Abstract
The aim of the present work was to check whether carbohydrate metabolism and partitioning contribute to the higher salt tolerance of the facultative halophyte Hordeum marinum compared to the glycophyte Hordeum vulgare. Seedlings with the same size from the two species were hydroponically grown at 0 (control), 150, and 300 mM NaCl for 3 weeks. H. marinum maintained higher relative growth rate, which was concomitant with a higher aptitude to maintain better shoot tissue hydration and membrane integrity under saline conditions compared to H. vulgare. Gas exchanges were reduced in the two species under saline conditions, but an increase in their water use efficiency was recorded. H. marinum exhibited an increase in leaf soluble sugar concentrations under saline conditions together with an enhancement in the transglucosidase DPE2 (EC 2.4.1.25) activity at 300 mM NaCl. However, H. vulgare showed a high increase in starch phosphorylase (EC 2.4.1.1) activity under saline conditions together with a decrease in leaf glucose and starch concentrations at 300 mM NaCl. In roots, both species accumulated glucose and fructose at 150 mM NaCl, but H. marinum exhibited a marked decrease in soluble sugar concentrations and an increase in starch concentration at 300 mM NaCl. Our data constitute an initiation to the involvement of carbohydrate metabolism and partitioning in salt responses of barley species and further work is necessary to elucidate how their flexibility confers higher tolerance to H. marinum compared to H. vulgare.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinity is an increasing problem that have been shown to decrease plant growth and crop yields and affect agricultural soil properties (Shahbaz et al. 2012). High salt concentrations (especially toxic ions such as Na+ and Cl−) exert various negative effects on plants, in particular oxidative and osmotic stresses, as well as ionic imbalances (Cardi et al. 2015). The ‘osmotic effects’ of salinity as referred to by Munns and Tester (2008) appear as early effects resulting from a salt-induced decrease in soil water potential around the root system, reducing, in this way, the plant’s ability to take up water, which can lead to cell dehydration (Islam et al. 2007). Salt-induced osmotic effects reduce transpiration rate through stomatal closure, which may affect CO2 fixation (Maggio et al. 2007; Chaves et al. 2009). The ‘ionic effects’ of salinity occur after relatively long-term exposure to salt (competitive ion uptake and transport within the plant) following intracellular accumulation of toxic ions of Na+ and Cl− within shoot tissues (Munns and Tester 2008; Harris et al. 2010). These effects disturb metabolic processes, photosynthetic efficiency, and plant growth and yield (Jusovic et al. 2018). The degree of damage depends among others on salinity level, exposure duration, and plant species (Munns and Tester 2008; Rozema and Flowers 2008). A variety of physiological and biochemical mechanisms has been evolved by plants to cope with salinity, including but not limited to (i) ion homeostasis (the regulatory mechanisms of ion uptake, transport, accumulation, and compartmentalization), (ii) osmotic adjustment (use of Na+ and Cl− in osmotic adjustment and biosynthesis of osmoprotectants and compatible solutes), (iii) reactive oxygen species (ROS) homeostasis (enzymatic and non-enzymatic antioxidant systems), and (iv) stress signaling (phytohormones and signaling molecules) (Gupta and Huang 2014). According to their degree of salinity tolerance, plants were divided into halophytes and glycophytes. Nevertheless, up to now, no clear definition was retained for halophytes (Munns and Tester 2008; Cheeseman 2015).
During the last decades, sea barley (Hordeum marinum Huds. or Hordeum maritimum With.) has attracted more and more attention as a promising plant at both fundamental and applied levels. This wild barley species was described as an annual facultative halophyte (Hafsi et al. 2007, 2010, 2011a, b; Lakhdar et al. 2008; Yousfi et al. 2010; Alamri et al. 2013; Chalbi et al. 2013; Ferchichi et al. 2018) and its responses to salinity was compared to those of its glycophytic relative Hordeum vulgare L. (cultivated barley) in several works (Garthwaite et al. 2005; Yousfi et al. 2010; Chalbi et al. 2013; Ferchichi et al. 2018). Garthwaite et al. (2005) compared the responses to increasing salinity for 16–21 days of eight wild barley species, including sea barley, to cultivated barley. They found that the majority of them showed a higher capacity to ‘exclude’ Na+ and Cl− from their shoots and to maintain higher leaf K+ than H. vulgare. The authors considered the most studied wild barley species more salt-tolerant than cultivated barley and retained the restriction of Na+ and Cl− entry to shoots as a criterion of salt tolerance in these species. The subjection of sea barley and cultivated barley to 0, 100, 200, and 300 mM NaCl for 60 h (osmotic shock) confirmed these statements and showed that H. vulgare adopted an energy-consuming strategy to combat salt osmotic effect using K+ and organic metabolites for osmotic adjustment, while H. marinum exhibited efficient metabolite management and metabolic nutrient regulation. Sea barley relied on Na+ for osmotic adjustment at moderate salinity, keeping in this way K+ and organic metabolites for metabolic purposes and used them only at high salinity (Yousfi et al. 2010). Islam et al. (2007) succeeded to transfer some salt-adaptive mechanisms from H. marinum to H. marinum–Triticum aestivum amphiploid. Chalbi et al. (2013) stated that sea barley maintained a less affected photosynthetic activity under long-term salinity compared to cultivated barley. They demonstrated also that despite the increase in the unsaturated-to-saturated fatty acid ratio and the double bond index observed in salt-treated H. vulgare plants, they showed more affected membrane integrity compared to H. marinum plants. Recently, Ferchichi et al. (2018) demonstrated in a metabolomic study that H. marinum experienced sequential metabolite and ion accumulation that allowed it a 2–3 week delay in showing stress damage symptoms in comparison with H. vulgare.
