Abstract
Background and aims
The mechanism for silicon-mediated salt tolerance is still not very clear. The aim of this study was to investigate the possible role of silicon in regulating carbohydrate metabolism in cucumber (Cucumis sativus L.).
Methods
Two cucumber cultivars (‘JinYou 1’ and ‘JinChun 5’) grown hydroponically were subjected to 75 mM NaCl stress in the absence or presence of added silicon (0.3 mM). Plant growth, oxidative damage, chlorophyll fluorescence and carbohydrate metabolism were investigated.
Results
Added silicon improved plant growth and photosynthetic performance, while alleviated oxidative damage of cucumber under salt stress. Salt stress increased the soluble sugar levels in both leaves and roots. Starch was accumulated in the leaves but decreased in the roots under salt stress. Added silicon decreased the soluble sugar levels in leaves through regulating the activities of carbohydrate metabolism enzymes. The starch content was decreased in leaves but increased in roots by added silicon under stress. Silicon addition increased the root sucrose content in ‘JinYou 1’ but decreased it in ‘JinChun 5’ under salt stress.
Conclusions
Silicon-mediated decrease of assimilate accumulation in leaves may alleviate photosynthetic feedback repression, while silicon-enhanced assimilate transport provides more energy storage in the roots, which is beneficial for salt stress tolerance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinity stress is one of the most serious constraints that adversely affect crop production (Zhu and Gong 2014). Salt stress imposes two stresses on plant tissues: one is osmotic stress caused by relatively high solute concentrations of the soil (growth medium) that disrupt homeostasis in water potential, and the other is ion-specific stress resulting from excess accumulations of Na+ and Cl− and altered K+/Na+ ratios (Golldack et al. 2011; Yin et al. 2016). Limited capacities of Na+ and Cl− exclusion from the plasma membrane and/or compartmentation into the vacuole lead to their excess accumulations in the cytosol (Blumwald 2000; Munns and Tester 2008). Moreover, due to the similarity of K+ and Na+ radii which makes it difficult for plants to discriminate between them, salt stress disrupts the acquisition of K+ nutrient (Blumwald 2000), which is one of the essential elements for plant growth and plays a critical role in maintaining enzyme activities (Jouyban 2012). The excess accumulations of salt ions and decreased K+/Na+ ratios disturb essential biochemical reactions in the cytosol by impairing enzyme activities and protein functions (Chinnusamy et al. 2005). How to increase the tolerance of crops to salt stress has received worldwide attention.
Silicon (Si) is the second most prevalent element in soil after oxygen and it is a beneficial element for plants, especially in adverse environmental conditions (Guntzer et al. 2012). Silicon application has been proven to be a promising method to reduce the damaging effects of salt stress on plants (Zhu and Gong 2014; Rizwan et al. 2015). Much work has been done to explore the mechanism(s) for silicon-mediated salt tolerance in plants. Most previous studies have been focused on how silicon decreases Na+ accumulation and maintains ion homeostasis in plants under salt stress. For example, Yin et al. (2013) found that silicon addition decreased Na+ accumulation in the leaves of sorghum. In grapevine, silicon application decreased both Na+ and Cl− accumulations in the roots under salt stress (Soylemezoglu et al. 2009). Gurmani et al. (2013) reported that Si application decreased Na+ transport and improved K+/Na+ ratio in salt-stressed wheat. However, silicon-mediated decrease in Na+ accumulation is not always the case (Romero-Aranda et al. 2006; Zhu and Gong 2014), suggesting the involvement of other mechanism(s). Recently, a number of studies have been focused on the alleviative effect of silicon on osmotic stress induced by salinity and found that silicon addition improved the plant water status, such as in tomato (Romero-Aranda et al. 2006; Li et al. 2015a), maize (Rohanipoor et al. 2013), sorghum (Yin et al. 2013), turfgrass (Esmaeili et al. 2015) and cucumber (Wang et al. 2015a). Chen et al. (2014) demonstrated that the alleviative effects of silicon in the osmotic stress phase were more pronounced than salt-induced ion toxicity in wheat. Liu et al. (2015) reported that added silicon alleviated the inhibition of root hydraulic conductance under salt stress in sorghum, and this was accounted for by increases in aquaporin expression and activity. However, they observed that salt stress alone also stimulated aquaporin expression, although the root hydraulic conductance was inhibited, which suggests a complex mechanism for silicon-mediated regulation of root hydraulic conductance. In cucumber, Wang et al. (2015a) found that silicon enhances the salt tolerance of cucumber through improving water balance and reducing Na+ accumulation in plants; however, Zhu et al. (2015) proposed that added silicon enhanced root water uptake in salt-stressed cucumber and it was less likely that silicon was actively involved in reducing Na+ accumulation. These results imply genotype-dependent reactions of silicon-mediated salt tolerance in cucumber plants. In addition, previous work by Zhu et al. (2004) also suggested that silicon-mediated salt tolerance of cucumber was attributed to the decreased oxidative stress. However, it remains unclear whether this is a direct action of silicon or just a consequence of stress alleviation. In zucchini squash (Cucurbita pepo L.), another important cucurbitaceae plant, silicon supply enhanced salt tolerance by inhibiting the translocation of Na and Cl to the leaves (Savvas et al. 2009). These studies suggest that the mechanism for silicon-mediated salt tolerance in plants is still not fully understood, and more work is still needed.
