res 206:106–114. https://doi.org/10.1016/j.fcr.2017.03.006
Fernie E, Tan DKY, Liu SY, Ullah N, Khoddami A (2022) Post-anthesis heat influences grain yield, physical and nutritional quality in wheat: a review. Agriculture 12(6):886. https://doi.org/10.3390/agriculture12060886
Gupta NK, Khan A, Maheshwari A, Narayan S, Chhapola OP, Arora A, Singh G (2015) Effect of post anthesis high temperature stress on growth, physiology and antioxidative defense mechanisms in contrasting wheat genotypes. Indian JPlant Physiol 20(2):103–110. https://doi.org/10.1007/s40502-015-0142-y
Hair F, Anderson J, Tatham L, Black C (2005) Multivariate data analysis (5th ed.) Prentice Hall.
Halford NG (2009) New insights on the effects of heat stress on crops. J Exp Bot 60:4215–4216. https://doi.org/10.1093/jxb/erp311
Hameed A, Bibi N, Akhter J, Iqbal N (2011) Differential changes in antioxidants, proteases, and lipid peroxidation in flag leaves of wheat genotypes under different levels of water deficit conditions. Plant Physiol Biochem 49(2):178–185. https://doi.org/10.1016/j.plaphy.2010.11.009
Hasanuzzaman M, Nahar K, Alam M, Roychowdhury R, Fujita M (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14:9643–9684. https://doi.org/10.3390/ijms14059643
Hasanuzzaman M, Nahar K, Alam MM, Fujita M (2014) Modulation of antioxidant machinery and the methylglyoxal detoxification system in selenium-supplemented Brassica napus seedlings confers tolerance to high temperature stress. Biol Trace Elem Res 161(3):297–307. https://doi.org/10.1007/s12011-014-0120-7
Hasanuzzaman M, Nahar K, Khan MIR, Al Mahmud J, Alam MM, Fujita M (2020) Regulation of reactive oxygen species metabolism and glyoxalase systems by exogenous osmolytes confers thermotolerance in Brassica napus. Gesunde Pfanz 72:3–16. https://doi.org/10.1007/s10343-019-00476-4
Hemantaranjan A, Malik CP, Bhanu AN (2018) Physiology of heat stress and tolerance mechanisms-an overview. J Plant Sci. Res. 34:51–64. https://doi.org/10.32381/JPSR.2018.34.01.7
Hossain A, Skalicky M, Brestic M, Maitra S, AshrafulAlam M, Syed MA, Hossain J, Sarkar S, Saha S, Bhadra P, Shankar T, Bhatt R, Chaki AK, Sabagh AEL, Islam T (2021) Consequences and mitigation strategies of abiotic stresses in wheat (Triticum aestivum L.) under the changing climate. Agronomy. 11(2):241. https://doi.org/10.3390/agronomy11020241
Hussain S, Ulhassan Z, Brestic M, Zivcak M, Zhou W, Allakhverdiev SI, Yang X, Safdar ME, Yang W, Liu W (2021) Photosynthesis research under climate change. Photosynth Res 150(1–3):5–19. https://doi.org/10.1007/s11120-021-00861-z
IPCC (2022) AR6: The Working Group II contribution, Climate Change 2022: Impacts, Adaptation and Vulnerability.
Jahan MS, Hasan MM, Alotaibi FS, Alabdallah NM, Alharbi BM et al (2022) Exogenous putrescine increases heat tolerance in tomato seedlings by regulating chlorophyll metabolism and enhancing antioxidant defense efficiency. Plants 11(8):1038. https://doi.org/10.3390/plants11081038
Kumari VV, Roy A, Vijayan R, Banerjee P, Verma VC, Nalia A, Pramanik M, Mukherjee B, Ghosh A, Reja MH et al (2021) Drought and Heat stress in cool-season food legumes in sub-tropical regions: consequences, adaptation, and mitigation strategies. Plants 10:1038. https://doi.org/10.3390/plants10061038
Lopes MS, Reynolds MP (2012) Stay-green in spring wheat can be determined by spectral reflectance measurements (normalized difference vegetation index) independently from phenology. J Exp Bot 63(10):3789–3798. https://doi.org/10.1093/jxb/ers071
Mondal S, Singh RP, Mason ER, Huerta-Espino J, Autrique E, Joshi AK (2016) Grain yield: adaptation and progress in breeding for early-maturing andheat-tolerant wheat lines in South Asia. Field Crops Res 192:78–85. https://doi.org/10.1016/j.fcr.2016.04.017
Nahar K, Hasanuzzaman M, Rahman A, Alam MM, Mahmud JA, Suzuki T, Fujita M (2016) Polyamines confer salt tolerance in mung bean (Vigna radiata L.) by reducing sodium uptake, improving nutrient homeostasis, antioxidant defense, and methylglyoxal detoxification systems. Front Plant Sci. 7:1104. https://doi.org/10.3389/fpls.2016.01104
Pandey GC, Mamrutha HM, Tiwari R, Sareen S, Bhatia S, Siwach P, Tiwari V, Sharma I (2015) Physiological traits associated with heat tolerance in bread wheat (Triticum aestivum L.). Physiol Mol Biol Plants. 21(1):93–99. https://doi.org/10.1007/s12298-014-0267-x
Pinto RS, Reynolds MP (2015) Common genetic basis for canopy temperature depression under heat and drought stress associated with optimized root distribution in bread wheat. Theor Appl Genet 128(4):575–585. https://doi.org/10.1007/s00122-015-2453-9
Pinto RS, Reynolds MP, Mathews KL, McIntyre CL, Olivares-Villegas JJ, Chapman SC (2010) Heat and drought adaptive QTL in a wheat population designed to minimize confounding agronomic effects. Theor Appl Genet 121(6):1001–1021. https://doi.org/10.1007/s00122-010-1351-4