Triose phosphates produced during carbon fixation are either stored as starch within the chloroplast or transported to the cytosol, where they contribute to sucrose synthesis (Hartman et al. 2017). Contrarily to starch synthesized in cells of storage organs (storage starch that can be stored over seasons and even over years), transitory starch in photosynthetic cells is synthesized and degraded within 1 day–night rhythm (Lu et al. 2005). The function of starch depends on the cell type from which it is derived, as well as on environmental conditions (Thalmann and Santelia 2017). Starch is also considered as a key molecule involved in the responses of plants to abiotic stresses; its remobilization constitutes a source of energy and carbon under potentially limited photosynthesis conditions. In addition, the released soluble sugars were reported to support plant growth and play a key role in osmotic adjustment, as well as in stress signaling (Van den Ende and El-Esawe 2014; Thalmann and Santelia 2017). Several enzymes are involved in starch metabolism, including starch phosphorylase (EC 2.4.1.1)—with its plastidial (Pho1) and cytosolic (Pho2) isoforms—that transfers glucosyl units from glucose-1-phosphate (G-1-P) to glycans containing α-1–4 linked glucan chains (Fettke et al. 2005a, b, 2012) and the transglucosidase DPE2 (EC 2.4.1.25) that transfers glucosyl residues from maltose to a polysaccharide with the release of glucose (Chia et al. 2004; Fettke et al. 2006). Thus, Pho2 and DPE2 are related to the degradation of starch and formation of sucrose in the cytosol, the transport metabolite of most plants. Furthermore, DPE2 also contributes to the release of glucose. For the plastidial phosphorylase, it has been reported that it contributes to starch metabolism under specific stress conditions such as cold (Orawetz et al. 2016).
Although carbohydrate concentrations were determined in mature leaves of H. marinum and H. vulgare under saline and non-saline conditions and their relative contribution to osmotic adjustment was estimated (Yousfi et al. 2010; Ferchichi et al. 2018), more importance should be given to their contribution to salt tolerance. This was the aim of the present study, in which we tried to find relationships between carbohydrate metabolism/partitioning and tolerance to moderate and high salinities in a comparative study between H. marinum and H. vulgare.
Materials and methods
Plant materials and growth conditions
Hordeum marinum seeds were collected in the Sebkha of Soliman (30 km south of Tunis, semi-arid area) and H. vulgare (var. Manel) seeds were provided by the National Institute of Agronomic Research of Tunis (INRAT). Seeds of both barley species were disinfected with calcium hypochlorite (2%) and germinated in petri dishes on filter paper moistened with distilled water. To obtain seedlings with the same size in the beginning of treatments, H. marinum germination was started 14 days before that of H. vulgare. Obtained seedlings were transferred in dark plastic containers filled with a continuously aerated Hewitt’s (1966) nutrient solution that was renewed twice a week. This pretreatment period took 40 days and seedlings received quarter strength, then half strength and finally complete nutrient medium. After 40 days for H. vulgare and 54 days for H. marinum, salt treatments were applied by adding NaCl to final concentrations of 0 mM (C: control), 150 mM (S1), and 300 mM (S2). Both pretreatment and treatment were conducted in a growth chamber with a light/dark temperature regime of 25/20 °C, a relative humidity of 60–80%, a light intensity of approximately 250 µmol photons m−2 s−1, and a photoperiod of 12 h. After 21 days of treatment, gas exchange parameters were measured then plants were harvested.
Growth and water content determination
Plants used for growth and water content analyses were cut into shoots and roots, weighed fresh, then oven-dried for 3 days at 70 °C, and weighed dry. Growth was measured as relative growth rate (RGR) as described by Rabhi et al. (2010).
Gas exchange measurements
Gas exchange parameters were measured in both species exposed to 0, 150, and 300 mM NaCl for 21 days of treatment, using a portable Licor gas analyzer (LC pro+, ADC Bio Scientific Ltd.). Measurements were taken in a greenhouse from the mid-lamina portion of fully expanded attached leaves. The measurements were carried out between 10.00 am and 1.00 pm at the following cuvette conditions: 800 µmol PPFD m−2 s−1, 30 °C leaf temperature, 0.35 mbar ambient CO2 partial pressure, and 26 mbar cuvette H2O partial pressure. Measured parameters were net CO2 assimilation (A), stomatal conductance (gs), transpiration rate (E), and water use efficiency (WUE). The latter was calculated as A⁄E ratio.
