Abstract
The studies regarding interaction of biochar and fertilizer and their effect on soil microbial biomass and enzyme activity are scarce. This study centers at analysis of soil microbial biomass carbon (MBC), nitrogen (MBN), and phosphorous (MBP) over a period of 2 years under mash bean-wheat cropping system. Overall, six treatments were performed which included (1) B0F0: no biochar no fertilizer; (2) B1F0: 5 tons ha−1 (hectare) biochar with no fertilizer; (3) B2F0: 10 tons ha−1 biochar (B2F0) with no fertilizer; (4) B0F1: no biochar with recommended N:P:K fertilizer (i.e., 23:45:25 kg ha−1 respectively); (5) B1F1: 5 tons ha−1 biochar with recommended fertilizer; (6) B2F1: 10 tons ha−1 biochar with recommended fertilizer. Results elucidated increased soil MBC which increased with increasing biochar concentration. Enzyme activity and microbial biomass were higher initially but decreased in the second year of biochar amendment (both with and without fertilizer) which may be attributed to positive priming effect at the start of the experiment. MBN was observed lowest with fertilizer application but increased with biochar application alone. Seasonal variation in MBC was less in biochar-amended soils suggesting that biochar induced a less extreme environment for microbes throughout the season. The biochar treatment (i.e., B2F0) enhanced the soil microbial biomass, phosphorus, and phosphatase activities while biochar with fertilizer treatment (B2F1) improved urease, dehydrogenase, and alkaline phosphatase activity significantly (P > 0.05) in mash bean crop. In wheat however, this treatment decreased urease activity while dehydrogenase activity remained unchanged. A positive correlation was observed between MBN and dehydrogenase activity with soil organic carbon, dissolved organic carbon, and soil moisture content. These outcomes imply that bagasse biochar improves soil conditions for microbes thereby plummeting temporal variations in carbon dynamics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biochar is a carbon (C)-rich solid residue produced by thermal degradation of organic materials under oxygen (O2) limited conditions for use specifically as an amendment to benefit soils (Lehmann and Joseph 2009). Amendments of biochar to soil have improved soil quality (Sohi et al. 2010; Sohi 2012; Kamran et al. 2018) and enhanced crop yields by improving soil physico-chemical properties including nutrient retention and increased water content (Glaser et al. 2002; Steiner et al. 2007), pH, and C levels (Chan et al. 2008) and by enhancing nutrient use efficiency (Van Zwieten et al. 2010; Ippolito et al. 2012). Various studies have reported that biochar application have boosted the soil biological activity (Anderson et al. 2011; Lehmann et al. 2011).
The effects of biochar application on soil biological processes varied with respect to feedstocks and its mechanism in various soils (Lehmann et al. 2011), which ensured high variability in response of soil microbial biomass to biochar additions reported by various studies (O'Neill et al. 2009; Grossman et al. 2010; Khodadad et al. 2011). Microbial biomass increased with biochar additions due to the presence of labile C fractions and unpyrolyzed feedstocks (Steinbeiss et al. 2009; Bruun et al. 2011; Zimmerman et al. 2011; Luo et al. 2013). In contrast, various studies reported no effect of biochar on soil microbial biomass (Castaldi et al. 2011; Zavalloni et al. 2011) as it is recalcitrant in nature (Kuzyakov et al. 2009). Dempster et al. (2012a) found that biochar amendments reduced soil microbial biomass due to toxic effect. Biochar application rates and soil type also affected response to soil microbial biomass (Lehmann et al. 2011).
Explanations for soil microbial biomass variation in response to biochar additions include increased available soil nutrients (dissolved organic matter—phosphorous (P), calcium (Ca), and potassium (K)—adsorption of toxic compounds, and improved soil pH and water content, all of which can affect the activity of soil microbes (Lehmann et al. 2011). The inner porosity of biochar might help soil microbes to evade grazers (Pietikäinen et al. 2000) and store carbon substrates and mineral nutrients (Saito and Muramoto 2002; Warnock et al. 2007).
Soil biological processes such as organic matter degradation, mineralization, and nutrient cycling are affected by the enzymes present in the soil due to microbial activities (Marx et al. 2001). Enzyme substrates are adsorbed by the biochar surfaces, thus enhancing the enzyme reactions leading to increased soil enzyme activity. Also the addition of biochar improves soil properties that indirectly increase soil enzyme activities (Zhang et al. 2016). Alkaline phosphatase has significant role in P cycling and is dominant in alkaline soils (Eivazi and Tabatabai 1977). Phosphatase enzyme liberates inorganic phosphates by hydrolysis of ester phosphate bonds which are assimilated by plants and microorganisms (Nannipieri et al. 2012). It converts organic P compounds to different inorganic forms. Urease enzyme is known for breaking the carbon-nitrogen bonds of some amides and urea (Bremner and Mulvaney 1978). Wu et al. (2012) reported increased urease activity in wheat straw biochar-amended soil and activity increased with increasing biochar rates. Further, the variation in urease activity is known to be effected considerably by soil pH and soil texture (Makoi and Ndakidemi 2008). The use of dehydrogenase activity as an index of overall microbial activity has been suggested by Palanisamy et al. (2017). The dehydrogenase activity indicates positive priming effect of biochar. Due to large surface area and porosity of biochar, it can stabilize native soil organic carbon (SOC) through direct sorption of dissolved organic matter and microbial enzymes on biochar surfaces and within pore spaces, which results in limiting soil enzyme activity (Bailey et al. 2011; Keith et al. 2011; Lammirato et al. 2011). Many other studies found immediate effect of biochar application on dehydrogenase activity which increased initially but decreased at the end of the experiment (Kuzyakov et al. 2009; Farrell et al. 2013; Bhaduri et al. 2016).