Photosynthesis is a process that converts light energy into chemical energy. Sucrose and starch are the main products of photosynthesis (Goldschmidt and Huber 1992). In the cytoplasm, one of the key enzymes for sucrose synthesis is sucrose-phosphate synthase (SPS) (Huber and Huber 1996). The main form of sugar transport in plants is sucrose, which is transported by sucrose transporter (Riesmeier et al. 1994). The main enzymes responsible for sucrose degradation are sucrose synthase (SUS) and invertase (Ranwala and Miller 1998). SUS can catalize both sucrose synthesis and decomposition, with the latter being its main role (Geigenberger and Stitt 1993). In starch metabolism, ADP-glucose pyrophosphorylase and amylase play important roles in starch synthesis and decomposition, respectively (Cardemil and Varner 1985; Ballicora et al. 2004). Continuous supply of available photosynthetic carbon is crucial for normal growth and development of plants. However, under stress conditions, the ability of plants to utilize light energy can be limited, which induces an overproduction of reactive oxygen species (Rossel et al. 2002) and causes oxidative damage. Moreover, environmental stresses also influence the source-to-sink transport of assimilates. On one hand, the inhibition of assimilate export leads to accumulations of soluble sugar and starch in source leaves, which causes photosynthetic feedback repression; on the other hand, it reduces carbon and energy supply for the sink tissues (Stitt 1991; Khelil et al. 2007). Under stress conditions, the accumulation of carbohydrates in sink tissues can play an important role in osmoprotection, osmotic adjustment, carbon storage and radical scavenging in plants (Parvaiz and Satyawati 2008). In view of the beneficial role of silicon on plant photosynthesis and growth under salt stress (see review by Zhu and Gong 2014), it is reasonable to speculate that silicon may have a regulatory effect on carbohydrate metabolism in plants. However, relevant information is still lacking.
Cucumber is an important horticultural crop and is highly sensitive to salinity (Huang et al. 2009). The positive effect of silicon on salt tolerance of cucumber has been observed in previous studies (Wang et al. 2015a; Zhu et al. 2015). In this study, the effects of silicon on the growth, oxidative damage, chlorophyll fluorescence and carbohydrate metabolism were investigated in cucumber seedlings. This study may help to understand the role of silicon in regulating carbohydrate metabolism and the mechanism for silicon-mediated tolerance to salt stress in plants.
Materials and methods
Plant material and growth conditions
Two cucumber (Cucumis sativus L.) cultivars ‘JinYou 1’ and ‘JinChun 5’ were used in this study, with the former being more sensitive to salt stress (Zhang and Wu 2009). Seeds were thoroughly rinsed with distilled water and germinated on moist gauze in petri dishes at 28 °C in dark for 2 days. The germinated seeds were sown in quartz sands and transferred to the university greenhouse. The temperature was set to 28 °C/18 °C, 12 h/12 h (day/night) in the greenhouse. When the second leaves were fully expanded, the seedlings were transferred to 15-L plastic containers filled with continuously aerated 1/4 strength of modified Hoagland nutrient solution based on Hoagland and Arnon (1950). Three days after transplanting, the strength of solution was increased to 1/2. The modified full strength Hoagland nutrient solution contained the following components: 4 mM Ca(NO3)2•4H2O, 6 mM KNO3, 1 mM NH4H2PO4, 2 mM MgSO4•7H2O, 46.24 μM H3BO3, 7.73 μM MnCl2•4H2O, 0.77 μM ZnSO4•7H2O, 0.20 μM CuSO4•5H2O, 0.45 μM Na2MoO4•2H2O, 71.24 μM EDTAFeNa•3H2O. Seven days after transplantation, salt treatment was started by adding 75 mM sodium chloride (NaCl) to the nutrient solution in the absence or presence of 0.3 mM silicon as sodium silicate (Na2SiO3·9H2O). The pH of the nutrient solution was adjusted to 6.0 every two days using 0.2 M H2SO4 or 1 M KOH. The culture solutions were renewed every 6 days. After 5, 10 and 15 days of salt treatment, the recent fully expanded leaves and roots were harvested, immediately frozen in liquid N2 and then stored at −80 °C until analysis.