Electrolyte leakage measurements
Electrolyte leakage (EL) was determined in fresh discs of fully expanded leaves through electrical conductivity (EC) measurements according to Dionisio-Sese and Tobita (1998). The leaf discs were immediately put into tubes containing 10 mL MilliQ water each. Their incubation for 2 h in a water bath at 32 °C allowed the determination of the initial electrical conductivity of the solution (EC1) by a Metrohm 712 conductivity meter. After incubation at 121 °C for 20 min and cooling to 25 °C, the final value (EC2) was determined. EL was then calculated as follows:
Sample preparation for starch and soluble sugar assays
At the harvest, samples from roots and fully expanded leaves were collected at the end of the light period (after 10–11 h of illumination) and frozen in liquid nitrogen then stored at − 80 °C until use. Soluble sugars were extracted in ethanol [80% (v/v)], then resuspended in double distilled water according to a modified method of Caporn et al. (1999). To an aliquot of 40–50 mg frozen material, an ethanol [80% (v/v)] volume of 0.85 mL was added and the mixture was incubated at 80 °C under continuous agitation for 15 min. After centrifugation at 20,000g for 10 min, the supernatant was collected. A second extraction was performed in the same way and the two supernatants were combined in a single ethanol extract. The latter was immediately evaporated in speed vacuum and the obtained pellet was resupended in 200 µL double distilled water for 10 min at 30 °C, then centrifuged for 10 min at 20,000g. The concentrations of glucose, sucrose, and fructose were spectrophotometrically determined in the supernatant through measurements of NAD+ reduction in the presence of specific enzymes at 340 nm. Four replicates from four different plants were used for each sugar assay. The pellet was used for starch assays.
Soluble sugar assays
A modified method of Caporn et al. (1999) was used for all soluble sugar assays. The assay buffer used to determine glucose concentrations was reconstituted from a reagent kit and contained: 200 mM imidazole/HCl (pH 6.9), 3 mM MgCl2, 5 mM NADP, 11 mM ATP, 0.5 unit mL−1 hexokinase, and 25 µL glucose-6-phosphate dehydrogenase suspension (Roche). A volume of glucose assay reagent was added to 5–20 µL sample to a final volume of 600 µL. Then, mixtures were agitated and incubated at room temperature for 15 min. Thereafter, absorbance was read at 340 nm versus deionized water.
For fructose assay, 2 units phosphoglucose isomerase (PGI) was added to the tube previously used for glucose determination. After incubation at room temperature for 15 min, absorbance was measured at 340 nm.
As for the determination of sucrose concentration, a suspension of 100 units invertase was added to the tube previously used for fructose assay. Absorbance was then read at 340 nm after incubation at room temperature for 10 min.
Starch assays
Starch pellet was washed with 1 mL cold double distilled water, centrifuged for 10 min at 20,000g, and shortly dried in speed vacuum. Thereafter, it was solubilized for 1 h in 0.5 mL KOH (0.2 M) at 95 °C. A subsequent neutralization was then performed by the addition of 88 µL acetic acid (1 M). After centrifugation for 10 min at 20,000g, an aliquot of 50 µL supernatant was mixed with 50 µL starch assay reagent containing 5 units amyloglucosidase, then incubated overnight at 55 °C. Subsequently, the starch content was determined using the hexokinase/glucose-6-phosphate dehydrogenase assay with an incubation of 15 min at room temperature. The absorbance was then measured at 340 nm (Stitt et al. 1989).
Sample preparation for enzyme assays
Frozen samples of roots and fully expanded leaves were homogenated in extraction buffer containing 100 mM Hepes–NaOH (pH 7.5), 1 mM EDTA, 2 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 10% (v/v) glycerol, 0.1% (w/v) Natriumsulfit, and 0.075% (w/v) Natriumdisulfit. Homogenates (or crude extracts) were then centrifuged for 12 min at 20,000g at 4 °C and the supernatants that are designated as crude extracts were collected. The concentrations of soluble proteins were determined using the microversion of Bio-Rad protein assay (Bio-Rad, Munich, Germany) and bovine serum albumin as standard.
Zymograms
Zymograms were performed according to Fettke et al. (2005b). Crude extracts were run on native PAGE gels. Thereafter, gels were incubated overnight at 37 °C in 100 mM citrate–NaOH (pH 6.5) containing 20 mM substrate. In the case of phosphorylases (Pho1 and Pho2), the substrate was glucose-1-phosphate (Sigma-Aldrich, Munich, Germany) and in the case of transglucosidase (DPE2), the substrate was maltose (Roth, Karlsruhe, Germany). Finally, gels were subjected to iodine staining.
Statistical analysis
Data were subjected to an ANOVA test using SPSS 16.0 software and means were compared according to Duncan’s test at 5% level of significance. Gas exchange data were presented as Log2(treated/control) and untransformed means were compared to the control using Student’s t test at 5% level of significance.
Results
Growth and water content
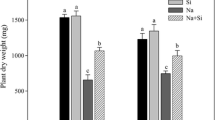
Under control conditions, H. marinum plants showed RGR values of 0.143 and 0.132 day−1, respectively in shoots and roots (Table 1). Both salinity levels decreased shoot RGR in this species by 16–19% and root RGR by 22–29%. As regards H. vulgare, shoot and root RGR values under control conditions were, respectively, 0.122 and 0.094 day−1. They decreased with the increasing salinity, keping root/shoot ratio statistically unchanged. The comparison of salt effects on the two barley species on the basis of whole plant RGR showed that S1 treatment reduced RGR of cultivated barley (ca. − 41%) more than did S1 and S2 in sea barley (− 21 and − 17%, respectively). Shoot water content exhibited the same trend as biomass in each species, whereas root water content was maintained constant regardless of the treatment in both of them (Table 1).