Biochar additions improve the ecosystem and its functioning (Lehmann et al. 2011; Biederman and Harpole 2013), including soil productivity and soil nutrient contents (Ding et al. 2016). The effect of biochar varies with the type or feed stock of biochar (Al-Wabel et al. 2018), soil, and climate. However, the present knowledge on soil microbial biomass dynamics due to biochar amendment is mostly based on the assessment of biochar application and no biochar treatment without considering the co-use of biochar and NPK specifically in legume-cereal cropping system and dearth in long-term field experimental data. The present field study with sequential biochar application and NPK lasted nearly 2 years in a mash bean-wheat cropping system, with whole inputs of biochar at 0%, 0.25%, and 0.5% biochar-C (carbon-equivalent). The aim of the present study is to study the effects of sugarcane bagasse biochar and NPK application on the seasonal variation in microbial biomass carbon (MBC), nitrogen (MBN), phosphorous (MBP), and different enzyme activities measured within a mash bean-wheat growing season during 2 years. The study helps to explore the microbial biomass dynamics with co-use of biochar and NPK application for enhanced carbon storage and higher crop yield in carbon-deficient soils of arid region.
Material and methods
Field experiment
Field experiment was carried out at Pir Mehr Ali Shah (PMAS)-Arid Agriculture University Rawalpindi Koont research farm (33° 1′ N to 36° 6′ N, 73° 30′ E to 73° 45′ E). The treatments were applied in randomized complete block design (RCBD). The soil of experimental area was sandy loam with pH 8.5 and moisture contents depend mainly on rainfall received in monsoon season. The average soil organic carbon (SOC) is less than 1%. The climate of the site is semi-arid to sub-tropical continental and sub-humid and has a bimodal rainfall incidence pattern, with two maxima in winter spring periods and late summer. Rainfall is erratic, around 60–70% of the entire rainfall usually occurred during the monsoon wet season (June to September) (Shafiq et al. 2005). Intense rain and undulating landscape are the major sources of soil erosion.
Biochar preparation and application
Sugarcane bagasse was used as a feedstock for biochar preparation. For this, bagasse was air-dried and pyrolysis was done in the sealed vessel entailing two metal barrels at 350 °C. The space between the barrels was ignited with natural gas. The process took 1 h, while the produced biochar was left to cool for an hour converting approximately 50% of the biomass into biochar (Gunther 2009). In the field, biochar mass was crushed to powder to pass through a 2-mm sieve to get a fine granular uniformity (Pan et al. 2011).
In the field, three treatments of biochar (0, 5, and 10 tons ha−1) were applied with and without chemical fertilizer, i.e., biochar at 0 tons ha−1 (B0F0), biochar at 5 tons ha−1 (B1F0), biochar at 10 tons ha−1 (B2F0), biochar at 0 tons ha−1 + NPK (B0F1), biochar at 5 tons ha−1 + NPK (B1F1), and biochar at 10 tons ha−1 + NPK (B2F1). The NPK was applied at 23 kg nitrogen (N), 45 kg phosphorous (P), and 25 kg potassium (K) per hectare, and treatments were assigned to field plots (1.5 m × 4.5 m) using RCBD. Biochar was applied one time before sowing of mash bean to the soil surface, thoroughly mixed, and then tilled to a depth of 12 cm, while fertilizer was applied to both crops during 2 years as recommended for both crops. Biochar treatments were applied to the mash bean crop, after which residual effects of the treatments on microbial biomass and activity were tested on the wheat crop in two growing cycles. The basic characteristics of the studied topsoil (0–15 cm) and biochar are given in Table 1.
Rhizosphere soil sampling
The rhizosphere soil samples were collected at crop maturity. Plants were randomly excavated at a depth of 10 cm from the replicate plots and tightly adhered soil attached to the plant root system was collected (Butler et al. 2003; Liu et al. 2008). The soil samples were collected in polyethylene bags and were transferred to the laboratory within 2 h of sampling. The samples were sieved (< 2 mm) and were stored at 4 °C. Soil samples for assessing microbial communities were not sieved and directly stored in freezer until isolation.
Biochar and soil characteristics
Biochar and soil moisture content (SMC) were determined gravimetrically. Soil and biochar samples were weighed, oven-dried, cooled, and then reweighed (Gardner et al. 1991). Electrical conductivity (EC) of soil from saturated paste was determined by a conductivity meter (Rhoades 1996) and soil pH was examined in 1 N KCl (potassium chloride) ratio of 1:1 soil suspension (Thomas 1996). EC and pH of biochar were analyzed in a 1:10 (w:v) water soluble extracts (Cayuela et al. 2013). Elemental composition (C/H/N/S) of the biochar was determined using a CHN Elemental Analyzer (Varian EL III) and the oxygen content was determined by weight difference. Proximate analysis including volatile matter and ash content was conducted according to the ASTM3174 (2011) and ASTM 3175 (2007), respectively, and fixed carbon content was determined by mass difference. Surface area and porosity of the biochar were determined via N2 adsorption using an ASAP 2020 instrument (Micromeritics Instrument). Prior to the measurement, the samples were passed through a 0.15-mm sieve and outgassed at 250 °C under vacuum for 4 h. Four data points, between relative pressures of 0.05 and 0.3, were used to construct the monolayer adsorption capacity. The total pore volume (Vtotal) was estimated from a single N2 adsorbed point at a N2 relative pressure of 0.99.
Surface area was measured via the Brunauer, Emmett, and Teller (BET) method that measures N2 gas sorption (0.162 nm2) at 77 K. Approximately 200 mg of ground sample (bagasse biochar) was out gassed at 120 °C for 16.5 h and then analyzed on an Autosorb-1 Surface Area Analyzer (Quantachrome Instruments). Five data points, with relative pressures of 0.05–0.3, were used to calculate the surface area (Brunauer et al. 1938). For the determination of soil organic carbon (SOC) contents, biochar samples were burnt to ashes in the muffle furnace at 500 °C for 4 h and calculated by using the formula described by Brake (1992).