Plant dry weight and chlorophyll fluorescence
After 15 days of salt stress, the plant dry weights were determined after oven drying at 80 °C for 72 h.
The leaf chlorophyll fluorescence parameters were measured using a portable fluorometer (PAM-2500; Walz, Effeltrich, Germany). The minimal (Fo) chlorophyll fluorescence emission was determined on dark-adapted leaves. The maximal chlorophyll fluorescence (Fm) was obtained during a subsequent saturating light pulse (8000 μmol m−2 s−1 for 0.8 s). The steady-state fluorescence (Fs) was recorded after the leaf being continuously illuminated with actinic light at an intensity of 300 μmol m−2 s−1. A second saturating pulse of white light (8000 μmol m−2 s−1 for 0.8 s) was imposed to determine the maximum fluorescence level in the light-adapted state (Fm′). The minimal fluorescence level in light-adapted state (Fo′) was determined by illuminating the leaf with far-red light (7 μmol m−2 s−1) for 3 s. The fluorescence parameters were calculated according to Shu et al. (2013) and Hu et al. (2014): maximal quantum yield of PSII photochemistry, Fv/Fm = (Fm − Fo)/Fm; effective quantum-use efficiency of PSII in light-adapted state, Fv’/Fm′ = (Fm′ − Fo′)/Fm′; quantum yield of PSII photochemistry, ΦPSII = (Fm′ − Fs)/fm′; photochemical quenching, qP = (Fm′ − Fs)/(Fm′ − Fo’); and nonphotochemical quenching, NPQ = (Fm − Fm′)/Fm′.
Malondialdehyde and hydrogen peroxide contents
Malondialdehyde (MDA) content in the leaves or roots was measured by thiobarbituric acid reaction according to Shi et al. (2014) with modifications. Fresh plant tissue (0.5 g) was homogenized in 10 ml of 0.1 % (w/v) trichloroacetic acid, and the extract was centrifuged at 5000 g for 10 min at 4 °C. To measure MDA, 1.5 ml of the supernatant was added into 1.5 ml of 0.5 % (w/v) thiobarbituric acid (TBA) made in 5 % trichloroacetic acid. The mixture was heated at 100 °C for 20 min and then quickly cooled in an ice bath. After centrifuging at 7888 g for 10 min, the absorbance of the supernatant was measured at 450 nm, 532 nm and 600 nm. The MDA concentration was calculated using the formula: C (μmol L−1) = 6.45 (OD532 - OD600) - 0.56 OD450 (Zheng and Tian 2006).
Hydrogen peroxide content in the leaves and roots was determined according to Gong et al. (2005). The plant tissue was ground in cold 0.1 % (w/v) trichloroacetic acid and the extract was centrifuged at 12,000 g for 15 min. A half ml of the supernatant was mixed with 0.5 ml of 10 mM potassium phosphate buffer (pH 7.0) and 1 ml of 1 M KI. The absorbance of the solution was recorded at 390 nm, and the content of H2O2 was determined using a standard curve.
Sugar and starch contents
Soluble sugar was measured using a sugar analysis system (HPLC, Shimadzu, Kyoto, Japan) according to Dong et al. (2011) with modifications. Briefly, the recent fully expanded leaves and roots were harvested and soluble sugars were extracted with 80 % (v/v) aqueous ethanol four times (75 °C, 10 min each). After evaporating the supernatant, the residue was redissolved in water, filtered through a 0.45 μm membrane HPLC filter (Whatman, Maidstone, UK) and used for soluble sugar analysis. The residue left after extracting soluble sugars was used for determination of starch content according to Yang et al. (2001).
Enzyme extraction and activity assay
Frozen samples were used for activity determination of sucrose phosphate synthase (SPS) (EC 2.4.1.14), sucrose synthase (SUS) (EC 2.4.1.13), acid invertase (AI, EC 3.2.1.26), neutral invertase (NI, EC 3.2.1.26), ADP-glucose pyrophosphorylase (ADP-GPPase, EC 2.7.7.27) and amylase activities (EC 3.2.1.1). One gram of tissue was extracted in 50 mM Hepes-NaOH (pH 7.5), 5 mM MgCl2, 1 mM Na-EDTA, 2.5 mM dithiothreitol, 0.5 mg ml−1 bovine serum albumin, and 0.05 % (v/v) Triton X-100 according to the method of Hubbard et al. (1989). The homogenates were centrifuged at 22,000 g at 4 °C for 10 min and the extracts were dialyzed at 4 °C for 20 h with three changes of dialysis buffer (Choudhury et al. 2010; Dong et al. 2011).