Membrane integrity
Membrane integrity was estimated through electrolyte leakage (EL) measurements; an increase in EL means a loss of membrane integrity. In H. marinum, EL increased from 6% in the control to about 10% in S1 and S2 treatments (Fig. 1a). In H. vulgare, recorded EL values were noticeably higher: they increased from 18% in the control to 39 and 70% in S1 and S2 treatments, respectively (Fig. 1b).
Gas exchange parameters
Net CO2 assimilation (A), stomatal conductance (gs), and transpiration rate (E) were significantly reduced by salt treatments in the two studied species (Fig. 2a, b). To mitigate this decrease in photosynthetic activity induced by salinity stress, both species increased their water use efficiency (WUE). Nevertheless, in sea barley, this adaptive response was observed only in S2 treatment.
Net CO2 assimilation (A), stomatal conductance (gs), transpiration rate (E), and water use efficiency (WUE) expressed as Log2(treated/control) in H. marinum and H. vulgare plants hydroponically grown for 3 weeks at 0 (C), 150 (S1), and 300 mM NaCl (S2). Values are means of four replicates. ns not significant; *P ≤ 0.05; **P ≤ 0.01 according to Student’s t test
Starch and soluble sugar concentrations
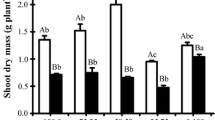
Figure 3 illustrates sugar concentrations in leaves of the two barley species in C, S1, and S2 treatments. Leaf glucose concentration was maintained unchanged in S1 treatment in both species, but it increased in S2 treatment in H. marinum by 64.4% and decreased in H. vulgare by 44.9%, in comparison with their controls (Fig. 3a, b). Leaf fructose concentration was maintained constant except in S1-treated plants of sea barley, in which it increased by 54.2% (Fig. 3c, d). The sharpest variation in leaf sugar concentrations was recorded in sucrose in H. marinum plants of S2 treatment (ca. + 179.8%) (Fig. 3e). Apart from this peak, no other significant change was observed in sucrose concentration in both species. As regards leaf starch concentration, it decreased only in cultivated barley in S2 treatment (Fig. 3h).
Concentrations of starch and soluble sugars in fully expanded leaves of H. marinum and H. vulgare plants hydroponically grown for 3 weeks at 0 (C), 150 (S1), and 300 mM NaCl (S2). Bars are means of four replicates ± SE. Bars labeled with at least one same letter are not significantly different according to Duncan’s test at P ≤ 0.05
Figure 4 shows sugar concentrations in roots of H. marinum and H. vulgare in C, S1, and S2 treatments. The two studied species exhibited high root glucose and fructose peaks in S1 treatment (Fig. 4a–d). In S2 treatment, root concentrations of these two soluble sugars were either maintained at the level of the control (case of H. vulgare) or markedly decreased (case of H. marinum). Root sucrose concentration of sea barley decreased by 30.3% in S1 treatment and by 93.3% in S2 treatment (Fig. 4e). In cultivated barley, root sucrose concentration showed no significant variation (Fig. 4f). As regards root starch concentration, it was doubled in S2-treated plants of H. marinum (Fig. 4g), while no other variation was observed in both species.
Concentrations of starch and soluble sugars in roots of H. marinum and H. vulgare plants hydroponically grown for 3 weeks at 0 (C), 150 (S1), and 300 mM NaCl (S2). Bars are means of four replicates ± SE. Bars labeled with at least one same letter are not significantly different according to Duncan’s test at P ≤ 0.05
Leaf enzyme activities
DPE2 activity showed contrasting trends in salt-treated plants of H. marinum and H. vulgare; it noticeably increased (ca. +120%) in S2 treatment in sea barley, and sharply decreased (up to − 82%) in S1 and S2 treatments in cultivated barley (Fig. 5a, b).
Zymograms of disproportionating enzyme 2 (DPE2) activity in fully expanded leaves of H. marinum and H. vulgare plants hydroponically grown for 3 weeks at 0 (C), 150 (S1), and 300 mM NaCl (S2). Bars are means of three replicates ± SE. Bars labeled with at least one same letter are not significantly different according to Duncan’s test at P ≤ 0.05
Pho1 activity decreased in H. marinum by 58% in S2 treatment and increased in H. vulgare to threefold and 12-fold the level of the control in S1 and S2 treatments, respectively (Fig. 6a, b). Pho2 activity was maintained unchanged in sea barley and increased under saline conditions by 147–163% in cultivated barley (Fig. 6c, d).