SOC was analyzed by the wet digestion process as described by Nelson and Sommers (1982). Wet digestion was performed with concentrated sulfuric (H2SO4) acid and 1 N K2Cr2O7 (potassium dichromate) solution. After digestion, the titration was carried out with 0.5 N ferrous ammonium sulfate solution. For total nitrogen (TN), the digestion was carried out with sulfuric acid (H2SO4) and allowed to distillation process with the addition of boric acid and NaOH. Nitrogen in the distillate was analyzed by the method given by Van-Schouwenberg and Walinge 1973. Dissolved organic carbon (DOC) was analyzed using total organic carbon (TOC)-V analyzer (Shimadzu Corp., Kyoto, Japan).
Microbial biomass analysis
MBC was measured by fumigation extraction method (Brookes et al. 1985). Ten-gram soil sample was fumigated for 24 h at 25 °C with ethanol-free chloroform (CHCl3) and extraction was performed with 0.5 M K2SO4. The similar process was carried out for non-fumigated soil samples. After the titration of extracts, MBC was calculated from the given formula: MBC = (Carbonfumigated − Carbonunfumigated) × 2.64.
For MBN, TN in the potassium sulfate (K2SO4) extract was determined after Kjeldahl-digestion. One gram of a digestion mixture (FeSO4 10: CuSO4–1: Se-0.1) and conc. H2SO4 (4.5 ml) was added after cooling, and digestion took 3 h. After cooling, the contents were mixed thoroughly after the addition of water (20 ml) and 10 M NaOH (25 ml). The digested material was moved into the steam distillation chamber of Kjeldahl by using 10 M NaOH and 2% H3BO3. Then, 40 ml of distillate collected and titrated to bluish red end point with 50 mM H2SO4 (Wu et al. 1990). The soil MBN was determined by using the equation; MBN = (Nfumigated − Nunfumigated) × 1.46.
For MBP (microbial biomass phosphorous), 2.5 g oven-dried soil sample was taken in duplicate. One was fumigated with alcohol-free CHCl3 (for 24 h at 25 °C), while second portion was incubated aerobically at 25 °C for 24 h at the same time. Both the portions were extracted by shaking for 30 min with 0.5 M NaHCO3 (adjusted to pH 8.5). Ten milliliter aliquot of clear extract was taken into a 50 ml volumetric flask and 8 ml of ascorbic acid solution and ammonium molybdate solution (color developing reagent). After 15 min of color development, reading was noted on spectrophotometer at 882-nm wavelength. Phosphorus standards (0, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, and 4.0 ppm) were prepared using KH2PO4 (Brookes et al. 1982; Joergensen et al. 1995). The MBP was calculated by using the following equation:
Enzyme activity
Dehydrogenase activity (DA) in the soil was measured by the reduction of TTC (2,3,5-triphenyl tetrazolium chloride) into TPF (triphenyl formazan). After filtration, the soil extract was analyzed at 546-nm wavelength on a spectrophotometer. The dehydrogenase activity (TPF μg g−1 dry weight (dwt) soil) was calculated as TPF (μg ml−1) × 45/dwt/5 (Alef 1995). For urease activity (UA), the soil extract was collected by using 50 ml KCl solution. After filtration, the ammonium content in the filtrate was analyzed by measuring the optical density at 690 nm (Kandeler and Gerber 1988).
For APA (alkaline phosphatase activity), 10 g moist soil was added in 100-ml volumetric flask and incubated (for 3 h at 37 °C) after addition of toluene (1.5 ml), disodium phenyl phosphate (10 ml), and buffer (20 ml). After incubation diluted to 100 ml and filtered 5 ml buffer and 1–8 ml of filtrate were transferred in 100-ml volumetric flask and again diluted to 25 ml. After dilution, added 2,6-dibromo quinonechloromide and incubated for 20–30 min at room temperature. Finally diluted with distilled water to 100 ml and analyzed the optical density (600 nm). Calibration curve was prepared by using 0–20 ml standard phenol solution. APA was expressed as phenol (μg g−1 dwt 2 h−1) (Alef 1995).
Statistical analysis
To determine the differences among treatments, two-way ANOVA (analysis of variance) was performed (Steel and Torrie 1997). Canonical correspondence analysis (CCA) was carried out to determine the correlation of the environmental variables with all parameters studied.
Results
Soil properties affected with biochar and NPK
The biochar amendment with and without fertilizer affected the soil properties. Significant variations in the SOC, DOC, TN, and soil moisture were recorded which increased with increasing biochar level (Table 2). Soil bulk density decreased significantly in both crops but improved SOC and TN in biochar treatments with fertilizer. In treatment of biochar without fertilizer (B2F0), SOC, TN, and SMC increased by 23%, 27%, and 56%, respectively in the mash bean crop. In the second year, similar trend was observed in mash bean crop. In wheat crop, the treatment of biochar with fertilizer (B2F1), SOC and SMC improved 32% and 39% as compared to control in the first year and the increase was 34% and 72% in the second year, respectively. DOC of soil increased in the mash bean crop while it significantly decreased in wheat crop. However, biochar application had no effect on soil EC and pH during both growing years.
Microbial biomass carbon
In mash bean, MBC significantly increased with increasing biochar level and addition of fertilizer further supplemented it (Table 3). The results elucidated that highest increase was observed with treatment B2F1, i.e., 25% as compared to control during the first year. In second year, the increase in MBC was slightly lower as compared to first year. However, highest MBC was recorded in treatment B2F1, i.e., 8% as compared to respective control. In wheat crop during first year, highest MBC was recorded in treatment B0F1 with 6% increase as compared to control while non-significant difference was recorded in counterparts. In second year, we observed that with addition of biochar MBC decreased significantly (i.e., 13%) and lowest MBC was recorded in B1F1.
