Abstract
The impact of oil pollution on coastal vulnerable ecosystems has been a major concern especially, in the Persian Gulf area. The current study was carried out to assess to what extent Avicennia marina can tolerate oil contamination and degrade crude oil polycyclic aromatic hydrocarbons (PAHs) from rhizosphere soil. Seeds of A. marina were grown in control and crude oil-contaminated (2.5, 5.0, 7.5, and 10% w/w) soil under ambient greenhouse conditions. Four-month-old plants were collected, measured for their biometry, and assayed for physiological characteristics in relation to degradation of PAHs. A. marina exposed to petroleum responded by allocating proportionally more biomass to root than shoot, activating enzymatic and non-enzymatic antioxidative mechanisms and removing of PAHs, particularly in lower concentrations of crude oil in the soil. The content of total PAHs in A. marina rhizosphere soil, grown on 2.5, 5.0, 7.5 and 10% oil-treated soils were, respectively, 37 ± 0.4, 21.84 ± 0.27, 12.78 ± 0.11 and 14.74 ± 0.03% lower than non-rhizosphere soil. Comparison of PAHs content of rhizospheric and non-rhizospheric soil also indicated that the highest rate of PAH removal was for acenaphthene (74.63 ± 0.78) in control, fluoranthene (71.18 ± 0.56) in 2.5%, and anthracene (69.45 ± 6.33, 55.66 ± 4.38 and 35.97 ± 0.22) in 5.0, 7.5 and 10% oil-contaminated soil and acenaphthene (74.63 ± 0.78) in control. Activities of peroxidase, ascorbate peroxidase, and polyphenol oxidase were more prominent in the roots of plants exposed to increasing concentrations of oil in soil than control plants. Conversely, the activity of superoxide dismutase decreased. These findings render A. marina as a phytoremediation candidate for small scale oil spills and residual oil pollution in coastal marine environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The hazardous effects of oil pollution have been a major concern and source of many investigations about the impact of large-scale oil spill into marine and coastal environments. Among the marine environments, fragile coastal and littoral ecosystems including mangrove forests of the Persian Gulf in southern Iran, site of the most transited oil shipping routes, are prone to damage from chronic floating oil pollution. Valuable mangrove forest ecosystems of the Persian Gulf include two prominent species, namely A. marina L. and Rhizophora mucronata Poir., the former prevalent in the southern and the latter occurring on the northern coastal regions of the Persian Gulf (Rashvand and Sadeghi 2014).
The coverage area of mangrove forests in the northern Persian Gulf has reduced significantly in the past thirty years. This reduction in coverage area can be contributed to climate change (Ward et al. 2016), spillage of petroleum and associated heavy metals in the marine environment, and industrialization and urban development (Nadim et al. 2008; Guo et al. 2016). Plants can accumulate heavy metals such as nickel and vanadium associated with crude oil and PAHs in their tissues, particularly in their roots. There are reports of large-scale oil spill incidences as late as 1991 in the Persian Gulf (Sheppard et al. 2010) as well as regular oil contamination from shipping through the Strait of Hormuz (Sadiq and McCain 2012) which could have damaged mangrove forests physiologically and ecologically. Such damage could be a result of the reallocation of cellular energy towards reducing abiotic stress, enzymatic regulation, or morphological and structural adjustments as indicated by many researchers (Ke et al. 2011; Naidoo et al. 2010; Sodré et al. 2013; Ralph and Burchett 1998). Other researchers (Youssef 2002; Olubodun and Eriyamremu 2018) have indicated that plants respond to petroleum and PAHs contamination in soil through regulating oxidative stress and scavenging of radical oxygen species (ROS) production.
Phytoremediation is defined as a method that uses plants to stabilize, extract, accumulate, degrade or transform contaminants in sediments, soils, or aquatic environments (Moreira et al. 2013). For practical purposes and maximum success in phytoremediation, it is crucial to use plants that are well adapted to the local environmental conditions and interacting microbial communities and endemic to the areas requiring treatment (Anderson et al. 1993; Shiri et al., 2015). For example, mangroves like A. marina (Forsk.) Vierh (Jia et al. 2016) and Kandelia obovata Sheue (Wang et al. 2014) have been reported to be able to clean up some PAHs in sediments (Jia et al. 2016). As PAHs represent some of the most frequent and persistent toxic contaminants in the Persian Gulf marine environment, their impact expectedly will be cast on vulnerable and fragile ecosystems, such as mangroves more widely. Knowledge of PAHs ecological and physiological impacts on mangroves is not only limited to the scale of investigations, but also our understanding of the physiological responses and phytoremediation capability of A. marina to oil contamination is limited. This investigation aims to determine the extent to which A. marina, the prevalent mangrove species growing in the northern Persian Gulf, can tolerate oil contamination, degrade PAHs in the soil in the vicinity of its roots (rhizospheric soil) compared with soil distant from roots (non-rhizospheric soil) and what is the extent of its stress-related enzyme activity and root growth and development under oil contamination.