Zymograms of plastidial (Pho1) and cytosolic (Pho2) α-glucan phosphorylase activities in fully expanded leaves of H. marinum and H. vulgare plants hydroponically grown for 3 weeks at 0 (C), 150 (S1), and 300 mM NaCl (S2). Bars are means of three replicates ± SE. Bars labeled with at least one same letter are not significantly different according to Duncan’s test at P ≤ 0.05
Discussion
H. marinum maintained its RGR at 89–93% of the control level at both high and moderate salinities, while H. vulgare was unable to maintain even 60% of its RGR at 150 mM NaCl (Table 1). Similar results were also obtained by Garthwaite et al. (2005) in the two barley species subjected to 150 and 300 NaCl for the same treatment period (3 weeks). In a previous work (Yousfi et al. 2010), some of us showed that since the early 60 h of salt treatment, H. marinum was able to maintain its capacity to produce biomass at the level of the control, while H. vulgare exhibited reduced shoot and root growth at high salinities (200 and 300 mM). EL data revealed also better membrane integrity in the facultative halophyte (H. marinum) compared to the salt-tolerant glycophyte (H. vulgare) (Fig. 1). Chalbi et al. (2013) stated that although increased unsaturation in membrane phospholipids is known to maintain membrane fluidity, it did not confer higher salt tolerance to cultivated barley in comparison with sea barley that showed no change in unsaturated–to–saturated fatty acid ratio and double bond index. Garthwaite et al. (2005) attributed this difference in salt tolerance to the higher aptitude of H. marinum to avoid leaf invasion by sodium and chloride ions and to maintain higher leaf potassium supply compared to H. vulgare.
Photosynthesis was impaired in the two barley species under moderate and high salinities (Fig. 2). Nevertheless, both species increased WUE in response to the salt-induced limitation in A. Such a response seems to be transitory in H. vulgare (it was not recorded after 30 days of treatment), whereas it seems permanent in H. marinum (it was observed after 30 days of treatment) (Chalbi et al. 2013). Stomatal closure and the enhanced WUE helped the latter maintain its shoot water content above 70% of the control level, whereas the former exhibited decreased shoot water content with the increasing salinity down to 41% of the control. H. marinum was found indeed to adapt since the early hours of salt treatment an efficient strategy to cope with the osmotic stress, the first phase of the biphasic salt stress, in comparison with H. vulgare that adapted an energy-consuming one (Yousfi et al. 2010). Osmotic adjustment in cultivated barley is ensured by K+ and organic metabolites (soluble sugars, proline, and free amino acids) at both moderate and high salinities. By contrast, in sea barley, it depends on salinity level: it is ensured by Na+ at moderate salinity with the involvement of organic metabolites at high salinity (Yousfi et al. 2010). Ferchichi et al. (2018) found that sea barley modulated its osmotic adjustment players with treatment period, too.
The halophyte H. marinum exhibited more marked variations in leaf soluble sugar concentrations compared to the glycophyte H. vulgare (Fig. 3). The former showed an increase in fructose concentration in S1 treatment and those of glucose and sucrose in S2 treatment, while the latter showed no soluble sugar accumulation. According to Ferchichi et al. (2018), leaf soluble sugars did not show an increase in their relative contribution to leaf total osmolality in response to 200 mM NaCl after 15 and 33 days of treatment in both H. marinum and H. vulgare. In fact, carbohydrate involvement in salt stress responses is not limited to osmotic adjustment. Sugars together with proline can be involved in protein and cell structure stabilization, especially under severe or prolonged stresses. A scavenging role of free radicals was also given to sugars, which protects cells from oxidative stress damages and maintains redox homeostasis (Singh et al. 2015). Furthermore, sugars were found to play a key role in stress signaling (Rolland et al. 2006). Comparative proteomics of the halophyte Thellungiella halophyla leaves at different salinity levels revealed that the majority of salt-responsive proteins are involved in carbohydrate metabolism; the most affected were starch and sucrose metabolisms that seem crucial for salt tolerance in halophytes (Wang et al. 2013).
Glucose accumulation in leaves of sea barley at 300 mM NaCl was concomitant with an increase in leaf DPE2 activity (Fig. 5). This enzyme is indispensable for transitory starch degradation and maltose metabolism that occur in source leaves at night (Fettke et al. 2006). In cultivated barley, leaf DPE2 activity was substantially reduced under salinity. These results suggest that this enzyme is involved in H. marinum responses to high salinity levels through the degradation of starch and the resulting accumulation of soluble sugars. In H. vulgare, DPE2 seems not involved and/or damaged by NaCl. As for Pho1 and Pho2, their activities were highly increased in cultivated barley, which suggests a possible role of these enzymes in the responses of carbohydrate metabolism to salinity. In sea barley, Pho1 activity was markedly decreased by only S2 treatment and Pho2 activity was not modified under saline conditions, which excludes their role in the responsive mechanisms of carbohydrate metabolism to salt stress. Ma et al. (2013) found that the expression of the genes encoding Pho1 and Pho2 (Pho1 and Pho2, respectively) varied with plant species, tissue, development stage, and environmental conditions (abiotic and biotic stresses). These authors stated that the effect of 100 mM NaCl on the expression of Pho1 and Pho2 in cultivated barley seedlings depended also on treatment duration. Zeeman et al. (2004) found that Pho1 was involved in leaf responses to a transient osmotic stress, but it was not necessary for starch degradation under non-stressful conditions. However, it was also shown that Pho1 is involved in starch metabolism under standard growth conditions (Malinova et al. 2014). The differential responses of DPE2, Pho1, and Pho2 led to the maintenance of leaf starch concentration at the level of the control at 150 mM NaCl in the two species and a decrease in starch level at 300 mM NaCl in H. vulgare.