Microbial biomass nitrogen
MBN results in mash bean elucidated that MBN increased with addition of biochar and fertilizer during first year of study (Table 3). Highest increase (68%) was observed in treatment B2F0 as compared to control. In second year, significant increase (29%) was recorded in the only treatment B2F1 while there was non-significant difference between other treatments. In wheat, we observed non-significant difference in MBN during both the years of study.
Microbial biomass phosphorus
In mash bean during the first year, data of MBP recorded revealed that the highest MBP was found in treatment B2F1 which was 40% higher as compared to control (Table 3). Addition of biochar increased the MBP overall. During the second year, similar trend was observed and the highest MBP was recorded in B2F1. In wheat crop during first year, MBP was found highest in treatment B2F1 which was 37% higher as compared to control. In second year similar trend was recorded and highest value was found in the same treatment with 29% increase.
Urease activity
In mash bean, the analysis of urease enzyme activity was done and data revealed that in first year, biochar addition overall increased urease enzyme activity and highest activity in treatment B2F1 with 20% increase as compared to control (Table 4). However, in second year, there was non-significant difference among the treatments and the control. The only treatment showing significant difference was B0F1 where the highest urease enzyme activity was recorded in second year. In wheat crop, addition of biochar decreased the urease activity overall as compared to control during both the years of study.
Dehydrogenase activity
The results of dehydrogenase activity in mash bean showed that addition of biochar was correlated with dehydrogenase activity (Table 4). The highest dehydrogenase activity was recorded when biochar was applied with fertilizer (B2F1) and 52% increase was recorded as compared to control. However, in second year, the increase in this treatment was only 19% as compared to control and the highest activity was recorded in B0F1 which was found to be 22% higher as compared to control. In wheat crop during first year, there was non-significant difference among the treatments on the account of dehydrogenase activity; however, the highest activity was recorded in treatment B0F1 which was 21% higher as compared to control. In second year, no significant difference was recorded in treatments as compared to control.
Alkaline phosphatase activity
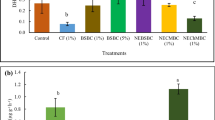
The analysis of alkaline phosphatase enzyme activity during first year in mash bean was found the highest in B2F0 with 54% increase as compared to control (Table 4). In second year, the same treatment was found the highest as compared to control. In wheat crop, similar trend was observed during both the years of study and the highest alkaline phosphatase activity was recorded with B2F0 with 42% increase during first year and 50.3% increase during second year. Other treatments were non-significantly different from the control.
Effect of biochar on biological yield
Biological yield in mash bean elucidated that biological yield (BY) increased with addition of biochar and fertilizer during both years of study (Table 5). The highest increase (37%) was observed in treatment B2F1 as compared to control. In second year, similar trend was noticed in the biochar treatment with significant difference between other treatments. Similarly, in wheat, we observed significant increase in BY with co-use of biochar and NPK during both the years of study. The highest increase (37%) was observed in treatment B2F1 as compared to control. However, in second year, the increase in this treatment was only 29% as compared to control.
Effect of biochar on grain yield
Mash bean grain yield showed that addition of biochar was correlated with grain yield (GY). The highest GY was recorded when biochar was applied with fertilizer (B2F1) and 23% increase was recorded as compared to control (Table 5). While in second year, 26% increase was observed with B2F1 as compared to control. In wheat crop, similar trend was observed during both the years of study and the highest GY was recorded with B2F1 with 43% increase during first year and 33% increase during second year. Other treatments showed significant differences among each other.
CCA of environmental variable and dependent variables
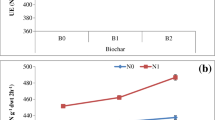
The CCA results in mash bean revealed that APE and MBN were best correlated with SMC in first axis (51%), while GY and UE were best correlated with BD (Fig. 1). However, in second year, soil BD was the main driver to enhance the MBC and UE in first axis while EC, pH, and SOC were the main drivers to increase APE and MBN. In wheat, UE, APE, MBC, and DE were best correlated with BD in first axis (75%), while APE, MBN, MBP, and DE were best correlated with SMC, SOC, and EC. In second year, MBC, UE, and MBN were best correlated with soil pH and BD in first axis while APE and MBN were best correlated with soil EC (Fig. 2).
Canonical correspondence analysis (CCA) of environmental and response variables of mash bean crop during 2 years. EC electrical conductivity, SMC soil moisture content, BD bulk density, SOC soil organic carbon, DOC dissolved organic carbon, TN total nitrogen, MBC microbial biomass carbon, MBN microbial biomass nitrogen, MBP microbial biomass phosphate, UE urease activity, DE dehydrogenase activity, APE alkaline phosphatase activity
Canonical correspondence analysis (CCA) of environmental and response variables of wheat crop during 2 years. EC electrical conductivity, SMC soil moisture content, BD bulk density, SOC soil organic carbon, DOC dissolved organic carbon, TN total nitrogen, MBC microbial biomass carbon, MBN microbial biomass nitrogen, MBP microbial biomass phosphate, UE urease activity, DE dehydrogenase activity, APE alkaline phosphatase activity
Discussion
Soil properties affected with biochar and NPK additions
Physical and chemical properties of soil have been shown to change in various studies with the biochar addition (Asai et al. 2009; Major et al. 2010). Different crops respond differently to the physical and chemical properties of biochar but crops also respond to soil type and climatic conditions (Gaskin et al. 2010; Van Zwieten et al. 2010; Haefele et al. 2011). Our study showed that biochar amendment improved the physico-chemical properties of soil, similar to the results of Asai et al. (2009) and Major et al. (2010). Biochar soil application increased the porosity and water content of the soil, and decreased the bulk density as reported by Ogawa and Okimori 2010, which promoted lateral root formation and increased the soil volume that was exploited by plant roots. The results of Genesio et al. (2012) study suggested that biochar changes the physical conditions of soil; its dark color alters thermal dynamics and leads to rapid germination, allowing more time for growth compared with controls. Pyrogenic bagasse reduced the soil bulk density and increased the available moisture which was in line with the results of Chen et al. (2010). In this study, biochar application had no effect on soil pH and EC since biochar was near neutral pH. However, the enhanced SMC was observed which might be attributed to the large surface area of biochar particles and the pores available to hold water molecules which adhere to biochar particles.
