Abstract
Drought stress severely affects plant growth and productivity. Black gram is an extensively cultivated legume crop worldwide. Its production has not improved much in the last decade as it is adversely affected by biotic and abiotic stresses among which drought is a major factor. Salicylic acid (SA) pre-treatment to a Vigna mungo variety significantly increases chlorophyll, proline, carbohydrate, and total phenolic content. APX, GPX and SOD activities also increase and CAT activity decreased. At molecular level, induced expression of various stress-related genes, i.e. heat shock protein (Hsp), calmodulin (CAM), malate dehydrogenase (MD), metallothionein (MT), mitogen-activated protein kinase (MAPK), tryptophan synthase (TSN), zinc finger (ZF), phenylalanine ammonia lyase (PAL) and WRKY proteins are analysed by quantitative RT PCR after 1 mM SA treatment under short-term drought stress. It is observed that 1 mM SA pre-treatment is optimum to increase tolerance against short-term drought stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Black gram, originated in India, is a short duration (90–120 days) legume crop, accounting for about 20% of world pulse production (Pandey 2019). Globally, higher production and consumption of black gram is reported in India, and here, it is the third important pulse (Pandey and Chakraborty 2016). It has a high nutritive value and is also used in sustainable cropping systems, medicinal preparations, nutraceuticals and cosmetics. The production of black gram has not improved in last decade mainly due to various stresses (Pandey and Chakraborty 2015). Drought stress has some typical features; it is a slow event, difficult to find out the starting and end points, no single indicator, difficult to quantify and is estimated to decrease crop productivity by half globally (Feng et al. 2020).
Plant response to drought may be instantaneous by change in protein phosphorylation and may extend to prolonged period by altered gene expression. The response intensity depends on different features, i.e. species and genotype of plants, length and severity of drought, age and stage of development of plants, organ and cell type and the sub cellular compartment of plants. Several biochemical and molecular parameters are modulated in response to drought.
Chlorophyll content is reduced under drought stress by chlorophyll degrading enzymes in sunflower (Kiani et al. 2008), Vaccinium myrtillus (Tahkokorpi et al. 2007) and also in cotton (Massacci et al. 2008). Reduced chlorophyll content is linked to reduced Rubisco activity, decreased gas exchange, instable protein complexes and damaged chlorophyll (Bota et al. 2004).
Reduced pigment content and closure of stomata under drought leads to reduction in photosynthesis and ultimately results in reduced carbohydrate content (Yazdanpanah et al. 2011).
In addition, increased lipid peroxidation and membrane injury index under drought disrupts leaf integrity and further reduces photosynthetic capacity (Zlatev et al. 2006).
Proline (a metal chelator) accumulation is a very early response of plants against water deficit. It inhibits lipid peroxidation, scavenges singlet molecular oxygen and stabilises protein structures (Ashraf and Foolad 2007). It is a key antioxidant and able to prevent programmed cell death (Chen and Dickman 2005).
Drought stress in plants increases reactive oxygen species (ROS) which can be disruptive if not brought under tolerable limits. H2O2 produced under stress is a signal molecule at lower concentrations but highly destructive at higher concentrations and may cause programmed cell death (Breusegem et al. 2001; Quan et al. 2008). H2O2 prevents CO2 fixation (up to 50%) in plants by oxidising the SH groups of the enzymes in Calvin cycle (Foyer and Shigeoka 2011).
To bring down the levels of ROS, plants have non enzymatic-enzymatic clean-up mechanism (Halliwell and Gutteridge 2006) where antioxidants play major role in the detoxification of ROS. Superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidase (APX) are key enzymatic antioxidants.
Polyphenolic compounds (secondary metabolites) protect plants against biotic and abiotic stress and phenylalanine ammonia lyase (PAL) play central role in their synthesis (Yang et al. 2016). Increase in phenolic content is reported under drought, high and low temperature, low soil fertility, high light intensity, water logging and UV radiations (Mole et al. 1988; Delalonde et al. 1996; Close and McArthur 2002; Ali and Abbas 2003; Gholizadeh 2011; Kabiri et al. 2014). Phenyl alanine ammonia lyase (PAL) is a component of antioxidative defence system and facilitates the protection against different stresses such as drought. Superoxide dismutase (SOD) works as a first line of defence against oxidative stress (Wang et al. 2020). Increased SOD activity is studied by Sharma and Dubey (2005) in rice, Zlatev et al. (2006) in common bean and also by Wang et al. (2008) in white clover and alligator weed against drought stress.
Catalase is a light sensitive enzyme that converts H2O2 into H2O and O2. Sharma and Dubey (2005) reported the decrease in CAT activity in rice seedlings under drought stress. Scavenging efficiency of CAT for H2O2 is lower when compared with peroxidase (Erdal and Dumlupinar 2010).
Ascorbate peroxidase (APX) uses ascorbate as a hydrogen donor to reduce the H2O2 in water-water and AA-GSH cycles (Asada 2000). APX has higher affinity (µM range) for H2O2 when compared with CAT and POD (mM range). Increased APX activity is reported by Yang et al. (2008) in Picea asperata and by Zlatev et al. (2006) in Phaseolus vulgaris against drought stress.
