Abstract
Coupling of biochar along with microbial inoculants could increase the phosphorus (P) availability and efficiency under the P-deficient environment. However, the effects of biochar and microbes on soil P retention remain still unconcerned in the subtropical environment. In the present study, AMF Glomus mosseae and Bacillus J 119 were applied as microbial material into two texturally different soils (soil A and soil B) amended with two different biochar (Rice husk biochar, RHBC; poplar wood chip biochar, PWBC). Both soils and biochar properties significantly affected the mycorrhizal root colonization. Soil amended with RHBC significantly improved the root colonization and root surface area in the no-P environment. Additionally, plant root and shoot biomass significantly enhanced in the combination of B + AMF. Moreover, B + AMF enhanced macronutrients (N, P, K, and Ca) and micronutrient concentration (Mg, Mn, Cu, and Zn) in plant root and shoot with biochars and in the no-P application. Overall, biochar application in both soils might increase the availability of nutrients especially P for maize plants. However, the responses of both biochar and microbial inoculants were varied with soil and biochar types which need in-depth investigations, especially its residual effects at field conditions in different climatic conditions before final recommendations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is a requirement for all living organisms. P is a non-renewable resource, which is essential for the present agriculture system. Currently, the global food industry depends on P which is taken from phosphate rock reserves which are confined to a few countries (Solovchenko et al. 2016). Phosphate rock is the main source of phosphorus in the world which is becoming very costly and scarce day by day (Cordell et al. 2011; George et al. 2016). Plant roots often react to P deficiency by assigning more carbon to roots, which results in enhanced root growth, lateral root formation, more exposure to the surface soil, more and lengthy root hairs and release of root exudates (Souri and Hatamian 2019). Thus, human beings would start to use P much more efficiently (Khan et al. 2018; Zhu et al. 2018).
Roots are linked with soil microbes using different processes to get various benefits such as nutrient acquisition (Naiji and Souri 2018). In soils, there are a lot of bacteria and fungi and these are beneficial for plant growth and development. The fungi are divided into two groups: pathogenic fungi, which cause negative effects on crops, and mycorrhizal fungi, which are good for crops, including arbuscular mycorrhizal fungi (AMF). The AMF symbiosis makes better plant mineral nutrition, plant water uptake, and resistance to contaminants (Smith and Read 2010). In addition to this, it is estimated that AMF usually enhances host plant resistance against phytopathogens, reduces the symptoms and disease severity, and ultimately helps the plant in survival and biomass production (Spagnoletti et al. 2018).
In the soil system, the AMF especially ectomycorrhiza and its hyphal bundles or ectomycorrhizal roots enhanced the diversity of bacterial communities and other soil microbiomes. (Hao et al. 2020). It has been noted that root mycorrhizal colonization can lead to improve phosphorus uptake by ten times over to root hairs only (Malik et al. 2019; Rafique et al. 2020). Moreover, inoculation with AMF can increase the plant performance and improve plant resistance to various biotic stresses (Mustafa et al. 2016).
Biochar (BC) is a key amendment that upgrades the fertility status of soil and is carbon-rich and also has a significant amount of certain nutrients (Hardy et al. 2019). As compared to other organic amendments, it is stable in soils for a prolonged period (Ding et al. 2019). Moreover, BC improve the P use efficiency of fertilizers by improving the soil physicochemical attributes (Rafique et al. 2019). Previously, the effect of BC and phosphorus solubilizing bacteria (PSB) on P fractions in the soil was well known (Alshankiti and Gill 2016; Ding et al. 2016; Aller 2017; Blanco-Canqui 2017; Ducey 2017). It is estimated that the combined application of BC and PSB increase the inorganic P fractions that was marked as plant available in the output composts (Wei et al. 2016; Li et al. 2020).
Limited knowledge about the co-application of BC with microbial inoculant for P adsorption into differently textured soils is present. We hypothesized that both BC along with AMF and bacillus strain would improve the soil nutrients availability to maize plant roots under the P-limited environment. Additionally, these microbial inoculants may also improve the root characteristics and increase the uptake of micro and macronutrients. Thus, the present study was carried out to evaluate the objectives that (1) how the biochar and microbial inoculants sustain the plant growth, root characteristics and improve the nutrients uptake and (2) whether BC, B and AMF may help to mediate the modern agriculture system where P-deficient environment is threatening the crop growth in near future.
Materials and methods
Biochar production
Two types of biochar feed stuff, i.e., rice husk (RH) and poplar wood chip (PW) were used. Biochar feedstuff (FS) was collected from the rice field situated 20 km from the COMSATS Islamabad Vehari Campus (2019 crop season) while poplar wood chip stuff was collected from the timber market, Vehari-Punjab, Pakistan. Both the feedstuff was sun dried for four days and then cut into small pieces of 2–3 cm. For biochar (BC) production, both FS were pyrolyzed at 400 °C for 4 h in a locally manufactured electrical heating system under anaerobic conditions (Gangil and Wakudkar 2013). Low-temperature BC production increases the bioavailability of carbon with higher N-mobilization and finally improved the soil microbial activities in the soil (Deenik et al. 2009; Wang et al. 2019). Hence, these biochar materials increased soil nutrient availability in phosphorus-limited soils. Both the feedstuff, i.e., RH and PW were not treated with any chemicals before the production of BC. After the production of both biochar materials i.e., rice rusk biochar (RHBC) and poplar wood chip biochar (PWBC) were allowed to cool down at room temperature and then passed through 2 mm sieve for uniform size and shape.