Effect of biochar on soil microbial biomass
Microbial biomass reflects the microbial turnover in the soil ecosystem which includes the growth, death, and organic matter decomposition. In the present study, MBC and MBN were increased significantly with biochar additions suggesting that biochar addition accelerates microbial growth. The results of MBC and MBN indicate a possible increase in microbial carbon use efficiency and a decrease in C turnover in response to biochar addition (Table 3). The enhanced microbial biomass with biochar addition may attribute to positive priming effect of biochar (data unpublished). Similarly, biochar contained labile organic carbon at the start of the experiment indicated enhanced microbial growth and activity. DOC was significantly higher in the mash bean crop when fresh biochar was applied; however, it decreased at the end of the experiment which could be linked with the microbial biomass reduction or remain unchanged over time. The small amounts of organic substrates of low molecular weight, such as glucose, amino acids, and root exudates, might trigger microbial activity and biomass render “apparent” or “real” priming effects. However, reported effects of biochar additions to microbial biomass are pretty inconsistent. Biochar may contain trace quantities of water soluble low molecular organic compounds among predominantly complex C substrates, which could trigger priming effects. In a short-term study of peanut, biochar application results in an increase of MBC due to increase of SOC (Bhaduri et al. 2016). Though, biochar has the potential to reduce microbial biomass as it contains volatile compounds (Deenik et al. 2010). Girvan et al. (2005) demonstrated that benzene concentrations of 40 mg kg−1 or higher can decrease the microbial biomass. Dempster et al. (2012b) found that biochar application induced toxic effects which hinder the soil microbial biomass.
MBP represents the immobilization of P by microbes, providing a mechanism of P retention in soils, and is an important contributor to the soil P pool (Liptzin and Silver 2009). Our finding of an increased MBP in high biochar-amended soil is more likely due to an enhanced nutrient availability, soil moisture, and microbial abundance and therefore increased enzyme levels in response to biochar addition. Our results suggest a greater availability of nutrients for microbe and plant use due to increased phosphatase activity in response to biochar addition. This is consistent with the findings of Biederman and Harpole (2013), whose meta-analysis found that, on average, biochar amendment resulted in increased soil MBP and P availability. Similarly, results of CCA confirmed the positive correlation of the enhanced MBP with SMC, SOC, and DOC (axis 2) in both crops.
Likewise, results of CCA revealed the soil BD was the main driver to enhance the microbial biomass in the mash bean crop. Biochar contains small pore spaces which may provide suitable habitat to microbes which caused increase microbial growth and activity. The internal porosity of biochar may help soil microbes to avoid grazers (Pietikäinen et al. 2000) and store C substrates and mineral nutrients (Saito and Muramoto 2002; Warnock et al. 2007). Various studies suggested that the increase in soil microbial activity in terms of soil MBC in biochar-treated soils (Lehmann et al. 2011; Masto et al. 2013) was mostly due to improvement of physico-chemical characteristics of the soil (Maestrini et al. 2015). In contrast, other studies reported that biochar has no effect on soil microbial biomass (Castaldi et al. 2011) due to its recalcitrance property (Kuzyakov et al. 2009). Biochar additions rates and soil type also affected response of soil microbial biomass (Lehmann et al. 2011). An explanation for soil microbial biomass change in response to additions of biochar includes enhanced availability of soil nutrients (i.e., DOC, P, Ca, and K), adsorption of toxic compounds, and improved soil water and pH status. All these changes have an impact on the activity of soil microorganisms (Lehmann et al. 2011).
Effect of biochar on soil enzyme activity
Similarly, the enzyme activity was boosted when biochar was applied, but it decreased at the end of the experiment, especially in case of urease, while the dehydrogenase activity remained unchanged. The dehydrogenase activity gives an indication of positive priming effect of biochar. Due to large surface area and porosity of biochar, it can stabilize native SOC through direct sorption of dissolved organic matter and microbial enzymes on biochar surfaces and within pore spaces, which results in hindering the activity of the soil enzymes (Bailey et al. 2011; Keith et al. 2011; Lammirato et al. 2011). Various other studies found immediate effect of biochar application resulting in an increase in dehydrogenase activity at initial phase while it suppressed at the end of the experiment (Kuzyakov et al. 2009; Farrell et al. 2013; Bhaduri et al. 2016). In the present study, biochar application suppressed native SOC mineralization rapidly. This could result from sorption of labile soil organic matter onto biochar particles, inhibitory effect on microbial activity of biochar-associated volatile organic compounds, and/or a brief shift to more labile substrates in biochar, relative to those in the low-C clay soil (Guenet et al. 2010; Lehmann et al. 2011; Spokas et al. 2011). In contrast, alkaline phosphatase activity increased gradually over the course of the experiment which suggested that biochar provides suitable habitat for the phytase-producing microbes. CCA results correlate that the SMC were the main drivers in controlling APA activity. In addition, microbial phosphatases release the organic bound phosphorous into inorganic P which could be available to plants (Azeem et al. 2015). As soils contain very large amount of organic phosphorus specially in alkaline soils (calcium and magnesium bound P), so microbes mounted or living in the pores of biochar particles may release the biochar-P and also from the organic matter which enhanced the activity of the enzyme which ultimately enhanced the crop growth and yield.