Guaiacol and pyrogallol work as electron donor for Guaiacol peroxidase (GPX) activity (Jebara et al. 2005). Increased GPX activity was reported by Zhang et al. (2006) and by Pan et al. (2006) in liquorice under stress conditions.
At molecular level, plants turn on or turn off a series of genes under different stresses. Heat shock protein (Hsp), calmodulin (CAM), malate dehydrogenase (MD), metallothionein (MT), mitogen-activated protein kinase (MAPK), tryptophan synthase (TSN), zinc finger (ZF), phenylalanine ammonia lyase (PAL) and WRKY directly or indirectly protect plants against stress conditions.
Pre-treatment with nitric oxide, ethylene and salicylic acid (SA) that are involved in plant signalling process have been used to increase tolerance to environmental stresses. SA is a phenolic compound and acts as a plant growth regulator; is able to affect various physiological, biochemical and molecular processes. Under stress condition, it protects photosynthetic pigments and enhances the activity of antioxidative enzymes (Gill et al. 2016; Wang et al. 2017). Involvement of SA against environmental stresses, particularly against biotic stresses is well established in black gram (Kundu et al. 2012).
In the present study, V. mungo plants are given SA pre-treatment and put under short-term drought stress to evaluate its effectiveness in increasing tolerance.
Materials and methods
Seeds of T9 plants were obtained from Division of Plant Biology, Bose Institute, Kolkata, India. Germinating seeds were transferred to soil filled plastic pots (capacity: 1L). The pots were kept in the growth chamber at (30 ± 2 °C). Three-week-old test plants (21 DAS) were grouped into 5 sets, each set consisted of 12–15 replicate plants as follows: set 1—healthy control plants; set 2—untreated plants; set 3, 4 and 5—leaves of T9 plants were pre-treated with either 0.5 mM, 1 mM or 3 mM of SA, respectively, by spraying until run-off and subsequently subjected to drought stress by withholding water after three days (72 h). Physiological, biochemical and molecular studies were done at the time of SA treatment, after 72 h of SA treatment, after 3 days of drought stress and after 24 h of re-watering (recovery).
Physiological study
The leaves of T9 plants were noted at the time of SA treatment, after SA treatment, after short drought stress and on recovery for any necrotic symptoms.
Biochemical study
Chlorophyll content (Arnon 1949), carbohydrate content (Hedge and Hofreiter 1962), H2O2 content (Alexieva et al. 2001) total phenolic content (Singleton et al. (1999) method with modifications as mentioned by Chakraborty et al. (2008), proline content (Bates et al. 1973), and lipid peroxidation (De Vos et al. 1989) were determined.
To determine the activities of antioxidant enzymes, fresh leaves (0.5 g) were homogenised in a mortar and pestle under ice-cold condition with 5 ml extraction buffer (50 mM phosphate buffer pH 7.0, 1 mM EDTA, 1 mM ascorbate, 1 mM PVP and 0.05% tritonX100). It was centrifuged at 5000 g for 20 min at 4 °C. Supernatant was collected and stored at − 20 °C for the assay. Activity of superoxide dismutase (Beauchamp and Fridovich 1971) as modified by Madamanchi et al. (1994); catalase (Miyagawa et al. 2000), ascorbate peroxidase (Chen and Asada 1989), guaiacol peroxidase (Kar and Mishra 1976) and PAL activity (Chakraborty et al. 2008) were determined.
Statistical analyses
Data are analysed by two-way analysis of variance to detect the overall significant (p < 0.05) differences between all means using SYSTAT (Ver. no. 13.00.05 SYSTAT software Inc. 2009).
Quantitative RT PCR
Quantitative RT PCR was performed and the activity of the following genes was observed: heat shock protein (Hsp), calmodulin (CAM), mitogen-activated protein kinase (MAPK), zinc finger (ZF), malate dehydrogenase (MD), tryptophan synthase (TSN), metallothionein (MT), phenylalanine ammonia lyase (PAL), rubisco activase (RA) and WRKY proteins, at the time of 1 mM SA treatment, after 72 h of SA treatment, after short-term drought stress and on recovery. Prior to RT PCR, RNA isolation and cDNA synthesis were done using Spectrum Plant Total RNA Kit (Sigma Aldrich, catalog no. STRN50) and high-capacity cDNA reverse transcription kit (Applied Biosystems, catalog no. 4368814), respectively. RT PCR was done using DyNAmo Color Flash SYBR Green qPCR kit (Thermo scientific, catalog no. F-416L) in a Qiagen Rotor Gene Q system. Quantitative study of the candidate genes was done according to Livak and Schmittgen (2001).
Results and discussion
Drought stress limits the plant growth and yields. Exogenous application of salicylic acid, an endogenous plant growth regulator, protects plants against stress conditions.
In the present study, wilting of leaves was observed in V. mungo plants under short-term drought stress. SA treatment at 1 mM concentration lowered wilting after 3 days of drought stress and on recovery while 3 mM SA treatment damaged the leaves and necrotic regions were observed. It is reported earlier the lower concentration of SA works as a defence strategy against drought stress, while higher concentration of SA generally damages the plants (Joseph et al. 2010).