Materials and methods
Soil substrate preparation
Soil was collected from the horizon of Bagho Mangrove Nursery site in Bandar Abbas, Hormozgan, Iran. The soil pH was 7.9 and its texture sandy-loam. Soil samples were sieved through a 2 mm mesh, and sterilized at 121 °C for 2 h.
Crude oil, obtained from Tehran Refinery (Sulfur content 1.21%, nitrogen 0.2%, asphalt 0.55%, Wax 7.3%, residual carbon 3.64%, H2S < 1 µg/g, nickel 8.3 µg/g, vanadium 28 µg/g, iron 5.4 µg/g, lead < 1 µg/g, sodium 27 µg/g, water content 0.05%), was added and mixed with soil thoroughly at concentrations of 2.5, 5.0, 7.5 and 10.0% (w/w). Pots (12 cm in diam.) containing 500 g of oil-contaminated soil (C) for each treatment were prepared. Similarly, sterilized non-contaminated soil (NC) in pots served as control. Soil in each pot attached to roots was considered as rhizospheric soil and soil close to pot margin and at a distance from roots as non-rhizospheric soil.
Plant growth conditions
Mature and uniform propagules of A. marina were collected from Tӑsbar Creek of Bandar Abbas-Hormozgan, surface sterilized with 1% sodium hypochlorite in water for 10 min, and washed thoroughly in sterilized distilled water. For each treatment and control, 15 pots were planted with one A. marina propagule in each pot, respectively. Plants in each pot were watered with 100 ml of tap water every other day. All experiments were carried out in greenhouse under a temperature regime of 21 and 18 °C during the day and night, respectively. Plants were harvested 120 days after planting. This time was selected as the time that the plant grown on highest concentration of crude oil in the soil form at least two leaves. Root and shoot lengths and fresh and dry weights (dried at 60 °C in the oven to constant weight) as well as number of leaves of each plant were determined. Representative fresh root samples were properly washed in running tap water and deionized water thoroughly before freezing in liquid nitrogen. For physiological analysis, three root sub-sample replicates were analyzed for each treatment using crushed tissue of ten propagules pooled.
Determination of H2O2 and MDA contents
H2O2 content in roots of oil-exposed and control plants were determined according to Velikova et al. (2000). The absorbance of the supernatant was measured spectrophotometrically (Analytik Jena Spekol 2000, Germany) at 390 nm. The H2O2 content was calculated by comparison with a standard calibration curve prepared using different concentrations of H2O2.
The lipid peroxidation was assessed according to the method of (Heath and Packer 1968) in 0.5 g tissue homogenized in 2.5 ml of 0.1% (m/v) trichloroacetic acid (TCA). The malondialdehyde (MDA) concentration was determined using spectrophotometer with absorption coefficient of 155 mM−1 cm−1.
Antioxidant enzyme activities
Root tissues were homogenized at 4 °C with a mortar and pestle in 0.1 M Tris–HCl buffer (pH 8.9). The homogenates were centrifuged at 13,000 g for 30 min at 4 °C, the resulting supernatants kept at − 80 °C to be used later for total protein determination and enzyme activity assays. A high-speed centrifuge (Beckman J2-MI high speed Centrifuge, Rotor No: 14) and UV–visible recording spectrophotometer (Analytik Jena Spekol 2000, Germany) were used.
The total protein content was determined according to the method described by Bradford (1976). Bovine serum albumin was used as standard. SOD (EC 1.15.1.1) activity in root was estimated by monitoring the inhibition of photochemical reduction of nitrobluetetrazolium (NBT) as described by Giannopolitis and Ries (1977). One unit of SOD was defined as the amount of enzyme which caused 50% inhibition of NBT reduction under the assay condition, and the results were expressed as [Unit mg−1 (protein)].
Peroxidase (POX; EC 1.11.1.7) activity was measured according to the method described by Abeles and Biles (1991). The POX activity was defined as l µM of benzidine oxidized per min per mg protein [Unit mg−1(protein)].
Polyphenol oxidase (PPO; EC 1.14.18.1) activity was determined according to the method described by Raymond et al. (1993) at 40 °C. The PPO activity was defined as 1 µM of pyrogallol oxidized per min per mg protein [Unit mg−1 (protein)].
Ascorbate peroxidase activity (APX; EC 1.11.1.11) was measured according to Jebara et al. (2005). The concentration of oxidized ascorbate was determined by the decrease in absorbance at 290 nm. The concentration of oxidized ascorbate was calculated using extinction coefficient (e = 2.8 mM cm−1). One unit of APX was defined as 1 µM oxidized ascorbate per min per mg protein [Unit mg−1(protein)].