Plant growth is also affected by the biochar-induced variations in soil physical and chemical properties predominantly SMC, BD, nutrient cycling, and carbon turnover (Dempster et al. 2012a, b; Taghizadeh-Toosi et al. 2012). Biochar improves the soil water holding capacity (Laird et al. 2010) and facilitates biomass gain (Kammann et al. 2011). Increased crop yield with biochar additions was reported in various studies which were attributed to improve soil health and nutrient uptake. Alburquerque et al. (2013) found 20–30% increase in wheat yield with the application of wheat straw and olive tree pruning biochar in a loamy sand-textured soil, attributed to enhanced nutrient availability. Availability of water content, reduction in soil bulk density, enhanced microbial growth, and carbon turnover may be attributed to enhanced crop biomass and yield in the arid climate. The results of our study presented that plant-based (bagasse) biochar with chemical fertilizer can be a better option to improve the soil health of dry areas.
Conclusion
The current study enhanced the understanding of the microbial biomass and enzyme activity as affected by the co-use of biochar and NPK additions in mash bean-wheat cropping system of the dry land of Pakistan. Biochar applications increased the SOC, TN, and crop yield, and decreased the soil bulk density. Seasonal fluctuation in MBC was less for biochar-amended soils than for control and NPK treatments, suggesting that biochar induced a less extreme environment for microorganisms throughout the season. The levels of soil microbial biomass phosphorus and phosphatase activities were most significantly increased with B2F0 treatment. The current study suggests that the use of bagasse biochar as a soil amendment could be adopted as an effective measure to achieve simultaneously high grain yield and lower carbon loss in mash bean and wheat crop lands of calcareous soil poor in organic carbon.
References
Alburquerque JA, Salazar P, Barron V, Torrent J, del Campillo MDC, Gallardo A, Villar R (2013) Enhanced wheat yield by biochar addition under different mineral fertilization levels. Agron Sust Develop 33:475–484
Alef K (1995) Dehydrogenase activity. In: Alef K, Nannipieri P (eds) Methods in applied soil microbiology and biochemistry. Academic Press Inc., San Diego, pp 228–230
Al-Wabel MI, Hussain Q, Usman AR, Ahmad M, Abduljabbar A, Sallam AS, Ok YS (2018) Impact of biochar properties on soil conditions and agricultural sustainability: a review. Land Degrad Develop 29(7):2124–2161
Anderson CR, Condron LM, Clough TJ, Fiers M, Stewart A, Hill RA, Sherlock RR (2011) Biochar induced soil microbial community change: implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia 54(5–6):309–320
Asai H, Samson KB, Stephan MH, Songyikhangsuthor K, Homma K, Kiyono Y (2009) Biochar amendment techniques for upland rice production in northern Laos 1. Soil physical properties, leaf SPAD and grain yield. Field Crops Res 111:81–84
Azeem M, Riaz A, Chaudhry AN, Hayat R, Hussain Q, Tahir MI, Imran M (2015) A review: release of organic phosphorus through microbial phytase activity. Arch Agron Soil Sci 60(6):751–766
Bailey VL, Fansler SJ, Smith JL, Bolton H (2011) Reconciling apparent variability in effects of biochar amendment on soil enzyme activities by assay optimization. Soil Biol Biochem 43:296–301
Bhaduri D, Saha A, Desai D, Meena HN (2016) Restoration of carbon and microbial activity in salt-induced soil by application of peanut shell biochar during short-term incubation study. Chemosphere 148:86–98
Biederman LA, Harpole WS (2013) Biochar and its effects on plant productivity and nutrient cycling: a meta-analysis. GCB Bioenergy 5(2):202–214
Brake JD (1992) A practical guide for composting poultry litter. MAFES Bulletin 981. Mississippi State University, USA, June. Dept. of Poultry Science, p 265
Bremner JM, Mulvaney RL (1978) Urease activity in soils. In: Burns RG (ed) Soil enzymes. Academic Press, New York, pp 149–196
Brookes PC, Powlson DS, Jenkinson DS (1982) Measurement of microbial biomass phosphorus in soil. Soil Biol Biochem 14:319–329
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method for measuring microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Brunauer S, Emmett P, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319
Bruun EW, Hauggaard-Nielsen H, Ibrahim N, Egsgaard Ambus HP, Jensen PA, Dam-Johansen K (2011) Influence of fast pyrolysis temperature on biochar labile fraction and short-term carbon loss in a loamy soil. Biomass Bioenergy 35:1182–1189
Butler JL, Williams MA, Bottomley PJ, Myrold DD (2003) Microbial community dynamics associated with rhizosphere carbon flow. Appl Environ Microbiol 60:6793–6800
Castaldi S, Riondino M, Baronti S, Esposito FR, Marzaioli R, Rutigliano FA, Vaccari FP, Miglietta F (2011) Impact of biochar application to a Mediterranean wheat crop on soil microbial activity and greenhouse gas fluxes. Chemosphere 85:1464–1471
Cayuela ML, Sánchez-Monedero MA, Roig A, Hanley K, Enders A, Lehmann J (2013) Biochar and denitrification in soils: when, how much and why does biochar reduce N2O emissions? Soil Res 15:26–28
Chan K, Van Zwieten L, Meszaros I, Downie A, Joseph S (2008) Using poultry litter biochars as soil amendments. Soil Res 46:437–444
Chen Y, Shinogi Y, Taira M (2010) Influence of biochar use on sugarcane growth, soil parameters, and groundwater quality. Soil Res 48:526–530