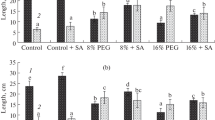
Reduction in chlorophyll content was observed under drought stress (Fig. 1a, b, c). When the plants were re-watered, increased chlorophyll content was observed in all plants except untreated plants when compared with drought-stressed plants of the same set. Higher chlorophyll content was observed in 1 mM SA-treated plants when compared to other concentrations. Alam et al. (2013) reported reduced chlorophyll content under drought stress.
In the present study, highest H2O2 content was observed in 3 mM SA-treated plants (Fig. 2a) and this may be the cause of necrotic regions observed in the leaves. SA treatment is reported to increase H2O2 and may lead to injury to plant tissue (Habibi 2012). H2O2 at lower concentration plays important role in signal transduction against stress conditions (Gong et al. 2005) while at higher concentration it is harmful to plants.
Total phenolic content was enhanced under drought stress as well as after SA treatment in the present study (Fig. 2b). Previously, Ali et al. (2007) reported enhanced level of total phenolics on SA treatment. Due to higher antioxidant properties that decrease ROS level under stress condition, phenolic compounds may facilitate plant defence against environmental stresses (Chakraborty et al. 2008).
Lipid peroxidation increased in drought-stressed plants after 3 days of short-term drought stress while lower level of lipid peroxidation was observed in 0.5 mM SA-treated plants and 1 mM SA-treated plants (Fig. 2c). Decrease in lipid peroxidation has been reported on SA treatment under drought stress (Alam et al. 2013; Kabiri et al. 2014).
Higher proline content was observed in 3 mM SA-treated plants, while it was lower in drought-stressed plants under short-term drought stress (Fig. 3a). Enhanced level of proline under drought stress is reported by Yazdanpanah et al. (2011) and Patel and Hemantaranjan (2012) on SA treatment and may act as a protective mechanism.
Drought stress adversely affected the ability of the plant metabolism and decreased carbohydrate content was observed after 3 days of drought stress (Fig. 3b). Treatment with SA could prevent the adverse effects of drought and an increase in carbohydrate concentration was observed. Increased carbohydrate content after SA treatment is previously reported by Yazdanpanah et al. (2011).
PAL activity increased on SA treatment in a concentration-dependent manner and also after 3 days of short-term drought stress (Fig. 4). The increase in PAL activity explains the increased phenolic concentration in the leaves and is previously reported by Ali et al. (2007).
The activity of the antioxidant enzymes, APX, GPX and SOD activity (Fig. 5a–c) increased, while CAT activity (Fig. 6) decreased on SA treatment. Increased activity of the antioxidant enzymes APX (Saruhan et al. 2012), GPX (Horvath et al. 2007) and SOD (Saruhan et al. 2012), and decreased activity of CAT (Shakirova, (2007) on SA treatment is due to the activation of antioxidant defence mechanism of plants to increase tolerance under stress conditions.
Induced Hsp (heat shock protein) expression is reported in biotic as well as abiotic stress conditions (Reddy et al. 2014; Pavlova et al. 2009), while there are no reports of their induction under normal conditions (Zhang et al. 2008). In the present study, increased Hsp expression was observed after SA treatment as well as under short-term drought stress and among all treatments higher fold change was observed for 1 mM SA-treated plants after short-term drought stress (Fig. 7a). HSPs work as molecular chaperones, highly conserved polypeptides and play pivotal role under biotic and abiotic stress (Kotak et al. 2007). HSPs also increase the membrane stability and scavenge ROS by regulating antioxidant enzymes. They help in protein folding and restrict irreversible mis fold of proteins to maintain cellular homeostasis against biotic and abiotic stress (Ahuja et al. 2010).
Intracellular plant Ca2+ level is influenced by different environmental stress conditions including salinity and drought stress, during plant growth and development (Xu et al. 2011). Intracellular Ca2+ plays important role in plant defence mechanisms as it acts as a signal molecule against stress conditions (Yang et al. 2010). Calmodulin (CAM) is a Ca2+ sensor, important in different calcium-dependent signalling pathways (Sun et al. 2001). AtCML9 (Magnan et al. 2008), AtCML24 (Delk et al. 2005) and AtCML42/43 (Chiasson et al. 2005) are modulated under different stress conditions.
In the present study, expression of CAM was induced after SA treatment and among all treatments higher expression was studied in 1 mM SA-treated plants under short-term drought stress (Fig. 7b).
Malate dehydrogenase (MD) expression was induced after SA treatment as well as under short-term drought stress in the present study (Fig. 7c). MD is a key enzyme of malate/aspartate shuttle and TCA (tri-carboxylic acid cycle). Different isoforms of MD differ in their localization and specificity for the NAD or NADP. Higher accumulation of MD is reported in soyabean leaf against drought and heat stress (Das et al. 2016). Increased expression of malate dehydrogenase like protein is reported in common bean against water-deficit conditions (Recchia et al. 2013).