Determination of phenolic contents
Method described by Sorahinobar et al. (2016) with minor modification was used for extracting root phenolic contents. 0.1 g ground root tissue was mixed and boiled with 80% methanol for 3 h. Total phenolic content was determined using Folin–Ciocalteu reagent according to (Akkol et al. 2008). 1 ml of methanolic extract was mixed with 5 ml Folin–Ciocalteu reagent and 4 ml of a 7.0% sodium carbonate solution. Similarly, gallic acid was used as standard control for the calibration curve. Mixtures were allowed to stand for 2 h before their absorbance was measured at 765 nm. Total phenolic values are expressed in terms of mg equivalent Gallic acid in 1 g FW.
Determination of PAL activity
Phenylalanine ammonia lyase (PAL; EC 4.3.1.24) activity was determined based on the rate of cinnamic acid production as described by Ochoa-Alejo and Gómez-Peralta (1993). One unit of PAL activity was expressed equal to 1 µmol of cinnamic acid produced per min.
Root anatomy
Hand cross sections of root were prepared. Sections were cleared in sodium hypochlorite and stained by carmine-vest (1% w/v in 50% ethanol) and methyl green (1% w/v, aqueous) and mounted in gelatin. Then, well-stained sections were photographed with an Olympus BH2 microscope and all the measurements and observations were performed 10 times on different sides by measurement software with five repeats at each part.
PAHs assessment
For collection of rhizospheric soil at harvest, plants were gently removed from the pots and their roots shaken to remove loose soil. The soil adhering to the root segments was collected as the rhizospheric soil. Non-rhizospheric soil was collected from marginal parts of pot not in contact with pot wall nor roots (with a 2 and 4 cm distance, respectively) and at least 5 cm depth.
PAHs were extracted from the soil samples according to MOOPAM (2000) with some modifications. Briefly, after freeze-drying of the soil samples, 2 g soil was extracted with dichloromethane: acetone (1:1) in an ultrasonic bath under the optimized conditions and the solvent was evaporated using a rotary evaporator. Clean-up of the extract was performed first, with acid-activated copper to remove the elemental sulfur followed by a silica–alumina column eluted by hexane and hexane–dichloromethane (90:10) as washing solvents. After removal of the solvent, the final residue was dissolved in 1 ml hexane. Analysis of PAHs was performed on an Agilent 6890 N GC system equipped with a 5973 mass detector and a MSD Chemstation software. Separation of PAHs was carried out on a HP-5 fused silica capillary column (30 m × 0.25 mm × 0.25 µm). All mass spectra were acquired in electron impact (EI) mode. p-Terphenyl-d14 was used as injection standard. All mass spectra were acquired in electron impact (EI) mode. The external standard addition method was used to calculate the recoveries. A known amount of 16 PAH standard mixture was added to a carefully weighed sediment and extraction and analysis of the spiked sample was performed exactly by the same procedure as the studied samples. The recoveries were 81–105%.
Raw index of PAH phytoremediation (Pi) was calculated as the percent change in concentration of initial (Ci) and final (Cf) PAH in both rhizospheric and non-rhizospheric soils between the times of the start of the experiment and the time of harvest of the plant as follows:
The differences between Pi (∆Pi) of non-rhizospheric and rhizospheric soils were used to express the capability of plant roots for PAH removal.
Statistical methods
Analytical experiments were conducted with three replicates per treatment. Data were subjected to one-way ANOVA. When, statistical difference between the means of the treatments existed, Duncan test at the 5% level and Pearson correlation index were applied using SPSS version 20. The graphs were designed by GraphPad Prism (Version 8.3.0; GraphPad Software, La Jolla CA, USA).
Results
Growth and morphometry
The height, shoot biomass and number of leaves of A. marina plant exposed to crude oil contamination were reduced. This reduction correlated negatively with the concentration of oil in the soil (Table 1). The biomass of A. marina significantly reduced under petroleum pollution (with a Pearson correlation coefficient of − 0.82 and − 0.71 for fresh and dry weights, respectively). With the increase of petroleum concentration in the soil, a higher root to shoot biomass ratio in line with the increase of root diameter and fewer root branching were observed (Fig. 1).