SPS activity was assayed according to Dong et al. (2011). The above extract (0.2 ml) was incubated with a reaction mixture containing 0.2 ml of 200 mM Hepes (pH 7.5), 0.1 ml of 25 mM fructose-6-phosphate, 0.1 ml of 100 mM MgCl2 and 0.1 ml of 50 mM UDP-glucose. The mixtures were incubated at 27 °C for 30 min, after which the reaction was terminated by boiling the mixture for 3 min. The amount of sucrose formed was determined by the resorcinol method (Vu et al. 1993) after cooling to room temperature. The SUS activity was assayed in the direction of UDP-fructose synthesis using a sucrose synthase assay kit (Genmed Scientifics Inc., USA). The soluble protein was measured following the method of Bradford (1976). Both SPS and SUS activities were presented as the amount of products formed per g of protein in a minute.
Acid invertases and neutral invertases were extracted and determined according to Miron and Schaffer (1991) with modifications. The tissues were homogenized in 50 mM Hepes-NaOH (pH 7.5), 0.5 mM Na-EDTA, 2.5 mM dithiothreitol, 3 mM diethyldithiocarbamic acid, 0.5 % (w/v) bovine serum albumin, and 1 % (w/v) insoluble polyvinyl pyrrolidone. The assay mixture for acid invertase contained 0.2 ml of enzyme extract, 0.6 ml of 0.1 M sodium acetate buffer (pH 4.5) and 0.2 ml of 0.75 M sucrose. The reaction was progressed in a 30 °C water bath for 30 min and stopped by boiling the mixture for 5 min in a water bath (Rosales et al. 2007). Reducing sugars released from sucrose were determined according to Rosales et al. (2007) by addition of dinitrosalicylic acid reagent. The neutral invertase activity was determined as for acid invertase but with 0.1 M K2HPO4–citrate buffer (pH 7.0) instead of sodium acetate buffer (Rosales et al. 2007). Both acid invertases and neutral invertases activities were presented as the amount of products formed per g of protein in a minute.
The β-amylase was extracted as described by Duffus and Rosie (1973) and the activity was determined using alkaline dinitrosalicylic acid (Bhatia and Asthir 2014). The ADP-GPPase was extracted according to Rosa et al. (2009) and its activity was determined using an ADP-GPPase assay kit (Genmed Scientifics Inc., USA).
Statistics
Data were subjected to analysis of variance using SPSS 19.0. The differences were considered significant at P < 0.05.
Results
Plant growth
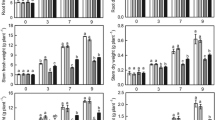
Under non-stress conditions, silicon addition did not change the dry mass accumulation in either ‘JinYou 1’ or ‘JinChun 5’ (Fig. 1). Salt stress severely decreased the plant dry weights of both cultivars after treatment for 15 days. Silicon application, on the other hand, significantly alleviated the deleterious effect of salt stress on the growth of both cultivars: the plant dry weights of ‘JinYou 1’ and ‘JinChun 5’ were increased by 62.3 % and 33.5 % respectively in NaCl + Si treatments compared with NaCl treatments alone (Fig. 1).
Effects of silicon on dry weight of cucumber seedlings under salt stress. The dry weights of seedlings were measured after 15 d of exposure to salt stress. C, control; Si, silicon; Na, salt treatment (NaCl); Na + Si, salt treatment plus silicon. Values are mean + SE of ten replicates. Different letters above bars indicate a significant difference (P < 0.05)
Malondialdehyde and hydrogen peroxide contents
Under non-stress conditions, silicon addition did not change the malondialdehyde (MDA) or hydrogen peroxide (H2O2) levels in either cucumber cultivar (Fig. 2). Under salt stress, the MDA and H2O2 contents in the roots and leaves of both cultivars were significantly increased as compared to their respective controls; while silicon addition significantly inhibited the increases, especially at late period of stress.