In roots, cultivated barley exhibited an increase in glucose and fructose concentrations in S1 treatment, while no variation was detected in starch and soluble sugar concentrations in S2 treatment (Fig. 4). These results suggest that root carbohydrate metabolism may play a role in plant responses to S1 treatments, but not to S2 one. As for sea barley roots, a marked accumulation of glucose and fructose was observed at 150 mM NaCl together with a decrease in sucrose concentration, starch level being maintained unchanged. This can be explained by a high capacity of plants to transport sucrose from source (leaves) to sink (roots) organs and a high activity of root invertase and/or sucrose synthase. These two enzymes catalyze sucrose cleavage into glucose and fructose (Koch 2004). At 300 mM NaCl, H. marinum showed a substantial decrease in all soluble sugars and a noticeable accumulation of starch. Hence, under these conditions, H. marinum accumulated carbohydrates in two different forms depending on plant organ: in leaves, where salt ions are not accumulated at high amounts, carbohydrates are accumulated as soluble sugars, while in roots, where salt ions can be accumulated at high amounts, carbohydrates are stored as starch. Starch is considered as a storage substance that can be mobilized when energy is not sufficiently supplied to cover energy demands (Krasensky and Jonak 2012), while soluble sugars can be immediately used for several purposes (Rolland et al. 2006; Singh et al. 2015). Our data constitute an initiation to the involvement of carbohydrate metabolism and partitioning in salt responses of barley species and further work is necessary to elucidate how their flexibility confers higher tolerance to H. marinum compared to H. vulgare.
Conclusion
Although carbohydrate metabolism is still in its infancy (Thalmann and Santelia 2017), the differential behaviors of the halophyte H. marinum and the glycophyte H. vulgare in terms of starch and soluble sugar distribution between source and sink organs, as well as the enzymes involved in starch metabolism can give insights into the importance of sugars in salt tolerance in barley species. This importance is not due to osmotic adjustment, but to other functions that need further investigations. The comparison between sea and cultivated barley species revealed a higher flexibility in carbohydrate metabolism and distribution in the former, which probably contributed to its higher salt tolerance in particular at high salinity levels.
Author contribution statement
WM participated in all steps of the work. NF, EB-H, and MB helped in the physiological study. SA-R, HM, and HQ helped in carbohydrate and enzyme assays. FC contributed to data analysis and interpretation. CA was the receipt of the grant as Head of the Laboratory of Extremophile Plants. JF and MR were the supervisors of the work.
Abbreviations
- A :
-
Net CO2 assimilation
- C:
-
Control
- E :
-
Transpiration rate
- EC:
-
Electrical conductivity
- EDTA:
-
Ethylenediaminetetraacetic acid
- EL:
-
Electrolyte leakage
- g s :
-
Stomatal conductance
- NAD+ :
-
Oxidized form of nicotinamide adenine dinucleotide
- PGI:
-
Phosphoglucose isomerase
- Pho1:
-
Plastidial phosphorylase isoform
- Pho2:
-
Cytosolic phosphorylase isoform
- PPFD:
-
Photosynthetic photon flux density
- RGR:
-
Relative growth rate
- ROS:
-
Reactive oxygen species
- S1:
-
150 mM NaCl
- S2:
-
300 mM NaCl
- WUE:
-
Water use efficiency
References
Alamri SA, Barrett-Lennard EG, Teakle NL, Colmer TD (2013) Improvement of salt and waterlogging tolerance in wheat: comparative physiology of Hordeum marinum-Triticum aestivum amphiploids with their H. marinum and wheat parents. Funct Plant Biol 40:1168–1178. https://doi.org/10.1071/FP12385
Caporn S, Brooks A, Press M, Lee J (1999) Effects of long-term exposure to elevated CO2 and increased nutrient supply on bracken (Pteridium aquilinum). Funct Ecol 13(Suppl. 1):107–115. https://doi.org/10.1046/j.1365-2435.1999.00013.x
Cardi M, Castiglia D, Ferrara M, Guerriero G, Chiurazzi M, Esposito S (2015) The effects of salt stress cause a diversion of basal metabolism in barley roots: possible different roles for glucose-6-phosphate dehydrogenase isoforms. Plant Physiol Biochem 86:44–54. https://doi.org/10.1016/j.plaphy.2014.11.001
Chalbi N, Hessini K, Gandour M, Naeit-Mohamed S, Smaoui A, Abdelly C, Ben Youssef N (2013) Are changes in membrane lipids and fatty acid composition related to salt-stress resistance in wild and cultivated barley? J Plant Nutr Soil Sci 176:138–147. https://doi.org/10.1002/jpln.201100413
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560. https://doi.org/10.1093/aob/mcn125