Root and shoot responded differently to the oil content of the soil. For example, root length and fresh weight increased in the soil containing up to 5.0% oil and decreased at higher concentrations of oil (7.5 and 10%). Also, microscopic examination of root cross sections revealed changes in root tissues exposed to oil in the rhizosphere, particularly, those at 10% oil in the soil. Root tissues of control plants showed a clear epidermis, cortex multi-layered with cortical parenchyma and aerenchyma with intercellular air spaces, three-layered pericycle bounded externally by an endodermis encircling the vascular bundles (Fig. 2a). Root of plants treated under 5% crude oil, however, showed clear epidermis, reduced cortical air spaces, intact endodermis, more compact and denser stele and disordered phloem (Fig. 2b). Root of plants grown in contaminated soil of 10% showed an increased number of epidermal cell layers, black deposits on epidermal cell walls and pericycle, and more evident aerenchyma and air spaces within tissues (Fig. 2c).
Physiological responses
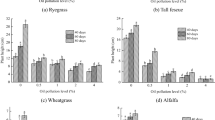
Plants grown under oil contamination showed different physiological responses depending on the level of soil contamination. For example, A. marina roots showed significant reduction in H2O2 and malondialdehyde (MDA) contents under 2.5 and 5% and conversely, increased in 10% contaminated soil (Fig. 3). In roots of A. marina, the induction levels of POX, APX, PPO and PAL enzymes were typically greater in plants grown in soil contaminated with higher concentrations of crude oil (Fig. 4); For example, the highest POX and APX and the least SOD enzymes activities and greatest protein contents occurred at 10% oil treatment compared with control. A higher level of PPO activity was also observed in plants grown in 5 and 10% oil treatment. Enhanced induction of phenolic contents in root occurred at 7.5% oil treatment and was greatest (about three folds that of respective control) at 10% oil treatment (Fig. 5).
The effect of crude oil contamination on total phenolic content and phenylalanine ammonia lyase activity in root of 4-month-old A. marina germinated and grown in different crude oil-contaminated soil. Columns indicate mean ± S.E. based on three replicates. Means with different letters indicate a significant difference p ≤ 0.05 using Duncan multiple range test
PAH phytoremediation
Figure 6 shows the GC chromatogram of the solution of 16 standard PAHs. Total concentration of PAHs was significantly reduced in both crude oil-contaminated rhizospheric and non-rhizospheric soil samples compared with control for 4 months and the reduction was greater in rhizospheric than non-rhizospheric soils (Fig. 7). Among the 16 standard toxic PAHs, naphthalene and acenaphthylene showed the highest content in soil (Table 2).
GC chromatogram of a 50 ppb standard solution of 16 priority PAHs under the optimized chromatographic condition. 1: Naphthalene; 2:Acenaphthylene; 3: Acenaphthene; 4: Fluorene; 5: Phenanthrene; 6: Anthracene; 7; Fluoranthene; 8: Pyrene; 9: Benzo(a) anthracene; 10: Chrysene; 11: Benzo (b) Fluoranthene; 12:Benzo(K) Fluoranthene; 13: Benzo(a)pyrene; 14: Indeno(1,2,3) pyrene; 15: Dibenz(a,h) Anthracene; 16: Benzo(g,h,i) perylene;
A. marina roots showed the highest ∆Pi (index to express the capability of root removing PAHs) for removing anthracene in 5, 7.5 and 10% oil-contaminated soil (Fig. 7). A. marina root removed some PAHs more than others at different concentrations of PAHs in soil with the greatest removal in 2.5% contaminated soil and the most total root biomass as follows:
-
Samples of negative control: Ace > Nap.
-
Samples of 2.5% treatment: Flu > Ant > BaA.
-
Samples of 5% treatment: Ant > BaA > Phe.
-
Samples of 7.5% treatment: Ant > BP > Phe and.
-
Samples of 10.0% treatment: Ant > A > Flu.
Average ∆Pi for roots of A. marina in removing PAHs under all treatments of oil in soil ranks as follows: Anthracene > Benzo(a)anthracene > Phenanthrene > Benzo(g,h,i)perylene > Fluoranthene > Acenaphthene > Pyrene > Fluorene > Acenaphthylene> enzo(b)Fluoranthene > Benzo(K) Fluoranthene > Benzo(a)pyrene Chrysene > Dibenz(a,h)Anthracene > Naphthalene > Indeno(1,2,3) pyrene.
Phytoremediation of the most PAHs (∆Pi) except Acenaphthylene and Benzo(K)fluoranthene showed negative correlation with root MDA content. Among the PAHs, phytoremediation of Naphthalene, Acenaphthene, Anthracene, Benzo(a) anthracene, Bezno(a)pyrene and Indeno-1,2,3-pyrene showed ≥ 80% correlation with leaves and stem dry weight. Strong negative correlation between Anthracene removal (∆Pi) with root MDA (− 0.98), H2O2 (− 0.97) and phenolic compounds (− 0.99) content as well as POX (− 0.92) and PAL (− 0.97) activities were observed.