A higher expression of Metallothionein (MT) was observed on 1 mM SA treatment under 3 days of short-term drought stress. Induced expression of MT was also studied after SA treatment but it was less when compared with 1 mM SA treatment under drought stress (Fig. 8a). MTs keep intracellular metal homeostasis and work as a ROS scavenger (Zhou et al. 2006). Yang et al. (2009) found the over expression of OsMT1a that increased the level of APX, CAT and POD in transgenic rice plants when compared to wild-type rice plants under drought stress. Accumulation of OsMT1a (Yang et al. 2009) and MT3 (cotton metallothionein protein) (Xue et al. 2009) are reported under water-deficit condition that protect plants from harmful effects of ROS. On the other hand, MT2 (metallothionein protein) in Arabidopsis is not induced by SA treatment (Murphy and Taiz 1995).
Mitogen-activated protein kinase (MAPK) cascades play pivotal role in various signalling pathways and respond to drought, wounding, salt stress, cold and oxidative stress (Ichimura et al. 2000; Yuasa et al. 2001). In the present work, MAPK expression was induced after SA treatment but not under short-term drought stress (Fig. 8b). Among all treatments, higher fold change was observed in 1 mM SA-treated plants on 3 days of drought stress. MAPK genes as AtMPK3, AtMPK4, AtMPK6, OsMPK1, OsMPK5, OsMPK12, GhMPK7 and p48 SIP kinase, etc. are reported to be induced by SA treatment and are essential for signal transduction (Li et al. 2012). Expression of OsMAPK44 is induced in rice against water deficit and salt stress (Jeong et al. 2006).
Tryptophan synthase is an important enzyme in tryptophan and indole synthesis. Higher expression of TSN gene was observed at 1 mM SA treatment after drought stress (fold change: 3.81) and lower expression in recovered drought-stressed plants (fold change: 0.33) (Fig. 9a). Here, expression of this gene was induced after drought stress (D) and SA treatment (1 mM SA and 1 mM D). There is no literature available on the effect of SA treatment on TSN expression. Camalexin (3-thiazol-2′-yl-indole) which is originated from tryptophan is a phytoalexin of Arabidopsis thaliana. Inducted expression of Camalexin is studied by plant pathogens and salicylic acid plays important role in it (Glawischnig 2007).
Zinc finger motifs are involved in RNA binding, transcriptional regulations, apoptosis, protein–protein interactions and growth and development of plants. These motifs are able to protect plants against salt and drought stress and suitable for engineering crop plants with enhanced resistance (Xu et al. 2008). Expression of various ZF proteins, i.e. ZFP252, ThZF1, GsZFP1, OsZnI, AtZAT6, AtTZF2, AtTZF3, GhTZF1 and OsTZF1 are reported under drought stress (Jan et al. 2013; Shi et al. 2014; Zhou et al. 2014). ZF activity was induced after SA treatment and also after short-term drought stress (Fig. 9c). Different ZF proteins as CaKR1, GhTZF1 and OsTZF1 are regulated by SA (Jan et al. 2013).
Collaborating the biochemical study, PAL gene was also induced on SA treatment and under short-term drought stress (Fig. 10a). Higher fold change was studied for 1 mM SA-treated plants after 3 days of drought stress. These findings are in accordance to the results obtained by Phimchan et al. (2014). Regulation of PAL activity by SA has been reported by Xu et al. 2012.
Rubisco gene expression was increased after SA treatment but after short-term drought stress, it decreased (Fig. 10b). Drought stress damages the Rubisco protein and Rubisco activase (RA) works as a chaperone and protects synthesis of chloroplast protein from drought-induced damage. Aranjuelo et al. (2011) and Zhou et al. (2007) reported reduced RA activity in plants under drought stress while some workers reported minor or even no hindrance in Rubisco activity (Pelloux et al. 2001; Flexas et al. 2006).
WRKY genes (group of transcription factors) are involved in plant development, dormancy, drought tolerance, embryogenesis and thermal hysteresis (Xie et al. 2005) and some of them are reported to be regulated by SA (Zhang et al. 2012). WRKY transcription factors play pivotal role in stress response or tolerance and also regulate different plant processes (Phukan et al. 2016). Ding et al. (2016) in wheat, Wang et al. (2015) in soybean and Wu et al. (2017) in common bean studied that WRKY proteins facilitate tolerance against abiotic stresses. Niu et al. (2012) studied the role of TaWRKY2 (wheat WRKY gene) in growth, development and also against stress condition. Increased level of WRKY21 is reported in Arabidopsis that facilitates the drought tolerance (Jiang et al. 2012). In the present study, expression of WRKY was induced under short term of drought stress and also after SA treatment (Fig. 10c). Higher fold change was observed for 1 mM D (1 mM SA-treated plants after 3 days of drought stress).
Conclusion
A summary of modulation of biochemical and molecular processes in V. mungo plants treated with SA and then put under drought stress is represented in Fig. 11. The role of SA against biotic and abiotic stresses as a plant defence molecule is well established but there is no literature available for the effect of SA pre- treatment in pulses under short-term drought stress. 1 mM SA treatment was found to suitable for T9 plants to tolerate short term of drought stress while higher concentrations, i.e. 3 mM damaged the plants.