Biochar and soil analysis
The RHBC and PWBC sample was taken and analyzed for pH, EC, CEC, total carbon contents (TC), total nitrogen contents (TN), NO3−1 and NH4 before application as treatments by following the standard methods.
In COMSATS University Vehari campus, soil sample was taken from the experimental site and is marked as soil A. and from Cholistan desert, Bahawalpur, Punjab, Pakistan sandy soil sample was collected and marked as soil B. The sandy soil samples were fetched from 0 to 20 cm depth and mixed vigorously until a homogenous mixture appeared. Then the samples were carried to the laboratory, dried in sun, and sieved (2 mm). Then various physiochemical analysis was done, i.e., pH, cation exchange capacity (CEC), electrical conductivity (EC), total organic carbon (TOC), CaCO3, mineral nitrogen, available phosphorous (P) and available potassium (K) and soil samples were added to pots. Organic matter was measured, while pH and EC were measured by pH meter and EC meter accordingly (Nelson and Sommers 1996). The soil–water mixture was formed by making the suspension (1:2.5; soil: water) and then placed it for 30 min at room temperature to be balanced and this mixture was employed for checking the EC and pH. EC meter was standardized with KCl solution (0.01 N) at 25 °C (Page 1982). The same soil filtrate was used to check the soil Na and K using flame photometry (Estefan et al. 2013). The CaCO3 was checked by adopting the method of Loeppert and Suarez (1996). The soil carbon contents were checked by wet oxidation of soil samples using hydrogen peroxides, sulfuric acid and chromic acid (Walkley and Black 1934). Soil NO3-N contents were determined by subtracting.
NH4+-N was calculated from soil samples by subtracting the total nitrogen NH4+-N. The whole process was done using extraction methods (40 g soil + 200 ml KCl;2 M). The distillation was performed on this soil mixture and then the titration process was done. Soil N was and measured by subtracting organic N contents from total N contents (Abbasi and Khaliq 2016). Total N concentration, i.e., soil and plant samples, was recorded by the Kjeldahl digestion method (MAFF 1973). Available P and K contents from the soil were and determined by following the process as described by Houba et al. (1989).
Arbuscular mycorrhizal fungi inoculum (AMF)
Growth medium containing three parts of sand and one part of vermiculite (mixture was pasteurized twice with steam for 1 h and then followed by cooling for 24 h) for the culture of Glomus mosseae (AMF) using the method is described by Brundrett et al. (1996). The AMF growing culture was allowed to grow for 4 months in a makeshift glass house with a temperature of 30 °C and photoperiod (14–16 h) and DI water was applied to the pot. After four months of growth, the AMF culture did not irrigate further, and the topes were cut. The pot contained sand, AMF spores, sporocarp, hyphae, and infected root segments and all the materials were sundried for three days in the glass house (Feldmann and Idczak 1992). The potential of this AMF inoculum was tested following the method of MP bioassay (Rashid et al. 1997) and was found approximately 300 propagules per g. Control was prepared by mixing all the stuff with 100 ml DI water into sand culture except the AMF inoculum and then filtrate passed through filter paper (8 µm) as described by Khan (1988).
Experimental setup
The experiment was carried out at the research area of COMSATS University Islamabad, Vehari in a completely randomized design (CRD) with factorial arrangements and replied four times. The study contained four treatments, i.e., control (CT), Bacteria (Phosphorus-solubilizing bacteria; PSB, Strain J 119, B), Arbuscular Mycorrhizal Fungi (AMF) and B + AMF. AMF inoculum of Glomus mosseae (5 g) from the pot culture (see above; 2.3) comprising sand, AMF roots and sores, infected root segments was added into the soil while the bacterial counts were 107–8 cfu/ml. Two types of soils were used, i.e., clay loam and loamy clay. Each pot was filled with 7 kg of soil (11-inch height and 11-inch width and 5-inch base) and the total pots were 32. Before filling the pots, the soil was sieved through 2 mm. Biochars, i.e., RHBC and PWBC were mixed with soil at the rate of 2% w/w. Maize (Zea mays L.) cultivar, i.e., P1429 was used (marketed by Dupont Company). The recommended dose of NPK was applied in the form of urea (0.81 g per pot), DAP (0.71 g per pot) and SOP (0.51 g per pot). Half dose of phosphorus was used than the recommended dose. Initially, three seeds were sown in the pots at the depth of 3 cm and then thinned to one for the remaining period of the experiment. The seeds were pretreated with fungicide. Distilled water was applied to achieve the 75% field capacity using the speedy moisture meter on daily basis. The pots were placed in the wirehouse having sunlight (D/N, 10/14 h), temperature (22–30 °C) and humidity (70–75%). To make negligible the effect of microclimate on the experiment, the pot position was changed fortnightly. The maize crop was sown on February 01, 2020, and harvested on April 14, 2020.
Sampling
The maize plant was harvested after 55 days of emergence. The fresh and dry biomass of the maize plants were determined. The above-ground maize plant biomass was collected by cutting plants near the soil surface. The stem samples were chopped to 2–3 cm and then dried in a hot air oven at 70 °C until a constant weight was achieved. The soil was moistened before taking the root samples. Roots were extracted from each pot, washed thoroughly with deionized water and then dry under the sun for 2 days. Then root samples were put into a hot air oven at 70 °C until a constant weight was achieved. The shoot and root samples were ground in the automatic grinder and then saved into plastic jars for elemental analysis.