Deenik JL, McClellan T, Uehara G, Antal MJ, Campbell S (2010) Charcoal volatile matter content influences plant growth and soil nitrogen transformations. Soil Sci Soc Am J 74:1259–1270
Dempster N, Gleeson B, Solaiman M, Jones L, Murphy V (2012a) Decreased soil microbial biomass and nitrogen mineralization with eucalyptus biochar addition to a coarse textured soil. Plant Soil 354:311–324
Dempster N, Jones L, Murphy V (2012b) Organic nitrogen mineralization in two contrasting agro-ecosystems is unchanged by biochar addition. Soil Bio Bioch 48:47–50
Ding Y, Liu Y, Liu S, Li Z, Tan X, Huang X, Zeng G, Zhou L, Zheng B (2016) A review: biochar to improve soil fertility. Agron sust develop 36(2):36
Eivazi F, Tabatabai MA (1977) Phosphatases in soils. Soil Biol Biochem 9:167–172
Farrell M, Thomas K, Kuhn K, Lynne M, Macdonald TM, Maddern DV, Murphy PA, Bhupinder PS, Karen B, Evelyn S, Jeff AB (2013) Microbial utilization of biochar-derived carbon. Sci Tot Environ 465:288–297
Gardner CMK, Bell JP, Cooper JD, Dean TJ, Hodnett MG, Gardner N (1991) Soil water content, physical methods. Marcel Dekker, New York, pp 25–30
Gaskin JW, Speir RA, Harris K, Das KC, Lee RD, Morris LA (2010) Effect of peanut hull and pine chip biochar on soil nutrients, corn nutrient status, and yield. Agron J 102:623–633
Genesio L, Miglietta F, Lugato E, Baronti S, Pieri M, Vaccari FP (2012) Surface albedo following biochar application in durum wheat. Environ Res Lett 7:25–36
Girvan MS, Campbell CD, Killham K, Prosser JI, Glover LA (2005) Bacterial diversity promotes community stability and functional resilience after perturbation. Environ Microbiol 7:301–313
Glaser B, Lehmann J, Zech W (2002) Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal-a review. Biol Fertl Soils 35:219–230
Grossman JM, O'Neill BE, Tsai SM, Liang B, Neves E, Lehmann J, Thies JE (2010) Amazonian anthrosols support similar microbial communities that differ distinctly from those extant in adjacent, unmodified soils of the same mineralogy. Microb Ecol 60:192–205
Guenet B, Leloup J, Raynaud X, Bardoux G, Abbadie L (2010) Negative priming matter mineralization in a smectite-rich soil. Environ Sci Technol 45:9611–9618
Gunther S (2009) Biochar 10, mother nature network. Plant Soil 8:236–242
Haefele MS, Konboon Y, Wongboon Amarante WS, Maarifat AA, Pfeiffer ME (2011) Effects and fate of biochar from rice residues in rice-based systems. Plant Soil 8:236–242
Ippolito JA, Laird DA, Busscher WJ (2012) Environmental benefits of biochar. J Environ Qual 41:967–972
Joergensen RG, Kubler H, Meyer B, Wolters V (1995) Microbial biomass phosphorus in soils of beech (Fagus sylvatica L.) forests. Biol Fert Soils 19:215–219
Kammann CI, Linsel S, Gößling JW, Koyro HW (2011) Influence of biochar on drought tolerance of Chenopodium quinoa wild and on soil-plant relations. Plant Soil 345:195–210
Kamran MA, Jiang J, Li JY, Shi RY, Mehmood K, Abdulaha-Al Baquy M, Xu RK (2018) Amelioration of soil acidity, Olsen-P, and phosphatase activity by manure-and peat-derived biochars in different acidic soils. Arab J Geosci 11(11):272
Kandeler E, Gerber H (1988) Short-term assay of soil urease activity using colorimetric determination of ammonium. Plant Soil 6:68–72
Keith A, Singh B, Singh BP (2011) Interactive priming of biochar and labile organic matter mineralization in a smectite-rich soil. Environ Sci Technol 45:9611–9618
Khodadad CLM, Zimmerman AR, Green SJ, Uthandi S, Foster JS (2011) Taxa specific changes in soil microbial community composition induced by pyrogenic carbon amendments. Soil Biol Biochem 43:385–392
Kuzyakov Y, Subbotina I, Chen HQ (2009) Black carbon decomposition and incorporation into microbial biomass estimated by 14C labeling. Soil Biol Biochem 41:210–219
Laird DA, Fleming P, Davis DD, Horton R, Wang B, Karlen DL (2010) Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 158:443–449
Lammirato C, Miltner A, Kaestner M (2011) Effects of wood char and activated carbon on the hydrolysis of cellobiose by β-glucosidase from Aspergillus niger. Soil Biol Biochem 43:1936–1942
Lehmann J, Joseph S (2009) Biochar for environmental management: an introduction. Biochar for environmental management: Sci Technol Earthscan, London, UK, pp 1–12
Lehmann J, Rillig M, Thies J, Masiello CA, Hockaday WC, Crowley DE (2011) Biochar effects on soil biota. Soil Biol Biochem 43:1812–1836
Liptzin D, Silver WL (2009) Effects of carbon additions on iron reduction and phosphorus availability in a humid tropical forest soil. Soil Biol Biochem 41(8):1696–1702
Liu W, Lu HH, Wu W, Wei QK, Chen YX, Thies JE (2008) Transgenic BT rice does not affect enzyme activities and microbial composition in the rhizosphere during crop development. Soil Biol Biochem 40:475–486
Luo Y, Durenkamp M, De Nobili M, Lin Q, Devonshire BJ, Brookes PC (2013) Microbial biomass growth, following incorporation of biochars produced at 350°C or 700°C, in a silty-clay loam soil of high and low pH. Soil Biol Biochem 57:513–523
Maestrini B, Nannipieri P, Abiven S (2015) A meta-analysis on pyrogenic organic matter induced priming effect. GCB Bioenergy 7:577–590
Major J, Rondon M, Molina D, Riha SJ, Lehmann J (2010) Maize yield and nutrition during 4 years after biochar application to a Colombian savanna Oxisol. Plant Soil 333:117–128
Makoi J, Ndakidemi PA (2008) Selected soil enzymes: examples of their potential roles in the ecosystem. Afr J Biotechnol 7:181–191