Effect of silicon on malondialdehyde and hydrogen peroxide contents of cucumber seedlings under salt stress. A-D, JinYou 1; E-H, JinChun 5. A, B, E and F, MDA content; C, D, G and H, H2O2. DW, dry weight; MDA, malondialdehyde. Values are mean + SE of four replicates. Different letters above bars indicate a significant difference at P < 0.05
Chlorophyll fluorescence parameters
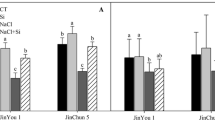
Compared with the control, salt stress reduced the maximum photochemistry efficiency of PSII (Fv/Fm), efficiency of excitation capture by open PSII reaction center (Fv’/Fm′), actual photochemical efficiency of PSII (ΦPSII) and coefficient of photochemical quenching (qP) (Table 1); while addition of silicon alleviated these decreases in stressed plants. Unlike the changes of other parameters, the nonphotochemical quenching (NPQ) was significantly increased by NaCl treatment, but it was decreased by added silicon in stressed plants. There were no significant differences in these parameters between the control plants and those treated with silicon alone.
Soluble sugar and starch contents
In the leaves of both cucumber cultivars, salt stress remarkably increased the concentrations of sucrose, fructose and glucose (Table 2). However, the concentrations in the silicon-treated seedlings were significantly lower than those without added silicon under salt stress, especially at later stress period (10 and 15 days). The ratio of hexose to sucrose was decreased by salt stress after 10 and 15 days of treatment in both cultivars, and it was increased by silicon addition in the stressed plants (Table 2). The starch concentrations were gradually increased in the leaves of both cultivars during the salt treatment, and the highest level appeared on the 15th day of treatment; while silicon addition inhibited the increase, except on the 5th day in ‘JinYou 1’, when the starch concentration was decreased under salt stress and added silicon inhibited the decrease (Table 2). In both cultivars, the ratios of sucrose to starch in the leaves were obviously increased, while added silicon inhibited the increases.
In the roots of ‘JinYou 1’, under stress conditions, significant increases in sucrose, fructose and glucose concentrations were observed (Table 2). Silicon application promoted the increases in sucrose and glucose levels, but it had little effect on the fructose concentration, except a slight increase after 5 days of treatment. In the roots of ‘JinChun 5’, salt stress significantly increased the levels of sucrose, fructose and glucose from 5th day on, while silicon addition basically inhibited the increases, except on the 15th day, when there was no significant difference in sucrose concentration between NaCl and NaCl+Si treatments. In the roots of both cultivars, the ratios of hexose to sucrose were decreased after 10 and 15 days of salt stress (Table 2). There was no significant difference in this ratio between NaCl and NaCl+Si treatments in both cultivars, except that silicon addition decreased the ratio in ‘JinYou 1’ after 15 days of stress (Table 2). The starch concentrations in the roots of both cultivars were decreased after 10 and 15 days of salt stress, but the accumulations were increased in the presence of added silicon (Table 2). The ratios of sucrose to starch in the roots of both cultivars were increased under salt stress (Table 2); while silicon addition did not change the ratio on the 5th and 10th days after stress start, but it increased the ratio on the 15th day in ‘JinYou 1’. In ‘JinChun 5’, silicon addition inhibited stress-induced increase in this ratio (Table 2).
The contents of total carbohydrates (sucrose, glucose, fructose and starch) in leaves were basically increased under salt stress, especially at late stress period; while added silicon partly inhibited the increases (Table 3). In the roots of ‘JinYou 1’, the total carbohydrate content was increased after 5 days of salt stress, but it was decreased after 15 days of stress; while silicon addition increased the total carbohydrate content from the 10th day onward after stress initiation. In the roots of ‘JinChun 5’, except on the 5th day, the total carbohydrate content was basically not altered by salt or silicon addition. It is noticeable that the ratio of total carbohydrate content in root to that in leaf was decreased under salt stress, and it was obviously higher in silicon-added plants (Table 3).
Activities of enzymes involved in sucrose and starch metabolism
(a) sucrose phosphate synthase (SPS)
Compared with the control, the SPS activities in both leaves and roots were significantly increased in the two cucumber cultivars under salt stress (Figs. 3a, 3e and 4a, 4e). In the leaves of both cultivars, salt-stress-induced increases in the SPS activities were significantly inhibited by added silicon after 10 and 15 days of treatment (Figs. 3a and 4a). In ‘JinYou 1’, added silicon further increased the SPS activity in the roots of stressed plants (Fig 3e). However, in the roots of ‘JinChun 5’, silicon addition inhibited stress-induced increase of the enzyme activity (Fig. 4e).