Nutrients analysis in root and shoot
The content of mineral nutrients of N, P, K, Zn, Ca, Mg, Mn, and Cu in the root and shoot of maize was measured by taking plant materials (1 g) in the 25 ml digestion flask. The plant sample was put overnight by adding 5 ml concentrated H2SO4 (Sigma Aldrich, USA). Plant samples were heated on the electric hot plate at 150 °C for I h. Then added 5 ml di-acid mixture (HClO4 and HNO3) with 2:1 ratio and heated the mixture for one more hour at 150 °C and cooled. The mixture was filtered using Whatman filter paper and diluted (with 50 ml DI water). No plant sample was added to prepare the blank sample. The nutrient concentrations (N, P, K, Zn, Ca, Mg, Mn, and Cu) were measured by atomic absorption spectrophotometer (Perkin Elmer, Singapore) using their respective standards (Richards 1954).
Root characteristics
After washing the maize roots with DI water, then scanned through high definition digital scanner (600 DPI). Grayscale images of roots were analyzed for root length, root surface area, and root volume using WINRHIZO Pro. Software (Regent Instruments, QC, Canada) (Himmelbauer 2004).
AMF root colonization
AMF root fungal infection was determined following the method of Phillips and Hayman (1970) with some modifications. Briefly, the maize roots were cut into small pieces (2–3 inches) with a hand cutter and then stained with non-vital stain trypan blue (0.05%). The fungal colonization was measured following the equation (Giovannetti and Mosse 1980);
Statistical analysis
The statistical analysis of the data was arranged in Excel and Statistix software (STATISTIX V.8.1, Analytical Software, Tallahassee, FL, USA) was used to find out the interaction of microbial inoculants and biochars on the nutrient’s availability to maize plants. The difference among means was tested using the LSD test at 5% probability level. Maize plant fresh and dry biomasses, AMF root colonization and the soil nutrients were the dependent variables.
Results
Root and shoot fresh weight (g)
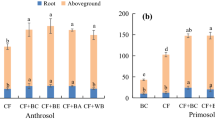
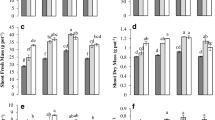
Root fresh weight (RFW) was enhanced significantly by 55% in B + AMF treatments of soil A (in no-P soil) along with RHBC addition. However, we compared between the biochar treatments (RHBC and PWBC) and noted that it was increased by 30% in RHBC in soil A compared to control (Fig. 1). Moreover, AMF Glomus mosseae inoculation increased RFW by 18% in RHBC amended soil A (in no-P application) while 8% increase in RFW was observed in PWBC amended soil over control treatments. Bacterial inoculants improved the RFW by 21% in RHBC amended soil A while only 11 in PWBC soil A (in limited-P application). As far as the influence of soil microbial inoculant was concentered in soil B was concerned, RHBC significantly increased RFW by 26% in AMF + B inoculant soil followed by 14% in AMF Glomus mosseae inoculant treatments (in no-P application) with respect to control (Fig. 1). The current study data indicated that RHBC had more potential in enhancing the RFW over to PWBC.
An almost similar data trend was noted in the case of shoot fresh weight (SFW) which was increased by 67% in B + AMF treatments of soil A with RHBC followed by 41% in alone AMF treatments in no-P treatments (Fig. 2B). However, AMF Glomus mosseae inoculation increased SFW by 52% in RHBC amended soil B (in no-P application) while 16% increase in SFW was observed in PWBC amended soil with respect to control treatments. Bacterial J119 significantly increased the SFW by 45% in soil A amended with RHBC while 18% in PWBC amended soil A (in limited-P application). In soil B, a better response of RHBC was recorded in soil A over to soil B amended with PWBC. Fungus with bacterial inoculation enhanced the SFW by 46% in RHBC amended soil and 21% was recorded in PWBC treatments (in no-P application). Bacillus strain J119 was improved the SFW by 46% in no-P treatments with RHBC while 16% in PWBC treatments with respect to control (Fig. 2).
Root and shoot dry biomass (g)
Root dry biomass (RDB) was enhanced by 67% in B + AMF treatments of soil A amended with RHBC (in no-P application), whereas in PWBC amended soil A improved the RDB by 41% with respect to control (Fig. 3). Moreover, when a half dose of P2O5 was added to soil A, then Bacillus strain J119 inoculant significantly contributed to 35% increase in RDB. As regards soil B, AMF inoculation significantly enhanced the RDB by 31% in RHBC amended soil (in no-P application) while by 11% in PWCB amended soil (in no-P application). However, a half dose of P2O5 along with the B + AMF Glomus mosseae and AMF-alone inoculation improved the RDB by 28% and 16% in RHBC root amended soil respectively, over control treatments (Fig. 4). RDB also improved in the RHBC amended soil by 21% and 14% for B + AMF Glomus mosseae and AMF-alone inoculation respectively than control (Fig. 4).
Root colonization (%)
The root colonization was relatively higher in soil A as compared to soil B but have a similar date trend of increase in both soils (Table 2). Additionally, soil A had better colonization in no-P soil treatments as compared to with limited-P treatments. Moreover, for all treatment combinations in both soils, the B + AMF Glomus mosseae inoculation has promoted root colonization than the sole inoculation of AMF and bacteria alone. In both soils, B-treatments had significantly less colonization (by 11%) with respect to CT-treatments in RHBC amended soil A (in no-P soil). The B + AMF inoculation showed 81% colonization, whereas it was 68% in AMF inoculant in RHBC amended soil A (in no-P application). Overall, it was observed that B + AMF inoculation was contributed more by 70–86% in both soils. In soil B, 55% higher root colonization was noted in B + AMF with RHBC amended soil, whereas 41% higher was recorded in PWBC amended soils (in no-P soil application).