References
Ahuja I, De Vos RCH, Bones AM, Hall RD (2010) Plant molecular stress responses face climate change. Trends Plant Sci 15:664–674
Alam MM, Hasanuzzaman M, Nahar K, Fujita M (2013) Exogenous salicylic acid ameliorates short-term drought stress in mustard (Brassica juncea L.) seedlings by up-regulating the antioxidant defense and glyoxalase system. Aust J Crop Sci 7(7):1053–1063
Alexieva V, Sergiev I, Mapellis S, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ 24:1337–1344
Ali RM, Abbas HM (2003) Response of salt stressed barley seedlings to phenyl urea. Plant Soil Environ 49(4):158–162
Ali MB, Hahn EJ, Paek KY (2007) Methyl jasmonate and salicylic acid induced oxidative stress and accumulation of phenolics in Panax ginseng bioreactor root suspension cultures. Molecules 12:607–621
Aranjuelo K, Molero G, Erice G, Avice JC, Nogue S (2011) Plant physiology and proteomics reveals the leaf response to drought in alfalfa (Medicago sativa L.). J Exp Bot 62(1):111–123
Arnon DI (1949) Copper enzymes in isolated chloroplasts: polyphenol oxidases in Beta vulgaris. Plant Physiol 24:1–15
Asada K (2000) The water-water cycle as alternative photon and electron sinks. Phil Trans R Soc Lond B Biol Sci 355:1419–1431
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59(2):206–216
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Bota J, Flexas J, Medrano H (2004) Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytol 162:671–681
Breusegem FV, Vranova E, Dat JF, Inze D (2001) The role of active oxygen species in plant signal transduction. Plant Sci 161(3):405–414
Chakraborty D, Sircar D, Mitra A (2008) Phenylalanine ammonia-lyase- mediated biosynthesis of 2–hydroxy–4- methoxy benzaldehyde in roots of Hemidesmus indicus. J Plant Physiol 165(10):1033–1040
Chen GX, Asada K (1989) Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol 30:987–998
Chen C, Dickman MB (2005) Proline suppresses apoptosis in the fungal pathogen Colletotrichum trifolii. P Natl Acad Sci USA 102(9):3459–3464
Chiasson D, Ekengren SK, Martin GB, Dobney SL, Snedden WA (2005) Calmodulin-like proteins from Arabidopsis and tomato are involved in host defense against Pseudomonas syringaepv. tomato. Plant Mol Biol 58(6):887–897
Close DC, McArthur C (2002) Rethinking the role of many plant phenolics: protection from photo damage not herbivores? Oikos 99(1):166–172
Das A, Eldakak M, Paudel B, Kim DW, Hemmati H, Basu C, Rohila JS (2016) Leaf proteome analysis reveals prospective drought and heat stress response mechanisms in soybean. Biomed Res Int 2016:6021047
De Vos CHR, Schat H, Vooijs R, Ernst WHO (1989) Copper-induced damage to the permeability barrier in roots of Silene cucubalus. Plant Physiol 135:164–179
Delalonde M, Rarret Y, Caumans MP (1996) Development of phenolic compounds in maize anthers (Zea mays) during cold pre-treatment prior to androgenesis. J Plant Physiol 149(5):612–616
Delk NA, Johnson KA, Chowdhury NI, Braam J (2005) CML24, regulated in expression by diverse stimuli, encodes a potential Ca2+ sensor that functions in responses to abscisic acid, daylength, and ion stress. Plant Physiol 139(1):240–253
Ding W, Fang W, Shi S, Zhao Y, Li X, Xiao K (2016) Wheat WRKY type transcription factor gene TaWRKY1 is essential in mediating drought tolerance associated with an ABA-dependent pathway. Plant Mol Biol Rep 34:1111–1126
Erdal S, Dumlupinar R (2010) Mammalian sex hormones stimulate antioxidant system and enhance growth of chickpea plants. Acta Physiol Plant 33(3):1011–1017
Feng W, Lu H, Yao T, Yu Q (2020) Drought characteristics and its elevation dependence in the Qinghai-Tibet plateau during the last half-century. Sci Rep 10:14323
Flexas J, Bota J, Galmes J, Medrano H, Ribas-Carbó M (2006) Keeping a positive carbon balance under adverse conditions: responses of photosynthesis and respiration to water stress. Physiol Plant 127:343–352
Foyer CH, Shigeoka S (2011) Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol 155(1):93–100
Gholizadeh A (2011) Effects of drought on the activity of phenylalanine ammonia lyase in the leaves and roots of maize inbreds. Aust J Basic Appl Sci 5(9):952–956
Gill RA, Na Z, Ali B, Farooq MA, Xu J, Gill MB, Mao B, Zhou W (2016) Role of exogenous salicylic acid in regulating physio-morphic and molecular changes under chromium toxicity in black- and yellow- seeded Brassica napus L. Environ Sci Pollut Res 23:20483–20496
Glawischnig E (2007) Camalexin. Phytochemistry 68(4):401–406
Gong H, Zhu X, Chen K, Wang S, Zhang C (2005) Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci 169:313–321