Effect of silicon addition on the activities of enzymes involved in sucrose synthesis and catabolism in ‘JinYou 1’. A-D, leaf; E-H, root. A and E, sucrose phosphate synthase (SPS); B and F, sucrose synthase; C and G, soluble acid invertase; D and H, soluble neutral invertase. Values are mean + SE of four replicates. Different letters above bars indicate a significant difference at P < 0.05
Effect of silicon addition on the activities of enzymes involved in sucrose synthesis and catabolism in ‘JinChun 5’. A-D, leaf; E-H, root. A and E, sucrose phosphate synthase (SPS); B and F, sucrose synthase (SUS); C and G, soluble acid invertase; D and H, soluble neutral invertase. Values are mean + SE of four replicates. Different letters above bars indicate a significant difference at P < 0.05
There were significantly correlations between the sucrose concentration and sucrose phosphate synthase activity in the roots of both cultivars (Fig. 5). Similar results were also observed in the leaves, except on the 5th day after salt treatment, when there were positive correlations with high correlation coefficients although they were not statistically significant at P = 0.05 level.
(b) sucrose synthase and invertases
Under salt stress, the activities of sucrose synthase were increased in both leaves and roots of the two cucumber cultivars (Figs. 3b, 3f and 4b, f). In the leaves, under salt stress, silicon addition did not affect the sucrose synthase activity in ‘JinYou 1’ (Fig. 3b), whereas it significantly inhibited the activity in ‘JinChun 5’ (Fig. 4b). In the roots of both cultivars, a decreased activity of sucrose synthase by added silicon was observed after 15 days of salt treatment (Figs. 3f and 4f).
In the leaves, the acid invertase activities were decreased at late period of salt stress, while added silicon alleviated the decreases in both cucumber cultivars (Figs. 3c and 4c). By and large, the activities of neutral invertases were not changed by added silicon in both cultivars under salt stress, except on the 15th day, when the activity was inhibited by silicon addition in ‘JinChun 5’ (Figs. 3d and 4d).
In the roots of ‘JinYou 1’, salt stress significantly increased the activities of both acid invertase and neutral invertase, and silicon application further promoted the increases (Fig. 3g, h). In the roots of ‘JinChun 5’, the activities of both invertases were basically increased under salt stress, while added silicon had no effect on their activities (Fig 4g, h).
(c) Amylases and ADP-GPPase
In the leaves of both cucumber cultivars, the amylase activities were enhanced under salt stress, except on the 5th day, when the amylase activity was decreased in ‘JinYou 1’ (Fig. 6a, c). However, the amylase activities were kept at the control levels in silicon-treated plants under stress in the two cultivars (Fig. 6a, c).
In the roots of ‘JinYou 1’, the amylase activity was only increased after 15 days of salt stress, and added silicon increased the activities from the 10th day onward (Fig. 6b). In ‘JinChun 5’, salt stress increased the amylase activities in roots throughout the stress period and silicon addition inhibited the increase, except on the 15th day, when the activity was not changed by added silicon under salt stress (Fig. 6d).
In the leaves of both cultivars, the activities of ADP-GPPase were significantly increased under salt stress, while added silicon partly inhibited the increase (Fig. 7a, b).
Discussion
The ameliorative effects of silicon on the growth inhibition of salt-stressed plants have been reported in quite some species like rice (Gong et al. 2006; Shi et al. 2013), wheat (Tuna et al. 2008), sorghum (Liu et al. 2015), sugarcane (Ashraf et al. 2010), aloe (Xu et al. 2015), tomato (Romero-Aranda et al. 2006; Li et al. 2015a), zucchini (Savvas et al. 2009) and cucumber (Zhu et al. 2004; Wang et al. 2015a; Zhu et al. 2015). In this study, the salt-induced growth inhibition in cucumber seedlings was significantly improved by silicon addition, which is consistent with previous findings.