Discussion
Change in shoot/root biomass ratio under oil treatment (Table 1) indicates reallocation of energy by plant towards extending roots and increasing root biomass while reducing leaf area and number compared with control. Reduced leaf area exacerbates leaf transpiration and, thus, limits the rate of photosynthesis and subsequently, reduces plant growth (Olubodun and Eriyamremu 2018). A. marina under oil contamination changed its root architecture by increasing root diameter and reducing lateral root branches. This change coincided with increased numbers of root cell layers (Fig. 2) leading to an overall change in surface area to volume ratio of the root which may contribute to lower absorption area for pollutants. These root architectural changes also may help the plant to prevent oil contaminants entering vascular system or be a special mechanism to trap them in the cell walls and vacuoles. Findings of this study corroborate reports of other researchers (Fry et al. 2018; Vives-Peris et al. 2020) in that plants can adaptively respond to belowground stresses by altering biomass allocation to the roots, to alleviate the stresses in a manner that optimizes the capture of soil nutrients and maximizes plant growth rate. Nie et al. (2010) showed that petroleum pollution in Phragmites australis, not only promoted the carbon allocation to plant roots but also enhanced the release of carbon from roots to activate soil microorganisms. In this process, as a result of the stressed physiological and biochemical state of A. marina plants, radical species of oxygen (ROSs) are produced as indicated by other investigators (Zhang et al. 2007; Yong and Tam 2007).
Pollution-stressed A. marina plants responded through the production of both enzymatic and non-enzymatic antioxidants. For example, Pearson correlation coefficients analysis of results revealed positive and consistent correlations between H2O2 and MDA (0.96) content of root with the activity of APX (with correlation of 0.77 and 0.66) and POX (0.83 and 0.75) enzymes under different degree of oil contamination, respectively. This indicates that A. marina combats ROSs through multi-faceted antioxidant enzyme activity and preserving of membrane integrity (Ke et al. 2011) as indicated by reduced contents of H2O2 and MDA (Fig. 3). Although, such finding is supported by findings of other researchers on other plants, still different plants may respond differently to oil contamination as shown by Sodré et al. (2013) on the reduced activity of SOD in Aegiceras corniculatum (Yong and Tam 2007; Zhang et al. 2007) which showed increased activity of SOD in Bruguiera gymnorrhiza. To better understand the biochemical pathway and mechanism of enzyme action, enzymatic antioxidant investigation has been carried out by researchers at the molecular level. Further detailed and comprehensive integrated biochemical analysis of the enzymatic pathways is needed to determine the share of enzymatic to non-enzymatic antioxidants.
Higher PAL activity in roots of oil-treated plants compared to non-treated control can facilitate the production of phenolic compounds, such as flavonoids, a group of complex non-enzymatic antioxidants commonly produced by many plant species. Our findings of increased content of phenolic compounds in roots of plants grown under 7.5 and 10% (w/w) crude oil is in agreement with that of other investigators (Zhou et al. 2009). Zhou et al. (2009) showed that exposure of alfalfa and fescue plants to PAHs increased the contents of phenolic compounds which they contributed to changes in gene expression of PAL enzyme.
The existence of PAHs in soil poses many challenges to plant roots, such as water stress, chemical toxicity and nutrient deficiency (Balasubramaniyam 2015). Azaizeh et al. (2011) reviewed the capability of plants in PAH removal. Among the PAHs, benzofluoranthenes, benzo (a) pyrene, benzo (a) anthracene, dibenzo (a, h) anthracene and indeno (1, 2, 3-cd) pyrene are the most potent toxic compounds and, therefore, targeted for phytoremediation with greater priority (Wild and Jones 1995). In this study, the difference between percentage change of PAHs in rhizospheric and non-rhizospheric soil of A. marina is introduced as a measure of the ability of the plant to remove PAHs from the oil-contaminated soil. The presence of root represents a greater capacity for removing PAHs as indicated by the differences in ∆Pi of rhizospheric and negative control soils, with the greatest reduction in anthracene content compared with removal in contents of other PAHs. This is to be expected as the chemical structures of PAHs differ. Although, there is no correlation between PAH’s solubility and diffusivity in water (Tansel et al. 2013) and each PAH has a different threshold for absorption and degradation, the higher removal of PAHs in rhizospheric soil in comparison with non-rhizospheric soil could be because of plant root uptake, facilitated enzymatic degradation (like PPO) (Liu et al. 2015) or degradation by rhizosphere microbial communities (Fang et al. 2001; Wieland et al. 2001; Corgié et al. 2003). The latter possibility is removed in this investigation as the soil was completely heat-sterilized before planting of A. marina propagules.