Marx MC, Wood M, Jarvis SC (2001) A microplate fluorimetric assay for the study of enzyme diversity in soils. Soil Biol Biochem 33:1633–1640
Masto RE, Kumar S, Rout TK, Sarkar P, George J, Ram LC (2013) Biochar from water hyacinth (Eichornia crassipes) and its impact on soil biological activity. Catena 111:64–71
Nannipieri P, Giagnoni L, Renella G, Puglisi E, Ceccanti B, Masciandaro G, Fornasier F, Moscatelli MC, Marinari S (2012) Soil enzymology: classical and molecular approaches. Biol Fertil Soils 48:743–762
Nelson DW, Sommers LE (1982) Total carbon, organic carbon and organic matter. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, Part 2 Chemical and microbiological properties. Agronomy monograph 9. Am. Society of Agronomy and Soil Science Society of America, Madison, Wisconsin, USA, pp 539–594
Ogawa M, Okimori Y (2010) Pioneering works in biochar research, Japan. Soil Res 48:489–500
O'Neill B, Grossman J, Tsai MT, Gomes JE, Lehmann J, Peterson J, Neves E, Thies JE (2009) Bacterial community composition in Brazilian Anthrosols and adjacent soils characterized using culturing and molecular identification. Microb Ecol 58:23–35
Palanisamy M, Iniyakumar M, Elaiyaraju P, Uthandi SM, Sivasubramanian V (2017) Effect of application of algal biochar on soil enzymes. J Algal Biomass Utln 8(4):1–9
Pan G, Lin Z, Li L, Zhang A, Zheng J, Zhang X (2011) Perspective on biomass carbon industrialization of organic waste from agriculture and rural areas in China. J Agric Sci Technol 13:75–82
Pietikäinen J, Kiikkilä O, Fritze H (2000) Charcoal as a habitat for microbes and its effects on the microbial community of the underlying humus. Oikos 89:231–242
Rhoades JD (1996) Salinity, electrical conductivity and total dissolved solids. In: D.L. Sparks. Methods of soil analysis part 3. Soil Sci. Soc. America. No. 5. Madison, Wisconsin, USA., p. 417–435
Saito M, Muramoto T (2002) Inoculation with arbuscular mycorrhizal fungi: the status quo in Japan and the future prospects. Plant Soil 244:273–279
Shafiq M, Rashid A, Mangrio AG (2005) Agricultural potential soil resources of the Pothwar Plateau. Soil Environ 24:109–119
Sohi SP (2012) Carbon storage with benefits. Science 338:1034–1035
Sohi S, Krull E, Lopez-Capel E, Bol R (2010) A review of biochar and its use and function in soil. Adv Agr 105:47–82
Spokas KA, Novak JM, Stewart CE, Cantrell KB, Uchimiya M, DuSaire MG (2011) Qualitative analysis of volatile organic compounds on biochar. Chemosphere 85:869–882
Steel RGD, Torrie JH (1997) Principles and procedures of statistics, a biometric approach. McGraw Hill Book Co. Inc. New York, USA, pp 178–182
Steinbeiss S, Gleixner G, Antonietti M (2009) Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol Biochem 41:1301–1310
Steiner C, Teixeira WG, Lehmann J, Zech W (2007) Long term effects of manure, charcoal and mineral fertilization on crop production and fertility on a highly weathered Central Amazonian upland soil. Plant Soil 291:275–290
Taghizadeh-Toosi A, Clough TJ, Sherlock RR, Condron LM (2012) Biochar adsorbed ammonia is bioavailable. Plant Soil 350:57–69
Thomas GW (1996) Soil pH and soil acidity. In: Sparks DL (eds) Methods of soil analysis part 3. Soil Sci. Soc. America, No.5, Madison, Wisconsin, USA, pp 475–490
Van Zwieten L, Kimber S, Morris S, Chan KY, Downie A, Rust J, Joseph S, Cowie A (2010) Effects of biochar from slow pyrolysis of paper mill waste on agronomic performance and soil fertility. Plant Soil 327:235–246
Van-Schouwenberg JCH, Walinge I (1973) Methods of analysis for plant material. Agric Univ Wageningen, Netherlands, pp 25–30
Warnock DD, Lehmann J, Kuyper TW, Rillig MC (2007) Mycorrhizal responses to biochar in soil concepts and mechanisms. Plant Soil 300:9–20
Wu J, Joergensen RG, Pommerening B, Chaussod R, Brooks PC (1990) Measurement of soil microbial biomass by fumigation-extraction an automated procedure. Soil Biol Biochem 22:1167–1169
Wu F, Jia Z, Wang S, Chang SX, Startsev A (2012) Contrasting effects of wheat straw and its biochar on greenhouse gas emissions and enzyme activities in a Chernozemic soil. Biol Fertil Soils 49:555–565
Zavalloni C, Alberti G, Biasiol S, Vedove GD, Fornasier F (2011) Microbial mineralization of biochar and wheat straw mixture in soil: a short term study. Appl Soil Ecol 50:45–51
Zhang YL, Chen LJ, Zhang YG, Wu ZJ, Ma XZ, Yang XZ (2016) Examining the effects of biochar application on soil phosphorus levels and phosphatase activities with visible and fluorescence spectroscopy. Guang pu xue yu guang pu fen xi= Guang pu 36(7):2325–2329
Zimmerman A, Gao B, Ahn MY (2011) Positive and negative mineralization priming effects among a variety of biochar-amended soils. Soil Biol Biochem 43:1169–1179
Acknowledgments
The research was done on PMAS-AAUR Research farm and also acknowledges the Department of Soil Science and SWC.
Funding
This study received financial support from the Higher Education Commission of Pakistan.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This article is part of the Topical Collection on Implications of Biochar Application to Soil Environment under Arid Conditions
Rights and permissions
About this article
Cite this article
Azeem, M., Hayat, R., Hussain, Q. et al. Effects of biochar and NPK on soil microbial biomass and enzyme activity during 2 years of application in the arid region. Arab J Geosci 12, 311 (2019). https://doi.org/10.1007/s12517-019-4482-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-019-4482-1