Rehman HU, Tariq A, Ashraf I, Ahmed M, Muscolo A, Basra SMA, Reynolds M (2021) Evaluation of physiological and morphological traits for improving spring wheat adaptation to terminal heat stress. Plants 10(3):455. https://doi.org/10.3390/plants10030455
Riaz MW, Yang L, Yousaf MI, Sami A, Mei XD, Shah L, Rehman S, Xue L, Si H, Ma C (2021) Effects of heat stress on growth, physiology of plants, yield and grain quality of different spring wheat (Triticum aestivum L.) genotypes. Sustainability. 13(5):2972. https://doi.org/10.3390/su13052972
Sairam RK, Deshmukh PS, Shukla DS (1997) Tolerance to drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J Agron Crop Sci 178:171–177. https://doi.org/10.1111/j.1439-037X.1997.tb00486.x
Savicka M, Škute N (2010) Effects of high temperature on malondialdehyde content, superoxide production and growth changes in wheat seedlings (Triticum aestivum L.). Ekologija 56:26–33. https://doi.org/10.2478/v10055-010-0004-x
Sharma A, Rawat RS, Verma JS, Jaiswal JP (2013) Correlation and heat susceptibility index analysis for terminal heat tolerance in bread wheat. J Central Eur Agri 14(2):535–544. https://doi.org/10.5513/JCEA01/14.2.1233
Shenoda JE, Sanad MNME, Rizkalla AA, El-Assal S, Ali RT, Hussein MH (2021) Effect of long-term heat stress on grain yield, pollen grain viability and germinability in bread wheat (Triticum aestivum L.) under field conditions. Heliyon. 7(6):e07096. https://doi.org/10.1016/j.heliyon.2021.e07096
Shewry PR (2009) Wheat. J Exp Botany 60(6):1537–1553. https://doi.org/10.1093/jxb/erp058
Shpiler L, Blum A (1991) Heat tolerance for yield and its components in different wheat cultivars. Euphytica 51(3):257–263. https://doi.org/10.1007/BF00039727
Ullah A, Nadeem F, Nawaz A, Siddique KHM, Farooq M (2022) Heat stress effect on reproductive physiology and yield of wheat. J Agron Crop Sci 208:1–17. https://doi.org/10.1111/jac.12572
Viswanathan C, Khanna-Chopra R (2001) Effect of heat stress on grain growth, starch synthesis and protein synthesis in grains of wheat (Triticum aestivum L.). Varieties differing in grain weight stability. J Agron Crop Sci 186:1–7. https://doi.org/10.1046/j.1439-037x.2001.00432.x
Wang QL, Chen JH, He NY, Guo FQ (2018) Metabolic reprogramming in chloroplasts under heat stress in plants. Int J Mol Sci 19:849. https://doi.org/10.3390/ijms19030849
Warrington IJ, Dunstone RL, Green LM (1977) Temperature effects at three developmental stages on the yield of the wheat ear. Aust J Agr Res 28(1):11–27. https://doi.org/10.1071/AR9770011
Weatherley PE (1950) Studies in the water relations of the cotton plant. The field measurement of water deficits in leaves. New Phytol 49:81–87. https://doi.org/10.1111/j.1469-8137.1950.tb05146.x
Xu Q, Paulsen AQ, Guikema JA, Paulsen GM (1995) Functional and ultrastructural injury to photosynthesis in wheat by high temperature during maturation. Environ Exp Bot 35:43–54. https://doi.org/10.1016/0098-8472(94)00030-9
Xu J, Lowe C, Hernandez-Leon SG, Dreisigacker S, Reynolds MP, Valenzuela-Soto EM, Paul MJ, Heuer S (2022) The Effects of brief heat during early booting on reproductive, developmental, and chlorophyll physiological performance in common wheat (Triticum aestivum L.). Front. Plant Sci. 13:886541. https://doi.org/10.3389/fpls.2022.886541
Yadav MR, Choudhary M, Singh J, Lal MK, Jha PK et al (2022) Impacts, tolerance, adaptation, and mitigation of heat stress on wheat under changing climates. Int J Mol Sci 23(5):2838. https://doi.org/10.3390/ijms23052838
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Res 14:415–421. https://doi.org/10.1111/j.1365-3180.1974.tb01084.x
Zhao Q, Zhou L, Liu J, DuX A-A-U, Huang F, Pan G, Cheng F (2018) Relationship of ROS accumulation and superoxide dismutase isozymes in developing anther with floret fertility of rice under heat stress. Plant Physiol Biochem 122:90–101. https://doi.org/10.1016/j.plaphy
Acknowledgements
The authors thankfully acknowledge the ICAR-RCER for providing the necessary facilities for the successful conduction of the experiment.
Funding
In-house project. Indian Council Of Agriculture Research, New Delhi, India.
Author information
Authors and Affiliations
Contributions
SKD: conceived the research, and designed experiments; JSM, and SK and SKD: performed the experiments; SKD, TKK and SKS: analyzed the data and prepared the draft; SKD and SKS: edited the draft and prepared the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
No ethical approval is required.
Consent for publication
All authors have seen the latest version of the manuscript and agree to its publication.
Additional information
Communicated by M. Oka.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dwivedi, S.K., Soni, S.K., Mishra, J.S. et al. Assessment of terminal heat tolerance ability of wheat genotypes based on chemometric analysis and agro-physiological traits. Acta Physiol Plant 46, 48 (2024). https://doi.org/10.1007/s11738-024-03677-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-024-03677-1