Habibi G (2012) Exogenous salicylic acid alleviates oxidative damage of barley plants under drought stress. Acta Biol Szeged 56(1):57–63
Halliwell B, Gutteridge JMC (2006) Free radicals in biology and medicine, 4th edn. Clarendon Press, Oxford
Hedge JE, Hofreiter BT (1962). In: Whistler RL, Be Miller JN (eds) Carbohydrate chemistry, vol 17. Academic Press, New York
Horvath E, Szalai G, Janda T (2007) Induction of abiotic stress tolerance by salicylic acid signalling. J Plant Growth Regul 26:290–300
Ichimura K, Mizoguchi T, Yoshida R, Yuasa T, Shinozaki K (2000) Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J 24(5):655–665
Jan A, Maruyama K, Todaka D, Kidokoro S, Abo M, Yoshimura E, Shinozaki K, Nakashima K, Shinozaki KY (2013) OsTZF1, a CCCH-tandem zinc finger protein, confers delayed senescence and stress tolerance in rice by regulating stress-related genes. Plant Physiol 161:1202–1216
Jebara S, Jebara M, Limam F, Aouani ME (2005) Changes in ascorbate peroxidase, catalase, guaiacol peroxidase and superoxide dismutase activities in common bean (Phaseolus vulgaris) nodules under salt stress. J Plant Physiol 162(8):929–936
Jeong MJ, Lee SK, Kim BG, Kwon TR, Cho WS, Park YT, Lee JO, Kwon HB, Byun MO, Park SC (2006) A rice (Oryza sativa L.) MAP kinase gene, OsMAPK44, is involved in response to abiotic stresses. Plant Cell Tiss Org 85(2):151–160
Jiang Y, Liang G, Yu D (2012) Activated expression of WRKY57 confers drought tolerance in Arabidopsis. Mol Plant 5:1375–1388
Joseph B, Jini D, Sujatha S (2010) Insight into role of exogenous salicylic acid on plants growth under salt environment. Asian J Crop Sci 2(4):226–235
Kabiri R, Nasibi F, Farahbakhsh H (2014) Effect of exogenous salicylic acid on some physiological parameters and alleviation of drought stress in Nigella sativa plant under hydroponic culture. Plant Protect Sci 50(1):43–51
Kar M, Mishra D (1976) Catalase, peroxidase and polyphenol oxidase activities during rice leaf senescence. Plant Physiol 57:315–320
Kiani SP, Maury P, Sarrafi A, Grieu P (2008) QTL analysis of chlorophyll fluorescence parameters in sunflower (Helianthus annuus L.) under well-watered and water stressed conditions. Plant Sci 175(4):565–573
Kotak S, Larkindale J, Lee U, von Koskull-Doring P, Vierling E, Scharf K (2007) Complexity of the heat stress response in plants. Plant Biol 10:310–316
Kundu S, Chakraborty D, Pal A (2012) Salicylic acid ameliorates susceptible Vigna mungo cultivar to Mungbean yellow mosaic India virus infection. Sci Cult 78(5–6):217–226
Li Y, Liu Y, Fu Y, Wei T, Guyader LL, Gao G, Liu RS, Chang YZ, Chen C (2012) The triggering of apoptosis in macrophages by pristine graphene through the MAPK and TGF-beta signaling pathways. Biomaterials 33(2):402–411
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real time quantitative PCR and the 2−ΔΔCt method. Methods 25:402–408
Madamanchi NR, Donahue JL, Cramer CL, Alscher RG, Pedersen K (1994) Differential response of Cu Zn superoxide dismutases in two pea cultivars during a short term exposure to sulphur dioxide. Plant Mol Biol 26:95–103
Magnan F, Ranty B, Charpenteau M, Sotta B, Galaud JP, Aldon D (2008) Mutations in AtCML9, a calmodulin-like protein from Arabidopsis thaliana, alter plant responses to abiotic stress and abscisic acid. Plant J 56(4):575–589
Massacci A, Nabiev SM, Pietrosanti L, Nematov SK, Chernikova TN, Thor K, Leipner J (2008) Response of the photosynthetic apparatus of cotton (Gossypium hirsutum) to the onset of drought stress under field conditions studied by gas-exchange analysis and chlorophyll fluorescence imaging. Plant Physiol Bioch 46(2):189–195
Miyagawa Y, Tamori M, Shigeoka S (2000) Evaluation of the defense system in chloroplasts to photooxidative stress caused by paraquat using transgenic tobacco plants expressing catalase from Escherichia coli. Plant Cell Physiol 41:311–320
Mole S, Ross JAM, Waterman PG (1988) Light induced variation in phenolic levels in foliage of rain-forest plants: I. Chemical changes. J Chem Ecol 14(1):1–21
Murphy A, Taiz L (1995) Comparison of metallothionein cene expression and nonprotein thiols in ten Arabidopsis ecotypes. Plant Physiol 109:945–954
Niu CF, Wei W, Zhou QY, Tian AG, Hao YJ, Zhang WK, Ma B, Lin Q, Zhang ZB, Zhang JS (2012) Wheat WRKY genes TaWRKY2 and TaWRKY19 regulate abiotic stress tolerance in transgenic Arabidopsis plants. Plant Cell Environ 35:1156–1170