Root characteristics
Data in Table 1 indicated that root length was significantly enhanced by 81% in soil A amended with RHBC (in limited-P application) with respect to control. Besides the biochar types, the microbial inoculants improved the root length and increased by 57% in B + AMF with RHBC amended soil A (in no-P application) over to control. In soil B, the soil amended with RHBC showed similar results with respect to root length improvement (by 31%) for B + AMF Glomus mosseae treated soil as compared to control (in no-P application). Like root length, the root surface area was significantly increased by the biochar types and microbial inoculants. The B + AMF inoculation promoted the root surface area by 58% in RHBC amended soil (in no-P application) while the increase was only 23% in case of limited-P application (Table 2) with respect to control. On the other hand, in soil B, the root surface area was not increased too much, but this increase was only 18% for B + AMF treatments in RHBC amended soil in comparison to control (in limited-P application). Similarly, the root volume tended to increase in both soils, i.e., soil A and soil B. The root volume was promoted up to 44% in RHBC amended soil A for B + AMF treatments (in no-P application), whereas only 18% root volume was recorded in the same treatment for limited-P soil (Table 2).
Root and shoot nutrients analysis
In soil A, AMF + B and AMF-alone significantly improved the nitrogen (N) contents by 29% and 14% respectively in RHBC amended soil (in no-P application) while in limited-P treatments, only B-alone treatments were significantly enhanced the N contents by 39%, and followed by B + AMF (by 29%) as compared to their respective control in plant shoot (Table 2). B-alone inoculation only increased the N contents by 7% as compared to no inoculants (control) in shoot samples, while similar effects were noted in plant root samples (Table 2). As we looked at the effect of PWBC, B + AMF and B-alone increased the N contents by 25% and 16% respectively compared to their control (in no-P application), while the N contents were improved along with the application of limited-P, and it was increased by 5% and 2% compared to no-P treatments. The behavior of biochar types in soil B was different, B + AMF and B-alone increased the N contents by 29% and 17% (in no-P application) while with limited-P, maximum N contents were recorded in B + AMF by 23% and alone B by 18% in RHBC amended soil over to their respective control. An almost similar trend was noted in root samples with a maximum noted in B + AMF, followed by B-alone treatments (Table 2).
In soil A, M and B + AMF treatments significantly improved shoot P by 33% and 20% respectively in RHBC amended soil (in no-P application), while in limited P, only B treatment significantly increased by 52% and AMF treatment by 24% than control. When PWBC was applied in soil A (in no-P application), B + AMF treatments improved P concentration in shoot parts by 12% compared to control (Table 2). Soil B response was quite different and 27% increase was noted in AMF treatments of RHBC amended soil (in no-P application) than control. In addition to RHBC in soil B, 13% increase in shoot P was observed in B treatment, whereas 32% improved in AMF treatments (in no-P application) than in control. Only 2% increase was observed for B treatment with limited P than control. Soil inoculants significantly enhanced the root P for soil A amended with PWBC (in no-P application). The P uptake in root portion was also increased by the addition of P2O5 in the soil. A significant increase of 31% in P contents was observed in AMF inoculated plants compared to control for soil B amended with RHBC (in no-P application).
In RHBC amended soil A, B + AMF and AMF-alone significantly increased the concentration of K in the root by 81% and 73% (in no-P application) as compared to their respective controls (Table 2). As far as soil B was concerned, root k concentration in B + AMF and AMF-alone was improved by 80% and 77% over to control, but this increase was dropped in AMF and B + AMF by 13% and 12% (in limited-P application). On the other hand, in PWBC amended soil A, a significant improvement was observed in K accumulation in the root by 69 and 63% in B + AMF and AMF treatments with no-P application, while this increase was decreased in limited-P application treatments with B + AMF and AMF amended soil by 39% and 26%. In soil A, shoot K concentration was increased in B + AMF and AMF-alone by 25% and 19% respectively in contrary to control (in no-P application), but K concentration in the shoot was decreased to 19% and 15% in soil B. An almost similar pattern was noted in PWBC amended soil A and soil B.
A significant increase in Ca contents (by 34% and 50%) was noticed in root and shoot inoculated with B + AMF treatment in soil A amended with RHBC (in no-P application) contrary to control (Table 3). While in soil B, this increase was only 16% and 39% in Ca contents for RHBC amended soil inoculated with B + AMF (in no-P application) contrary to control. Similarly, in PWBC amended soil A, Ca contents were significantly increased by 40% (in no-P application) in the root, but in soil B, this was improved by 20% over to control. Moreover, in the shoot, Ca contents were enhanced by 42% in soil A and by 34% in soil B (in no-P application) while by 25% in soil A and by 32% in soil B with the limited-P application (Table 3).
In no-P application treatments, Mg contents were increased in the root by 59% (in soil A) and 16% (in soil B), while a less increase in Mg contents was noticed in PWBC that was by 37% (in soil A) and 12% (in soil B) (Table 4). In soil A, the concentration of Mn in root was improved according to biochar type while the use of PWBC decreased Mn uptake in plant root samples (Table 4). A similar trend was observed in soil B. The AMF, i.e., Glomus mosseae significantly increased the Mn uptake. The presence of Cu contents in different treatments of RHBC amended soil A with no-P application ranged from 19.53 to 11.70 ppm, whereas in PWBC amended soil A, it ranged from 13.46 to 6.40 ppm (Table 3). Biochar types had a nominal effect on the increase of Cu uptake, RHBC amended soil had more Cu in comparison to PWBC amended soil. However, a significant improvement (40%) was observed in B + AMF treatments with no-P soil amended with RHBC. Moreover, a similar trend was noted by Zn contents in the root. Plants grown in soil A had more Zn contents in their root and shoots and soils while the limited P have less concentration of Zn than no-P applied to soil (Table 4). A significant increase (32 and 38%) in Zn uptake was noticed in B + AMF treatment of soil A and soil B amended with RHBC (in no-P application) in comparison to control.