Cheeseman JM (2015) The evolution of halophytes, glycophytes and crops, and its implications for food security under saline conditions. New Phytol 206:557–570. https://doi.org/10.1111/nph.13217
Chia T, Thorneycroft D, Chapple A, Messerli G, Chen J, Zeeman SC, Smith SM, Smith AM (2004) A cytosolic glucosyltransferase is required for conversion of starch to sucrose in Arabidopsis leaves at night. Plant J 37:853–863. https://doi.org/10.1111/j.1365-313X.2003.02012.x
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9. https://doi.org/10.1016/S0168-9452(98)00025-9
Ferchichi S, Hessini K, Dell’Aversana E, D’Amelia L, Woodrow P, Ciarmiello LF, Fuggi A, Carillo P (2018) Hordeum vulgare and Hordeum maritimum respond to extended salinity stress displaying different temporal accumulation pattern of metabolites. Funct Plant Biol 45(11):1096–1109. https://doi.org/10.1071/FP18046
Fettke J, Eckermann N, Tiessen A, Geigenberger P, Steup M (2005a) Identification, subcellular localization and biochemical characterization of water-soluble heteroglycans (SHG) in leaves of Arabidopsis thaliana L.: distinct SHG reside in the cytosol and in the apoplast. Plant J 43:568–585. https://doi.org/10.1111/j.1365-313X.2005.02475.x
Fettke J, Poeste S, Eckermann N, Tiessen A, Pauly M, Geigenberger P, Steup M (2005b) Analysis of cytosolic heteroglycans from leaves of transgenic potato (Solanum tuberosum L.) plants that under- or overexpress the Pho 2 phosphorylase isozyme. Plant Cell Physiol 46:1987–2004. https://doi.org/10.1093/pcp/pci214
Fettke J, Chia T, Eckermann N, Smith A, Steup M (2006) A transglucosidase necessary for starch degradation and maltose metabolism in leaves at night acts on cytosolic heteroglycans (SHG). Plant J 46:668–684. https://doi.org/10.1111/j.1365-313X.2006.02732.x
Fettke J, Fernie AR, Steup M (2012) Transitory starch and its degradation in higher plant cells. In: Tetlow IJ (ed) Essential reviews in experimental biology: starch: origins, structure and metabolism. SEB, London, pp 311–374
Garthwaite AJ, Von Bothmer R, Colmer TD (2005) Salt tolerance in wild Hordeum species is associated with restricted entry of Na+ and Cl− into the shoots. J Exp Bot 56:2365–2378. https://doi.org/10.1093/jxb/eri229
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int. J. Genomics. https://doi.org/10.1155/2014/701596
Hafsi C, Lakhdhar A, Rabhi M, Debez A, Abdelly C, Ouerghi Z (2007) Interactive effects of salinity and potassium availability on growth, water status, and ionic composition of Hordeum maritimum. J Plant Nutr Soil Sci 170:469–473. https://doi.org/10.1002/jpln.200625203
Hafsi C, Romero-Puertas MC, Gupta DK, del Río LA, Sandalio LM, Abdelly C (2010) Moderate salinity enhances the antioxidative response in the halophyte Hordeum maritimum L. under potassium deficiency. Environ Exp Bot 69:129–136. https://doi.org/10.1016/j.envexpbot.2010.04.008
Hafsi C, Atia A, Lakhdar A, Debez A, Abdelly C (2011a) Differential responses in potassium absorption and use efficiencies in the halophytes Catapodium rigidum and Hordeum maritimum to various potassium concentrations in the medium. Plant Prod Sci 14:135–140. https://doi.org/10.1626/pps.14.135
Hafsi C, Romero-Puertas MC, del Río LA, Abdelly C, Sandalio LM (2011b) Antioxidative response of Hordeum maritimum L. to potassium deficiency. Acta Physiol Plant 33:193–202. https://doi.org/10.1007/s11738-010-0537-3
Harris BN, Sadras VO, Tester M (2010) A water-centred framework to assess the effects of salinity on the growth and yield of wheat and barley. Plant Soil 336:377–389. https://doi.org/10.1007/s11104-010-0489-9
Hartman MD, Figueroa CM, Arias DG, Iglesias AA (2017) Inhibition of recombinant aldose-6-phosphate reductase from peach leaves by hexose-phosphates, inorganic phosphate and oxidants. Plant Cell Physiol 58:145–155. https://doi.org/10.1093/pcp/pcw180
Hewitt EJ (1966) Sand and water culture methods used in the study of plant nutrition. Commonw Bur Hortic Tech Commun 22:431–446
Islam S, Malik AI, Islam AKMR, Colmer TD (2007) Salt tolerance in a Hordeum marinum–Triticum aestivum amphiploid, and its parents. J Exp Bot 58:1219–1229. https://doi.org/10.1093/jxb/erl293
Jusovic M, Velitchkova MY, Misheva SP, Börner A, Apostolova EL, Dobrikova AG (2018) Photosynthetic responses of a wheat mutant (Rht-B1c) with altered DELLA Proteins to salt stress. J Plant Growth Regul 37:645–656. https://doi.org/10.1007/s00344-017-9764-9
Koch K (2004) Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Opin Plant Biol 7:235–246. https://doi.org/10.1016/j.pbi.2004.03.014