Pan Y, Wu LJ, Yu ZL (2006) Effect of salt and drought stress on antioxidant enzymes activities and SOD isoenzymes of liquorice (Glycyrrhiza uralensis Fisch). Plant Growth Regul 49(2–3):157–165
Pandey S (2019) Review on medicinal importance of Vigna genus. Plant Sci Today 6(4):450–456
Pandey S, Chakraborty D (2015) Biochemical response of three Vigna mungo varieties (T9, RBU38 and VM4) under drought stress. Plant Sci Today 2(2):60–64
Pandey S, Chakraborty D (2016) Agro - morphological response of three Vigna mungo varieties (T9, RBU38 and VM4) to soil water deficit. Int J Sci Res Agric Sci 3(2):36–41
Patel PK, Hemantaranjan A (2012) Salicylic acid induced alteration in dry matter partitioning, antioxidant defense system and yield in chickpea (Cicer arietinum L.) under drought stress. Asian J Crop Sci 4(3):86–102
Pavlova EL, Rikhvanov EG, Tauson EL, Varakina NN, Gamburg KZ, Rusaleva TM, Borovskii GB, Voinikov VK (2009) Effect of salicylic acid on the development of induced thermotolerance and induction of heat shock protein synthesis in the Arabidopsis thaliana cell culture. Russ J Plant Physl 56(1):68–73
Pelloux J, Jolivet Y, Fontaine V, Banvoy J, Dizengremel P (2001) Changes in Rubisco and Rubisco activase gene expression and polypeptide content in Pinus halepensis M. subjected to ozone and drought. Plant Cell Environ 24:123–131
Phimchan P, Chanthai S, Bosland PW, Techawongstien S (2014) Enzymatic changes in phenylalanine ammonia-lyase, cinnamic-4- hydroxylase, capsaicin synthase, and peroxidase activities in capsicum under drought stress. J Agric Food Chem 62:7057–7062
Phukan UJ, Jeena GS, Shukla RK (2016) WRKY transcription factors: molecular regulation and stress responses in plants. Front Plant Sci 7:760
Quan LJ, Zang B, Shi WW, Li HY (2008) Hydrogen peroxide in plants: a versatile molecule of the reactive oxygen species network. J Integr Plant Biol 50(1):2–18
Recchia GH, Caldas DGG, Beraldo ALA, da Silva MJ, Tsai SM (2013) Transcriptional analysis of drought-induced genes in the roots of a tolerant genotype of the common bean (Phaseolus vulgaris L.). Int J Mol Sci 3(14):7155–7179
Reddy PS, Kishor PBK, Seiler C, Kuhlmann M, Eschen-Lippold L, Lee J, Reddy MK, Sreenivasulu (2014) Unraveling regulation of the small heat shock proteins by the heat shock factor HvHsfB2c in barley: its implications in drought stress response and seed development. PLoS ONE 9(3):e89125
Saruhan N, Saglam A, Kadioglu A (2012) Salicylic acid pre-treatment induces drought tolerance and delays leaf rolling by inducing antioxidant systems in maize genotypes. Acta Physiol Plant 34:97–106
Shakirova FM (2007) Role of hormonal system in the manifestation of growth promoting and antistress action of salicylic acid. In: Hayat S, Ahmad A (eds) Salicylic acid—a plant hormone. Springer, Dordrecht, pp 69–89
Sharma P, Dubey RS (2005) Modulation of nitrate reductase activity in rice seedlings under aluminium toxicity and water stress: role of osmolytes as enzyme protectant. J Plant Physiol 162(8):854–864
Shi H, Wang X, Ye T, Chen F, Deng J, Yang P, Zhang Y, Chan Z (2014) The Cysteine2/Histidine2-type transcription factor zinc finger of Arabidopsis thaliana 6 modulates biotic and abiotic stress responses by activating salicylic acid-related genes and c-repeat-binding factor genes in Arabidopsis. Plant Physiol 165(3):1367–1379
Singleton VL, Orthofer R, Lameula-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and oxidants by means of folin-ciocalteau reagent. Methods Enzymol 299:152
Sun D, Tang W, Ma L (2001) Extracellular calmodulin: a polypeptide signal in plants? Sci China Ser C 44(5):449–460
Tahkokorpi M, Taulavuori K, Laine K, Taulavuori E (2007) After effects of drought related winter stress in previous and current year stems of Vaccinium myrtillus L. Environ Exp Bot 61(1):85–93
Wang JP, Li YL, Zhang JG (2008) Effect of high-temperature and excessive-light stress on APX activity in apple peel. Acta Agr Boreali-Sin 23:144–147
Wang F, Chen HW, Li QT, Wei W, Li W, Zhang WK et al (2015) GmWRKY27 interacts with GmMYB174 to reduce expression of GmNAC29 for stress tolerance in soybean plants. Plant J 83:224–236
Wang J, Islam F, Li L, Long M, Yang C, Jin X, Ali B, Mao B, Zhou W (2017) Complementary RNA-sequencing based transcriptomics and iTRAQ proteomics reveal the mechanism of the alleviation of quinclorac stress by salicylic acid in Oryza sativa ssp. japonica. Int J Mol Sci 18:1975
Wang J, Zhang C, Shi Y, Long M, Islam F, Yang C, Yang S, He Y, Zhou W (2020) Evaluation of quinclorac toxicity and alleviation by salicylic acid in rice seedlings using ground-based visible/near-infrared hyperspectral imaging. Plant Methods 16:30