Discussion
In the current study, both soils with different textures and chemical properties were used to understand the behavior of two biochars (RHBC and PWBC) on the growth and nutrient update of maize crop in the presence of limited P and microbes. Moreover, both soils were inoculated with microbial materials, i.e., Bacillus J 119 (B), AMF Glomus mosseae (AMF) and a combination of both inoculants (B + AMF). The study of the root phenotype and root inoculated microbes is very important in the current era of agriculture in subtropical conditions but poorly explored topic.
The higher root colonization was observed in no-P soils perhaps more carbon utilization toward the formation and maintaining of fungal tissues and thus, it is getting the benefits of additional P. Moreover, from the mycorrhization process, both hosts, i.e., plant and fungal are equally getting benefits in the form of resources but usually, it is dependent on the other factors (Wang et al. 2016). Additionally, the mycorrhizal colonization of fungi also altered the root morphology and architecture, i.e., root length, root volume and root surface area and enhanced the P acquisition in a low P environment. Biochar, i.e., RHBC also increased the root colonization (Fig. 1a and b) that could be due to transfer of fungal spores through air currents. Besides that, an increase in root colonization and root architecture in microbial and biochar amended soil was also observed in different types of soils and biochar in the previous studies (House and Bever 2019; Malik et al. 2019). Better root architecture may decrease the metabolic cost of plants toward soil exploration resulted into less nutrients and carbon demand for growth and construction of typical root structure (Naiji and Souri 2018; Saghaiesh and Souri 2018).
Soil nutrient status especially P availability in soil media increased the mycorrhizal phenotype (Omondi et al. 2016). In our case, no-P soil treatments have developed mutualism with maize plants and commensalism, or parasitism occurred in P available soils (White and Millner 2017). The mechanism involved in the reduced development of symbiosis into nutrient-rich soil media was described by Raya-Hernández et al. (2020). Our results depicted that microtrichial colonization was more prominent into no-P soils as compared to P-limited soils (Liu et al. 2019). Thus, microbial inoculation and biochar dose improved the overall growth and survival of maize plants in nutrient-deficient environment (Chen et al. 2017b). Past studies described that microbial inoculation (B + AMF) increased the plant growth and development and nutrient adsorption. This inoculation increased the root surface area and networking, which are the major precursors in biochar mineralization. Additionally, dry and compacted soils may restrict the root growth and length leading to poor root hair development and density.
Present study results indicated that symbiosis of AMF with maize root changed the root structure and P-acquisition efficiency of plants. However, Glomus mosseae (AMF) efficiency was more prominent in P-limited treatments as compared to control or no-P treatments (Table 1). Root cortical aerenchyma (RCA) decreased the metabolic cost (C and N) of the root tissues, and maize plants with higher RCA have resulted in poor root cell respiration, larger soil exploration and higher interaction with soil nutrients under the suboptimal presence of P to maize plants than that had poor RCA (Schneider et al. 2017, Galindo‐Castañeda et al. 2018, Galindo‐Castañeda et al. 2019).
P deficiency could be resulted in disrupted carbohydrate metabolism and restricted leaf growth (Timlin et al. 2017). Limited applied P had shown a positive effect on the maize biomass production and more P accumulation into their body parts (Figs. 1 and 2). Xu et al. (2016) recorded a linear correlation between the higher P availability and more P uptake by maize plants. Usually, the addition of biochar into soil improved the phosphate-binding with cations, i.e., Ca2+, Mg2+, Mn2+, Zn 2+, Fe 3+ and Al3+ into the soil media (Dugdug et al. 2018). Biochar may increase the soil phosphate holding capacity and result in more retention of P for crop plants under no-P and limited-P environment (Yao et al. 2013). Past studies depicted that biochar application increased nutrient availability, i.e., P and K to plant roots (Chintala et al. 2014; Cordell and White 2014). The present study results stated that, irrespective of microbial inoculation, biochar application significantly enhanced N, HPO3−, K and Ca2+ availability in the soil media. Moreover, the effects of biochars (RHBC and PWBC) on plant biomass and nutrient uptake were less limited in limited-P treatments than in the no-P-limited treatments. Such soils had enough macro- and micronutrients for plant growth that may mask the biochar and microbial effects which were clearly seen in no-P treatments (Galindo‐Castañeda et al. 2018, Mohamed et al. 2018; Spagnoletti et al. 2018). Recently, Li et al. (2020) depicted that biochar application in P-limited environment increased plant growth as compared to no biochar in P-limited soils. Additionally, biochar may sustain the nutrient availability throughout the plant growth by retaining nutrients into soil media that can improve the plant photosynthesis and plant growth (Alori et al. 2017).