Krasensky J, Jonak C (2012) Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J Exp Bot 63:1593–1608. https://doi.org/10.1093/jxb/err460
Lakhdar A, Hafsi C, Rabhi M, Debez A, Montemurro F, Abdelly C, Jedidi N, Ouerghi Z (2008) Application of municipal solid waste compost reduces the negative effects of saline water in Hordeum maritimum L. Bioresour Technol 99:7160–7167. https://doi.org/10.1016/j.biortech.2007.12.071
Lu Y, Gehan JP, Sharkey TD (2005) Daylength and circadian effects on starch degradation and maltose metabolism. Plant Physiol 138(4):2280–2291. https://doi.org/10.1104/pp.105.061903
Ma J, Jiang QT, Zhang XW, Lan XJ, Pu ZE, Wei YM, Liu C, Lu ZX, Zheng YL (2013) Structure and expression of barley starch phosphorylase genes. Planta 238:1081–1093. https://doi.org/10.1007/s00425-013-1953-6
Maggio A, Raimondi G, Martino A, de Pascale S (2007) Salt stress response in tomato beyond the salinity tolerance threshold. Environ Exp Bot 59:276–282. https://doi.org/10.1016/j.envexpbot.2006.02.002
Malinova I, Mahlow S, Alseekh S, Orawetz T, Fernie AR, Baumann O, Steup M, Fettke J (2014) Double knockout mutants of Arabidopsis grown under normal conditions reveal that the plastidial phosphorylase isozyme participates in transitory starch metabolism. Plant Physiol 164:907–921. https://doi.org/10.1104/pp.113.227843
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Orawetz T, Malinova I, Orzechowski S, Fettke J (2016) Reduction of the plastidial phosphorylase in potato (Solanum tuberosum L.) reveals impact on storage starch structure during growth at low temperature. Plant Physiol Biochem 100:141–149. https://doi.org/10.1016/j.plaphy.2016.01.013
Rabhi M, Hajji S, Karray-Bouraoui N, Giuntini D, Castagna A, Smaoui A, Ranieri A, Abdelly C (2010) Nutrient uptake and management under saline conditions in the Xerohalophyte: Tecticornia indica (Willd.) subsp. indica. Acta Biol Hung 61:486–497. https://doi.org/10.1556/ABiol.61.2010.4.11
Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57:675–709. https://doi.org/10.3389/fpls.2014.00113
Rozema J, Flowers T (2008) Crops for a salinized world. Science 322:1478–1480. https://doi.org/10.1126/science.1168572
Shahbaz M, Ashraf M, Al-Qurainy F, Harris PJC (2012) Salt tolerance in selected vegetable crops. Crit Rev Plant Sci 31(4):303–320. https://doi.org/10.1080/07352689.2012.656496
Singh M, Kumar J, Singh S, Singh VP, Prasad SM (2015) Roles of osmoprotectants in improving salinity and drought tolerance in plants: a review. Rev Environ Sci Biotechnol 14:407–426. https://doi.org/10.1007/s11157-015-9372-8
Stitt M, Lilley RM, Gerherdt R, Heldt HW (1989) Metabolite levels in specific cells and subcellular compartments of plant leaves. Methods Enzymol 174:518–552. https://doi.org/10.1016/0076-6879(89)74035-0
Thalmann M, Santelia D (2017) Starch as a determinant of plant fitness under abiotic stress. New Phytol 214:943–951. https://doi.org/10.1111/nph.14491
Van den Ende W, El-Esawe SK (2014) Sucrose signaling pathways leading to fructan and anthocyanin accumulation: a dual function in abiotic and biotic stress responses? Environ Exp Bot 108:4–13. https://doi.org/10.1016/j.envexpbot.2013.09.017
Wang X, Chang L, Wang B, Wang D, Li P, Wang L, Yi X, Huang Q, Peng M, Guo A (2013) Comparative proteomics of Thellungiella halophila leaves from plants subjected to salinity reveals the importance of chloroplastic starch and soluble sugars in halophyte salt tolerance. Mol Cell Proteom 12:2174–2195. https://doi.org/10.1074/mcp.M112.022475
Yousfi S, Rabhi M, Hessini K, Abdelly C, Gharsalli M (2010) Differences in efficient metabolite management and nutrient metabolic regulation between wild and cultivated barley grown at high salinity. Plant Biol 12:650–658. https://doi.org/10.1111/j.1438-8677.2009.00265.x
Zeeman SC, Thorneycroft D, Schupp N, Chapple A, Weck M, Dunstan H, Haldimann P, Bechtold N, Smith AM, Smith SM (2004) Plastidial a-glucan phosphorylase is not required for starch degradation in Arabidopsis leaves but has a role in the tolerance of abiotic stress. Plant Physiol 135:849–858. https://doi.org/10.1104/pp.103.032631
Acknowledgements
This work was supported by the Tunisian Ministry of Higher Education and Scientific Research (LR10CBBC02) and the University of Potsdam (Germany).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Montanaro.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Medini, W., Farhat, N., Al-Rawi, S. et al. Do carbohydrate metabolism and partitioning contribute to the higher salt tolerance of Hordeum marinum compared to Hordeum vulgare?. Acta Physiol Plant 41, 190 (2019). https://doi.org/10.1007/s11738-019-2983-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-019-2983-x