Wu J, Chen J, Wang L, Wang S (2017) Genome-wide investigation of WRKY transcription factors involved in terminal drought stress response in common bean. Front Plant Sci 8:380
Xie Z, Zhang ZL, Zou X, Huang J, Ruas P, Thompson D, Shen QJ (2005) Annotations and functional analyses of the rice WRKY gene superfamily reveal positive and negative regulators of abscisic acid signaling in aleurone cells. Plant Physiol 137:176–189
Xu DQ, Huang J, Guo SQ, Yang X, Bao YM, Tang HJ, Zhang HS (2008) Overexpression of a TFIIIA-type zinc finger protein gene ZFP252 enhances drought and salt tolerance in rice (Oryza sativa L.). FEBS Lett 582(2):1037–1043
Xu GY, Pedro R, Wang ML, Xu ML, Cui YC, Li LY, Zhu YX, Xia XJ (2011) A novel rice calmodulin-like gene, OsMSR2, confers improved drought, salt tolerance and enhanced ABA sensitivity in Arabidopsis. Planta 234:47–59
Xu F, Deng G, Cheng S, Zhang W, Huang X, Li L, Cheng H, Rong X, Li J (2012) Molecular cloning, characterization and expression of the phenylalanine ammonia-lyase gene from Juglans regia. Molecules 17:7810–7823
Xue T, Li X, Zhu W, Wu C, Yang G, Zheng C (2009) Cotton metallothionein GhMT3a, a reactive oxygen species scavenger, increased tolerance against abiotic stress in transgenic tobacco and yeast. J Exp Bot 60:339–349
Yang Y, Han C, Liu Q, Lin B, Wang J (2008) Effect of drought and low light on growth and enzymatic antioxidant system of Picea asperata seedlings. Acta Physiol Plant 30(4):433–440
Yang Z, Wu Y, Li Y, Ling HQ, Chu C (2009) OsMT1a, a type 1 metallothionein, plays the pivotal role in zinc homeostasis and drought tolerance in rice. Plant Mol Biol 70(1–2):219–229
Yang L, Ji W, Zhu Y, Gao P, Li Y, Cai H, Bai X, Guo D (2010) GsCBRLK, a calcium/calmodulin-binding receptor-like kinase, is a positive regulator of plant tolerance to salt and ABA stress. J Exp Bot 61(9):2519–2533
Yang C, Hu LY, Ali B, Islam F, Bai QJ, Yun XP, Yoneyama K, Zhou WZ (2016) Seed treatment with salicylic acid invokes defence mechanism of Helianthus annuus against Orobanche cumana. Ann Appl Biol 169:408–422
Yazdanpanah S, Baghizadeh A, Abbassi F (2011) The interaction between drought stress and salicylic and ascorbic acids on some biochemical characteristics of Satureja hortensis. Afr J Agric Res 6(4):798–807
Yuasa T, Ichimura K, Mizoguchi T, Shinozaki K (2001) Oxidative stress activates ATMPK6, an Arabidopsis homologue of MAP kinase. Plant Cell Physiol 42(9):1012–1016
Zhang J, Jia W, Yang J, Ismail AM (2006) Role of ABA in integrating plant responses to drought and salt stresses. Field Crop Res 97(1):111–119
Zhang JH, Wang LJ, Pan QH, Wang YZ, Zhan JC, Huang WD (2008) Accumulation and sub cellular localization of heat shock proteins in young grape leaves during cross-adaptation to temperature stresses. Sci Horti 117:231–240
Zhang C, Grosic S, Whitham SA, Hill JH (2012) The requirement of multiple defense genes in soybean Rsv1–mediated extreme resistance to soybean mosaic virus. MPMI 25(10):1307–1313
Zhou G, Xu Y, Li J, Yang L, Liu JY (2006) Molecular analyses of the metallothionein gene family in rice (Oryza sativa L.). J Biochem Mol Biol 39(5):595–606
Zhou Y, Lam HM, Zhang J (2007) Inhibition of photosynthesis and energy dissipation induced by water and high light stresses in rice. J Exp Bot 58:1207–1217
Zhou T, Yang X, Wang L, Xu J, Zhang X (2014) GhTZF1 regulates drought stress responses and delays leaf senescence by inhibiting reactive oxygen species accumulation in transgenic Arabidopsis. Plant Mol Biol 85:163–177
Zlatev ZS, Lidon FC, Ramalho JC, Yordanov IT (2006) Comparison of resistance to drought of three bean cultivars. Biol Plantarum 50(3):389–394
Acknowledgements
DC acknowledges University Grants Commission, New Delhi for the project funding and SP acknowledges the same for fellowship.
Author information
Authors and Affiliations
Contributions
Sonali Pandey conducted the study, compiled the results. Dipjyoti Chakraborty conceived the study and participated in its design and coordination. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors disclose no conflict of interest.
Additional information
Communicated by W. Zhou.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pandey, S., Chakraborty, D. Salicylic acid increases tolerance of Vigna mungo cv. T9 to short-term drought stress. Acta Physiol Plant 45, 25 (2023). https://doi.org/10.1007/s11738-022-03492-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-022-03492-6