AMF (Glomus mosseae) can help in ameliorating the P stress in maize under suboptimal nutrient conditions in early and late plant growth stages as compared to control (Tian et al. 2013; Gerlach et al. 2015). Additionally, AMF (Glomus mosseae) alone or in combination is considered as P-solubilize (Chen et al. 2017a). Moreover, AMF can help in pathway formation into the soil for P uptake through root and thus, improve the P uptake in the no-P environment as was seen in the case of our study (Table 2 and 3) (Tian et al. 2013), but the availability of the nutrients is strongly related to soil physicochemical properties and soil hydraulic conductance (Hatamian et al. 2020, Shooshtari et al. 2020). Hao et al. (2020) described the role of AMF toward its P and water supply which is inconsistent in this study. However, biochar interaction with bacteria, such as Bacillus species J 119, improved the P concentration than no microbial inoculation or control treatments. In the current study, soil inoculation with bacteria J 119 enhanced the plant biomass and nutrient uptake because of its positive interaction with the maize plant roots (Kaur and Reddy 2015). The future P crises may be handled by microbial inoculation which could ensure the availability of soil P and residual P to plant roots.
Conclusion
The results of the present study suggest that arbuscular mycorrhizal fungi (Glomus mosseae) and bacteria (Bacillus J 119) were applied as microbial inoculants into two texturally different soils (soil A and soil B) amended with two different biochar, i.e., RHBC and PWBC. The addition of PWBC into soil significantly decreased root colonization in limited-P pots. Moreover, the shoot and root fresh and dry biomass were increased in RHBC amended soil. Additionally, a higher concentration of nutrients was also recorded in the roots and shoot portions. the It was worth seen that the properties of both soils and biochar significantly affected the mycorrhizal root colonization. Moreover, the combined application of AMF+B along with the biochar enhanced macronutrients (N, P, K and Ca) and micronutrient concentration (Mg, Mn, Cu, and Zn) in shoot and root of plant grown in limited-P soil. Furthermore, field studies about the residual effect of biochar and microbial inoculants in long-term experiments are wanted before its final recommendation.
Author contribution statement
Manuscript write up: HMRJ, MA, RQ, NM. Data analysis: HMRJ, RN, MAS. Formal analysis: SK, AF, HR. Software analysis: HMRJ, MS, AR. Review and Improvement: FN, KM, NM. HMRJ, RN, MA, RQ, MAS, FN, AR, MS, AF, HR, SK, KM, NM.
References
Abbasi MK, Khaliq A (2016) Nitrogen mineralization of a loam soil supplemented with organic–inorganic amendments under laboratory incubation. Front Plant Sci 7:1038
Aller DRS, Laird D, Cruse R, Hatfield J (2017) Impacts of fresh and aged biochars on plant available water and water use efficiency. Geoderma 307:114–121
Alori ET, Glick BR, Babalola OO (2017) Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front Microbiol 8:971
Alshankiti A, Gill S (2016) Integrated plant nutrient management for sandy soil using chemical fertilizers, compost, biochar and biofertilizers. J Arid Studis 26(3):101–106
Blanco-Canqui H (2017) Biochar and soil physical properties. Soil Sci Soc Am J 81:687–711
Brundrett M, Bougher N, Dell B, Grove T, Malajczuk N (1996) Working with mycorrhizas in forestry and agriculture. Australian Centre for International Agricultural Research Canberra
Chen M, Yang G, Sheng Y, Li P, Qiu H, Zhou X, Huang L, Chao ZJFips, (2017a) Glomus mosseae inoculation improves the root system architecture, photosynthetic efficiency and flavonoids accumulation of liquorice under nutrient stress. Front Plant Sci 8:931
Chen Y-L, Xu Z-W, Xu T-L, Veresoglou SD, Yang G-W (2017b) Chen B-DJSB, Biochemistry Nitrogen Deposition and Precipitation Induced Phylogenetic Clustering of Arbuscular Mycorrhizal Fungal. Communities 115(233):242
Chintala R, Schumacher TE, McDonald LM, Clay DE, Malo DD, Papiernik SK, Clay SA, Julson JL (2014) Phosphorus sorption and availability from biochars and soil/b iochar mixtures. Clean: Soil, Air, Water 42(5):626–634
Cordell D, White S (2014) Life’s bottleneck: sustaining the world’s phosphorus for a food secure future. Annu Rev Environ Resour 39:161–188
Cordell D, Rosemarin A, Schröder JJ, Smit AJC (2011) Towards global phosphorus security: a systems framework for phosphorus recovery and reuse options. Chemosphere 84(6):747–758
Deenik JL, McClellan A, Uehara G. 2009 Biochar volatile matter content effects on plant growth and nitrogen transformations in a tropical soil. Proceedings of the Western Nutrient Management Conference pp 26–31
Ding Y, Liu Y, Liu S, Li Z, Tan X, Huang X, Zeng G, Zhou L, Zheng B (2016) Biochar to improve soil fertility a review. Agron Sus Dev 36(2):36
Ding J, Yin Y, Sun A-Q, Lassen SB, Li G, Zhu D, Ke X (2019) Effects of biochar amendments on antibiotic resistome of the soil and collembolan gut. J Hazard Mater 377:186–194
Ducey TF, Bauer PJ, Sigua GC, Hunt PG, Miller JO, Cantrell KB (2017) Manure-derived biochars for use as a phosphorus fertilizer in cotton production. J Cotton Sci 21:259–264