Salinity and other various abiotic stresses can induce overproductions of reactive oxygen species by limiting the plant’s ability to use light energy through photosynthesis (Rossel et al. 2002). Excess H2O2 in plant cells can lead to the occurrence of lipid peroxidation, which can be assessed in terms of malondialdehyde (MDA) content (Shi et al. 2014). In this study, silicon addition decreased the H2O2 accumulation and MDA contents in salt-stressed cucumber, suggesting that added silicon may have improved the photosynthetic performance of stressed plants. Chlorophyll fluorescence has been widely used to monitor photosynthetic performance in plants (Gorbe and Calatayud 2012). Fluorescence quenching can be separated into photochemical and nonphotochemical components (Maxwell and Johnson 2000; Baker 2008). In general, Fv/Fm (maximal quantum yield of PSII photochemistry), Fv’/Fm′ (effective quantum-use efficiency of PSII in light-adapted state), ΦPSII (quantum yield of PSII photochemistry), and qP (photochemical quenching) have been calculated to reflect photochemical quenching, and NPQ is a non-photochemical-quenching parameter (Zhang et al. 2009). In this study, the Fv/Fm was significantly decreased under salt stress, while it was partly improved by added silicon. Silicon-mediated increase in Fv/Fm has also been observed in rice under drought stress (Chen et al. 2011). Silicon addition resulted in a less decrease in qP (photochemical quenching coefficient) in salt-stressed cucumber (Table 1), suggesting that more PSII reaction centers were in an open state, which allowed more excitation energy to be used for electron transport in silicon-added plants (Maxwell and Johnson 2000). In addition, the efficiency of excitation energy captured by open PSII (Fv’/Fm′) was increased by silicon addition under salt stress, suggesting that silicon decreased dissipation of excitation energy as heat in the PSII antennae, as was the case observed in NPQ (Table 1). The decrease of NPQ by added silicon implies an alleviation of damage under salt stress. ΦPSII reflects electron transport rate (Shu et al. 2013) and it was higher with silicon addition compared with salt stress alone (Table 1), indicating silicon-mediated improvement in the capacity to convert photon energy into chemical energy. Chloroplast ultrastructure can be damaged by salinity stress (Parida and Das 2005). Liang (1998) reported that silicon could ameliorate the damage of chloroplast ultrastructure induced by salt stress. In Cd-stressed maize, Vaculík et al. (2015) found that silicon addition can alleviate cadmium toxicity by enhanced photosynthetic rate and ΦPSII, which may be due to silicon-mediated enhancement of thylakoid formation in the chloroplasts of bundle sheath’s cells. In this study, the improvement of the photosynthetic performance in cucumber under salt stress may have been partly due to the protective effect of silicon on the structure of photosynthetic apparatus. Besides, silicon-mediated decrease in reactive oxygen species accumulation in cucumber under salt stress may also be related to enchanced capabilities of antioxidant defense, as observed in wheat, barley, tomato and rice under drought stress or salt stress (Gong et al. 2005; Gunes et al. 2007; Pei et al. 2010; Li et al. 2015a). It can be seen from these results that added silicon improved the photosynthetic performance of cucumber plants under salt stress, which might allow a constant supply of assimilates to the growing tissues.
Carbohydrate metabolism in leaves
Sucrose is one of the main products of photosynthesis (Choudhury et al. 2010). Maintenance of the balance among sucrose production, translocation, partition and use are important for the normal growth of plants. However, under environmental stresses, accumulations of soluble sugars such as sucrose, fructose and glucose are usually observed (Gil et al. 2013; Li et al. 2015b; Richter et al. 2015; Wei et al. 2015), resulting in growth retardation. Sucrose catabolism to supply hexoses is necessary for cell growth and development (Tymowska-Lalanne and Kreis 1998; Hütsch et al. 2015). In this study, in the leaves of both cultivars, the higher sucrose accumulation and lower hexose/sucrose ratio caused by salt stress could be attributed to the higher SPS activity and lower AI activity after 10 and 15 days of treatment. The activity of SUS but not invertases (AI + NI) in the leaves was increased under stress (Figs. 3b–d and 4b–d), which corresponded with the increased hexose levels after 10 and 15 days treatment in both cultivars under salt stress (Table 2), suggesting that SUS might play a more important role in sucrose hydrolysis in salt-stressed cucumber leaves. Despite of the increases of SUS and NI activities under salt stress (Figs. 3b, d and 4b, d), the leaf sucrose levels were still increased (Table 2), which suggests that the sucrose synthesis might have increased more than its degradation, as a good correlation was observed between the SPS activities and sucrose concentrations in both cultivars (Fig. 5). Balibrea et al. (2000) did not find any correlation between the total sucrolytic activity (AI + NI + SUS) and both sucrose and hexose contents in salt-sensitive tomato cultivars. As leaf sugar content is a result of the balance between synthesis, degradation and export, the changes of their levels are complex. Except sucrose synthesis and degradation, it is possible that salt stress inhibited the sucrose export. These studies suggest a complex sucrose metabolism, which remains to be investigated in more detail. In this study, the relatively low AI activity (Figs. 3c and 4c) observed in the leaves of salt-stressed cucumber could be explained by a higher concentration of sucrose hydrolysis products, especially fructose, which was suggested to inhibit the activities of acid invertases (Winter and Huber 2000). In this study, although the accumulation of soluble sugars, especially sucrose in the leaves of both cucumber cultivars (Table 2) might have contributed to osmotic adjustment under salt stress. However, the accumulation of sugars may also result from its lower utilization in the growing tissues and alternation of source and sink metabolism (Pelleschi et al. 1997; Alaoui-Sossé et al. 2004; Khelil et al. 2007), which may cause the feedback inhibition in photosynthesis of source leaves and result in growth reduction of cucumber seedlings under salt stress, as observed in this study (Table 1; Fig. 1).