The values of reduction in total PAHs content correlate fairly well with Hidayati et al. (2018) and further support the idea of phytoremediation capability A. marina to remove petroleum contamination from soil as mentioned by other researcher (Farrias et al. 2008). Furthermore, it is obvious that the oil treated A. marina plants have developed a special (non-concentration-dependent) strategy to remove PAHs. For example, the anthracene, benzo(a)anthracene, phenanthrene, benzo(g,h,i) perylene, and fluoranthene with the initial concentration rank of 13, 4, 2, 11 and 3 respectively have shown the highest removal among the soil PAHs. Jia et al. (2016) also demonstrated that the phenanthrene and pyrene degradation was significantly greater in the A. marina rhizospheric than in the non-rhizospheric sediments. In another study, Sampaio et al. (2019) confirmed the capability of R. mangle L. mangrove plants in PAH phytoremediation from diesel oil-contaminated soil with priority given to acenaphthene, fluorine and naphthalene, respectively. Negative ∆Pi for some PAHs may be due to the interconversion.
Although, we did not find significant correlations between PAHs removal from the A. marina rhizosphere soil (as indicated using ∆Pi) and the PAHs molecular weight, number of rings, water solubility, toxicity factor (Dandajeh et al, 2019), octanol–water partitioning coefficient as well as organic carbon partitioning coefficient, but we found that positive correlation between PAHs ∆Pi and shoot biomass along with its negative correlation with root MDA content that can be an indicator of the transfer of PAHs from root to shoot means lower oxidative stress in roots and higher toxicity in shoot. It seems that A. marina use phytoextraction strategy (Bashir et al., 2017) to eliminate PAHs from rhizosphere.
Conclusions
A. marina seeds germinated and grown in pots containing different levels of crude oil-contaminated soil showed biomass reduction, especially in aboveground organs. Increased root to shoot ratio of A. marina in response to oil contamination has revealed alteration of the carbon allocation pattern with more towards root than shoot to combat oil stress. Change in contents and activity of H2O2, MDA, phenolic compounds, POX, APX, PPO, and PAL enzymes demonstrated a strategy of the plant to harness oil-induced oxidative stress. Results of the PAH concentration assay of rhizospheric and non-rhizospheric contaminated soil determined that plants have developed a special strategy to eliminate special kinds of PAHs and those with the highest concentration in soil were among the top targets of removal. Taken together, current and previous findings suggest that A. marina has a good potential for removing PAHs from coastal areas; however, more pilot field studies of A. marina roots are underway.
Author contribution statement
HZM designed and supervised the findings of this work. BM carried out the experiments and analyzed experimental results with the assistance of MSH and MS. BM and MS wrote the manuscript with the help of JR. All authors read and approved the manuscripts.
References
Abeles FB, Biles CL (1991) Characterization of peroxidases in lignifying peach fruit endocarp. Plant Physiol 95:269–273. https://doi.org/10.1104/pp.95.1.269
Akkol EK, Göger F, Koşar M, Başer KHC (2008) Phenolic composition and biological activities of Salvia halophila and Salvia virgata from Turkey. Food Chem 108:942–949. https://doi.org/10.1016/j.foodchem.2007.11.071
Anderson TA, Guthrie EA, Walton BT (1993) Bioremediation in the rhizosphere. Environ Sci Technol 27:2630–2636. https://doi.org/10.1021/es00049a001
Azaizeh H, Castro PM, Kidd P (2011) Biodegradation of organic xenobiotic pollutants in the rhizosphere. In: Schröder P, Collins C (eds) Organic xenobiotics and plants. Springer, Dordrecht, pp 191–215. https://doi.org/https://doi.org/10.1007/978-90-481-9852-8_9
Balasubramaniyam A (2015) The influence of plants in the remediation of petroleum hydrocarbon-contaminated sites. Pharm Anal Chem Open Access 1:1–11. https://doi.org/10.4172/2471-2698.1000105
Bashir ME, El-Maradny A, El-Sherbiny M, Rasiq KT, Orif M (2017) Bio-concentration of polycyclic aromatic hydrocarbons in the grey mangrove (Avicennia marina) along eastern coast of the red sea. Open Chem 15:344–351. https://doi.org/10.1515/chem-2017-0038