Dugdug AA, Chang SX, Ok YS, Rajapaksha AU, Anyia A (2018) Phosphorus sorption capacity of biochars varies with biochar type and salinity level. Environ Sci Pollut Res Int 25(26):25799–25812
Estefan G, Sommer R, Ryan J (2013) Methods of soil, plant, and water analysis. A manual for the West Asia and North Africa region 3
Feldmann F, Idczak E (1992) 18 Inoculum Production of Vesicular-arbuscular Mycorrhizal Fungi for Use in Tropical Nurseries. In: Methods in microbiology. Elsevier. pp 339–357
Galindo-Castañeda T, Brown KM, Lynch JPJP, Cell E (2018) Reduced root cortical burden improves growth and grain yield under low phosphorus availability in maize. Plant Cell Environ 41(7):1579–1592
Galindo-Castañeda T, Brown KM, Kuldau GA, Roth GW, Wenner NG, Ray S, Schneider H, Lynch JPJP, Cell E (2019) Root cortical anatomy is associated with differential pathogenic and symbiotic fungal colonization in maize. Plant Cell Environ 42(11):2999–3014
Gangil S, Wakudkar HM (2013) Generation of bio-char from crop residues. Int J Emerg Technol Adv Eng 3(3):566–570
George TS, Hinsinger P, Turner BL (2016) Phosphorus in soils and plants–facing phosphorus scarcity. Springer
Gerlach N, Schmitz J, Polatajko A, Schluter U, Ffahnenstich H, Witt S, Fernie AR, Uroic K, Scholz U, Sonnewald U, Bucher M (2015) An Integrated Functional Approach to Dissect Systemic Responses in Maize to Arbuscular Mycorrhizal Symbiosis 38(8):1591–1612
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84(3):489–500
Hao X, Zhu Y-G, Nybroe O, Nicolaisen MHJFim, (2020) The composition and phosphorus cycling potential of bacterial communities associated with hyphae of Penicillium in soil are strongly affected by soil origin. Front Microbiol. https://doi.org/10.3389/fmicb.2019.02951
Hardy B, Sleutel S, Dufey JE, Cornelis J-T (2019) The long-term effect of biochar on soil microbial abundance activity and community structure is overwritten by land management. Front Environ Sci. https://doi.org/10.3389/fenvs.2019.00110
Hatamian M, Rezaei Nejad A, Kafi M, Souri MK, Shahbazi KJC, Agriculture BTi (2020) Interaction of lead and cadmium on growth and leaf morphophysiological characteristics of European hackberry (Celtis australis) seedlings. Chem. Biol. Technol. Agric. 7:1–8
Himmelbauer ML (2004) Estimating length, average diameter and surface area of roots using two different image analyses systems. Plant Soil 260(1–2):111–120
Houba V, Van der Lee J, Novozamsky I, Walinga I (1989) Soil and Plant Analysis Part 5:4–10
House GL, Bever JDJRE (2019) Biochar soil amendments in prairie restorations do not interfere with benefits from inoculation with native arbuscular mycorrhizal fungi. Restor Ecol 28(4):785–795
Kaur G, Reddy MS (2015) Effects of phosphate-solubilizing bacteria, rock phosphate and chemical fertilizers on maize-wheat cropping cycle and economics. Pedosphere 25(3):428–437
Khan AG (1988) Inoculum density of Glomus mosseae and growth of onion plants in unsterilized bituminous coal spoil. Soil Biol Biochem 20(5):749–753
Khan A, Lu G, Ayaz M, Zhang H, Wang R, Lv F, Yang X, Sun B, Zhang SJA, ecosystems, environment, (2018) Phosphorus efficiency, soil phosphorus dynamics and critical phosphorus level under long-term fertilization for single and double cropping systems. Agric Ecosyst Environ 256:1–11e
Li H, Li Y, Xu Y, Lu X (2020) Biochar phosphorus fertilizer effects on soil phosphorus availability. Chemosphere 244:125471
Liu M, Che Y, Wang L, Zhao Z, Zhang Y, Wei L, Xiao YJC (2019) Rice straw biochar and phosphorus inputs have more positive effects on the yield and nutrient uptake of lolium multiflorum than arbuscular mycorrhizal fungi in acidic cd-contaminated Soils. Chemosphere 235:32–39
Loeppert RH, Suarez DL (1996) Carbonate and gypsum, J.M. Bigham (Ed.), Methods of soil analysis, part 3-chemical methods, American Society of Agronomy, Madiscon, SSSA. Book Series No 5.
MAFF U (1973) The analysis of Agricultural Materials. MAFF Technical Bulletin No. 27. HMSO,
Malik Z, Shah Z, Tariq MJSJoA, (2019) Biochar Improves Viability of Arbuscular Mycorrhizal Fungi (Amf) in Soil and Roots of Wheat (Triticum aestivum) and Maize (Zea mays L) under Various Cropping Systems. Sarhad J Agric 35(3):834–846
Mohamed EA, Farag AG, Youssef SAJJoEP. (2018) Phosphate solubilization by Bacillus subtilis and Serratia marcescens isolated from tomato plant rhizosphere. journal of Environmental Protection. 9(03): 266
Mustafa G, Randoux B, Tisserant B, Fontaine J, Magnin-Robert M, Sahraoui AL-H, Reignault PJM (2016) Phosphorus supply, arbuscular mycorrhizal fungal species, and plant genotype impact on the protective efficacy of mycorrhizal inoculation against wheat powdery mildew. Mycorrhiza 26(7):685–697
Naiji M, Souri MKJASPHC (2018) Nutritional value and mineral concentrations of sweet basil under organic compared to chemical fertilization. Acta Sci Pol Hortorum Cultus 17(2):167–175
Nelson DW, Sommers LE (1996) Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis. Part 3. Chemical Methods; SSSA Book Series; Sparks, DL., Ed. SSSA and ASA., Madison, WI, USA, No. 5.
Omondi MO, Xia X, Nahayo A, Liu X, Korai PK, Pan GJG (2016) Quantification of biochar effects on soil hydrological properties using meta-analysis of literature data. Geoderma 274:28–34
Page, (1982) Albert Lee Miller, Robert H Keeney. American Society of Agronomy, Dennis R. Methods of soil analysis
Phillips JM, Hayman D (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55(1):158–161
Rafique M, Ortas I, Rizwan M, Chaudhary HJ, Gurmani AR, Hussain Munis MF (2020) Residual effects of biochar and phosphorus on growth and nutrient accumulation by maize (Zea mays L.) amended with microbes in texturally different soils. Chemosphere 238:124710
Rafique M, Ortas I, Ahmed IA, Rizwan M, Afridi MS, Sultan T, Chaudhary HJJJoem (2019) Potential impact of biochar types and microbial inoculants on growth of onion plant in differently textured and phosphorus limited soils. 247:672-680
Rashid A, Ahmed T, Ayub N, Khan A (1997) Effect of forest fire on number, viability and post-fire re-establishment of arbuscular mycorrhizae. Mycorrhiza 7(4):217–220
Raya-Hernández AI, Jaramillo-López PF, López-Carmona DA, Díaz T, Carrera-Valtierra JA, Larsen JJASE (2020) Field evidence for maize-mycorrhiza interactions in agroecosystems with low and high P soils under mineral and organic fertilization. Appl Soil Ecol 149:103511
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. Soil Sci 78(2):154
Saghaiesh SP, Souri MKJASP-HC (2018) Root growth characteristics of Khatouni melon seedlings as affected by potassium nutrition. Acta Sci. Pol. Hortorum Cultus 17(5):191
Schneider HM, Postma JA, Wojciechowski T, Kuppe C, Lynch JPJPP (2017) Root cortical senescence improves growth under suboptimal availability of N. P, and k 174(4):2333–2347
Shooshtari FZ, Souri MK, Hasandokht MR, Jari SKJC, Agriculture BTi (2020) Glycine mitigates fertilizer requirements of agricultural crops: case study with cucumber as a high fertilizer demanding crop. Biotechnology Advance 7(1):1–10
Smith SE, Read DJ (2010) Mycorrhizal symbiosis, 3rd edn. Academic Press, Elsevier Science. https://books.google.com.pk/books?id=qLciOJaG0C4C. ISBN 0080559344, 9780080559346.
Solovchenko A, Verschoor AM, Jablonowski ND, Nedbal LJBa, (2016) Phosphorus from wastewater to crops: an alternative path involving microalgae. Biotechnol Adv 34(5):550–564
Souri MK, Hatamian MJJoPN. (2019) Aminochelates in plant nutrition: a review. Journal of Plant Nutrition. 42(1): 67–78
Spagnoletti FN, Leiva M, Chiocchio V, Lavado RSJJoPN, Science S, (2018) Phosphorus fertilization reduces the severity of charcoal rot (Macrophomina phaseolina) and the arbuscular mycorrhizal protection in soybean. J Plant Nutr Soil Sci 181(6):855–860
Tian H, Drijber RA, Li X, Miller DN, Wienhold BJ (2013) Arbuscular mycorrhizal fungi differ in their ability to regulate the expression of phosphate transporters in maize (Zea mays L). Mycorrhiza 23(6):507–514
Timlin DJ, Naidu TCM, Fleisher DH, Reddy VR (2017) Quantitative effects of phosphorus on maize canopy photosynthesis and biomass. Crop Sci 57(6):3156–3169
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 37(1):29–38
Wang Q, He N, Liu Y, Li M, Xu l, (2016) Strong pulse effects of precipitation events on soil microbial respiration in temperate forests. Gerderma 275:67–73
Wang LC, Parikh SJ, Scow KM (2019) Impact of biochar on water retention of two agricultural soils – a multi-scale analysis. Gerderma 340:185–191
Wei Y, Zhao Y, Wang H, Lu Q, Cao Z, Cui H, Zhu L (2016) Wei ZJBt an optimized regulating method for composting phosphorus fractions transformation based on biochar addition and phosphate-solubilizing bacteria inoculation. Bioresour Technol 221:139–146
White K (2017) Millner PJQRoB Book Review of Biochar Application: Essential Soil Microbiology. Q R Biol 92:336–337
Xu G, Zhang Y, Sun J, Shao H (2016) Negative interactive effects between biochar and phosphorus fertilization on phosphorus availability and plant yield in saline sodic soil. Sci Total Environ 568:910–915
Yao Y, Gao B, Chen J, Yang LJEs, technology, (2013) Engineered biochar reclaiming phosphate from aqueous solutions: mechanisms and potential application as a slow-release fertilizer. Environ Sci Technol 47(15):8700–8708
Zhu J, Li M (2018) Whelan MJSotTE phosphorus activators contribute to legacy phosphorus availability in agricultural soils: a review. Sci Total Environ 612:522–537
Acknowledgements
The authors would like to acknowledge the ORIC, COMSATS University Islamabad, for their lavish funding to conduct the study and also facilitate elemental analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Ferrarese-Filho.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Javeed, H.M.R., Naeem, R., Ali, M. et al. Coupling biochar with microbial inoculants improves maize growth and nutrients acquisition under phosphorous-limited soil. Acta Physiol Plant 44, 110 (2022). https://doi.org/10.1007/s11738-022-03440-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-022-03440-4