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Corgié S, Joner EJ, Leyval C (2003) Rhizospheric degradation of phenanthrene is a function of proximity to roots. Plant Soil 257:143–150. https://doi.org/10.1023/A:1026278424871
Dandajeh HA, Talibi LN, Hellier P (2019) Influence of combustion characteristics and fuel composition on exhaust PAHs in a compression ignition engine. Energies 12(13):2575. https://doi.org/10.3390/en12132575
Fang C, Radosevich M, Fuhrmann JJ (2001) Atrazine and phenanthrene degradation in grass rhizosphere soil. Soil Biol Biochem 33:671–678. https://doi.org/10.1016/S0038-0717(00)00216-9
Farrias CO, Hamacher C, Wagener ADLR, Scofield ADL (2008) Origin and degradation of hydrocarbon in mangrove sediments (Rio de Janeiro, Brazil) contaminated by an oil spill. Org Geochem 39:289–307. https://doi.org/10.1016/j.orggeochem.2007.12.008
Fry EL, Evans AL, Sturrock CJ, Bullock JM, Bardgett RD (2018) Root architecture governs plasticity in response to drought. Plant Soil 433(1–2):189–200. https://doi.org/10.1007/s11104-018-3824-1
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I occurrence in higher plants. Plant Physiol 59:309–314. https://doi.org/10.1104/pp.59.2.309
Guo J, Chen X, Bao H, Li Y (2016) Photosynthetic and physiological responses of mangroves under an environmental deterioration. Acta Physiol Plant 38(6):140. https://doi.org/10.1007/s11738-016-2157-z
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Hidayati N, Hamim H, Sulistyaningsish YC (2018) Phytoremediation of petroleum hydrocarbon using three mangrove species applied through tidal bioreactor. Biodiversitas 19(3):786–792. https://doi.org/10.13057/biodiv/d190305
Jebara S, Jebara M, Limam F, Aouani ME (2005) Changes in ascorbate peroxidase, catalase, guaiacol peroxidase and superoxide dismutase activities in common bean (Phaseolus vulgaris) nodules under salt stress. J Plant Physiol 162:929–993. https://doi.org/10.1016/j.jplph.2004.10.005
Jia H, Wang H, Lu H, Jiang S, Dai M, Liu J, Yan C (2016) Rhizodegradation potential and tolerance of Avicennia marina (Forsk.) Vierh in phenanthrene and pyrene contaminated sediments. Mar Pollut Bull 110:112–118. https://doi.org/10.1016/j.marpolbul.2016.06.075
Ke L, Zhang C, Wong YS, Tam NFY (2011) Dose and accumulative effects of spent lubricating oil on four common mangrove plants in South China. Ecotox Environ Safe 74:55–66. https://doi.org/10.1016/j.ecoenv.2010.09.011
Liu R, Dai Y, Sun L (2015) Effect of rhizosphere enzymes on phytoremediation in PAH-contaminated soil using five plant species. PLoS ONE 10(3):1–14. https://doi.org/10.1371/journal.pone.0120369
MOOPAM (Manual of Oceanographic Observation and Pollution Analysis), (2000) Regional Organization for the Protection of Marine Environment. Kuwait: Regional Organization for the Protection of Marine Environment
Moreira ITA, Oliveira OM, Triguis JA, Queiroz AF, Ferreira SL, Martins CM, Silva AC, Falcão BA (2013) Phytoremediation in mangrove sediments impacted by persistent total petroleum hydrocarbons (TPH’s) using Avicennia schaueriana. Mar Pollut Bull 67(1–2):130–136. https://doi.org/10.1016/j.marpolbul.2012.11.024
Nadim F, Bagtzoglou AC, Iranmahboob J (2008) Coastal management in the Persian Gulf region within the framework of the ROPME programme of action. Ocean Coast Manag 51:556–565. https://doi.org/10.1016/j.ocecoaman.2008.04.007
Nie M, Yang Q, Jiang L-F, Fang C-M, Chen J-K, Li B (2010) Do plants modulate biomass allocation in response to petroleum pollution? Biol Lett 6:811–814. https://doi.org/10.1098/rsbl.2010.0261
Ochoa-Alejo N, Gómez-Peralta JE (1993) Activity of enzymes involved in capsaicin biosynthesis in callus tissue and fruits of chili pepper (Capsicum annuum L.). J Plant Physiol 141:147–152. https://doi.org/10.1016/S0176-1617(11)80751-0
Olubodun O, Eriyamremu E (2018) Effect of different crude oil fractions on growth and oxidative stress parameters of maize radicle. Int J Plant Soil Sci 2:144–154. https://doi.org/10.9734/IJPSS/2013/4102
Ralph P, Burchett M (1998) Impact of petrochemicals on the photosynthesis of Halophila ovalis using chlorophyll fluorescence. Mar Pollut Bull 36:429–436. https://doi.org/10.1016/S0025-326X(97)00207-5
Rashvand S, Sadeghi SM (2014) Distribution, characteristics and economic importance of mangrove forests in Iran. In: Faridah-Hanum I., Latiff A., Hakeem KR, Ozturk M, Mangrove Ecosystems of Asia, 1rd edn. Springer, New York, pp 95–126
Raymond J, Rakariyatham N, Azanza J (1993) Purification and some properties of polyphenoloxidase from sunflower seeds. Phytochem 34:927–931. https://doi.org/10.1016/S0031-9422(00)90689-7
Sadiq M, McCain JC (2012) The gulf war aftermath: An environmental tragedy. Springer, Netherlands, p 298
Sampaio CJS, de Souza JRB, Damião AO, Bahiense TC, Roque MRA (2019) Biodegradation of polycyclic aromatic hydrocarbons (PAHs) in a diesel oil-contaminated mangrove by plant growth-promoting rhizobacteria. Biotech 9(4):155. https://doi.org/10.1007/s13205-019-1686-8
Sheppard C, Al-Husiani M, Al-Jamali F, Al- Yamani F et al (2010) The Gulf: a young sea in decline. Mar Pollut Bull 60:13–38. https://doi.org/10.1016/j.marpolbul.2009.10.017
Shiri M, Rabhi M, El Amrani A, Abdelly C (2015) Cross-tolerance to abiotic stresses in halophytes: application for phytoremediation of organic pollutants. Acta Physiol Plant 37(10):209. https://doi.org/10.1007/s11738-015-1954-0
Sodré V, Caetano VS, Rocha RM et al (2013) Physiological aspects of mangrove (Laguncularia racemosa) grown in microcosms with oil-degrading bacteria and oil contaminated sediment. Environ Pollut 172:243–249. https://doi.org/10.1016/j.envpol.2012.09.003
Sorahinobar M, Niknam V, Ebrahimzadeh H, Soltanloo H, Moradi B, Bahram M (2016) Lack of association between Fusarium graminearum resistance in spike and crude extract tolerance in seedling of wheat. Eur J Plant Pathol 144(3):525–538
Tansel B, Lee M, Tansel DZ (2013) Comparison of fate profiles of PAHs in soil, sediments and mangrove leaves after oil spills by QSAR and QSPR. Mar Pollut Bull 73:258–262. https://doi.org/10.1016/j.marpolbul.2013.05.011
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Vives-Peris V, López-Climent MF, Pérez-Clemente RM, Gómez-Cadenas A (2020) Root involvement in plant responses to adverse environmental conditions. Agronomy 10:942. https://doi.org/10.3390/agronomy10070942
Wang W, Zhang X, Huang J, Yan C, Zhang Q, Lu H, Liu J (2014) Interactive effects of cadmium and pyrene on contaminant removal from co-contaminated sediment planted with mangrove Kandelia obovata (S., L.) Yong seedlings. Mar Pollut Bull 84:306–313. https://doi.org/10.1016/j.marpolbul.2014.04.046
Ward RD, Friess DA, Day RH, MacKenzie RA (2016) Impacts of climate change on mangrove ecosystems: a region by region overview. Ecosyst Health Sust 2:e01211. https://doi.org/10.1002/ehs2.1211
Wieland G, Neumann R, Backhaus H (2001) Variation of microbial communities in soil, rhizosphere, and rhizoplane in response to crop species, soil type, and crop development. Appl Environ Microbiol 67:5849–5854. https://doi.org/10.1128/AEM.67.12.5849-5854.2001
Wild SR, Jones KC (1995) Polynuclear aromatic hydrocarbons in the United Kingdom environment: a preliminary source inventory and budget. Environ Pollut 88:91–108. https://doi.org/10.1016/0269-7491(95)91052-M
Yong Y, Tam N (2007) Effects of used lubricating oil on two mangroves Aegiceras corniculatum and Avicennia marina. J Environ Sci 19:1355–1360. https://doi.org/10.1016/S1001-0742(07)60221-6
Youssef T (2002) Evidence for reduced post-spill recovery by the halophyte Sporobolus iocladus (Nees ex Trin.) Nees in oil-contaminated sediments. Mar Pollut Bull 44:334–339. https://doi.org/10.1016/S0025-326X(01)00265-X
Zhang C, Leung K, Wong Y, Tam N (2007) Germination, growth and physiological responses of mangrove plant (Bruguiera gymnorrhiza) to lubricating oil pollution. Environ Exp Bot 60:127–136. https://doi.org/10.1016/j.envexpbot.2006.09.002
Zhou X, Cébron A, Béguiristain T, Leyval C (2009) Water and phosphorus content affect PAH dissipation in spiked soil planted with mycorrhizal alfalfa and tall fescue. Chemosphere 77:709–713. https://doi.org/10.1016/j.chemosphere.2009.08.050
Acknowledgments
This research was supported by the Tarbiat Modares University and Norwegian University of Science and Technology (NTNU). We thank over colleagues from the Cell, Molecular Biology and Genomics group of NTNU who provided insight and expertise that greatly assisted the research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by M. Capuana.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moradi, B., Zare Maivan, H., Seyed Hashtroudi, M. et al. Physiological responses and phytoremediation capability of Avicennia marina to oil contamination. Acta Physiol Plant 43, 18 (2021). https://doi.org/10.1007/s11738-020-03177-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-020-03177-y