Although the positive effects of silicon on plant growth under stress conditions have been widely reported (Ma 2004; Liang et al. 2007; Rizwan et al. 2015), yet how silicon regulates sugar metabolism in plants is largely unknown. In this study, by and large, silicon addition did not change the levels of soluble sugar in the leaves (Table 2), nor did it alter the activities of enzymes involved in their synthesis and catalysis in non-stress conditions (Figs. 3–4). However, under salt stress, addition of silicon changed the soluble sugar metabolism. In the leaves of both cucumber cultivars, silicon addition alleviated the increase of soluble sugar level (Table 2). Under salt stress, the silicon-mediated decrease in the leaf sucrose level might have been related to the decreases of SPS activities (Figs. 3a and 4a) and increases in AI activities (Fig. 3c and 4c). Silicon addition had little effect on the SUS activity in ‘JinYou 1’ (Fig. 3b) but decreased it in ‘JinChun 5’ (Fig. 4b) under salt stress. These results suggested that the effect of silicon on the enzymes activities involved in carbohydrate metabolism is quite complex and species-dependent. In leaves, a constant but low level of hexoses has been proposed to be necessary in growing leaves in order to maintain a respiratory substrate level without risking the metabolic activity being perturbed by large changes in photo-assimilate abundance in nearby cells (Kingston-Smith et al. 1999). The inhibition of increase in soluble sugar may contribute to the alleviation of feedback repression of photosynthesis (Khelil et al. 2007), as observed in this study (Table 1) and our previous work (Zhu et al. 2015) that added silicon improved the leaf photosynthesis under salt stress. Moreover, it should be noticed that the ratio of hexose/sucrose in leaves, which can be considered to be a measure of sucrose utilization (Khelil et al. 2007), was higher with silicon addition compared with NaCl treatment alone after 10 days of stress (Table 2), suggesting that silicon addition improved sucrose utilization of cucumber leaves. The increase of sucrose utilization may provide more energy required for plant growth (Balibrea et al. 2000; Dong et al. 2011), as the growth improvement by added silicon under salt stress observed in this study (Fig. 1).
A high level of sucrose can promote the biosynthesis of starch (Yuan et al. 2015). Starch serves as a main carbohydrate store in the majority of higher plants and can be rapidly degraded to release soluble sugars for mobilization and utilization (Tetlow 2004; Li et al. 2015c). The activity of ADP-GPPase is of prime importance in starch synthesis, while amylase catalyzes the initial step in the hydrolysis of starch into glucose, maltose and low molecular weight di- and oligosaccharides (Muller-Rober et al. 1990; Bhatia and Asthir 2014; Wang et al. 2015b). In this study, the ADP-GPPase activities were promoted in the leaves of both cultivars under salt stress (Fig. 7), which might have been responsible for the accumulation of starch. Enhancement of β-amylase activity by osmotic stress has been reported previously (Datta et al. 1999; Todaka et al. 2000). In cucumber, Todaka et al. (2000) found that the β-amylase activity was increased under water stress, followed by increases in sucrose and maltose levels. In this study, the β-amylase activity was induced by salinity (except for a decrease in the leaves of ‘JinYou 1’ after 5 days of treatment) (Fig. 6), which may have contributed to the increase of sucrose content in cucumber seedlings under salt stress. The increased β-amylase activities were likely to result in a decrease in starch accumulation in the leaves of salt-stressed cucumber. Nevertheless, the starch content was increased in the leaves (Table 2). It can be assumed that salt stress induced more starch synthesis by ADP-GPPase than its degradation by β-amylase. Under salt stress, silicon addition reversed both starch biosynthesis and hydrolysis back to the control, therefore decreased starch accumulation in the leaves of both cultivars. Except an alteration of balance between synthesis and degradation, starch accumulation in leaves may also result from a decrease in leaf expansion, phloem loading, or capacity of assimilate transport under stress condition (Alaoui-Sossé et al. 2004). In the present study, added silicon decreased the leaf starch content but increased the root starch content under salt stress, suggesting that added silicon increased sugar export from the source leaves. Since excess accumulation of starch in leaves can lead to a feedback repression of photosynthesis (Han et al. 2008), silicon-mediated decrease of starch accumulation in cucumber leaves may have contributed to the higher photosynthetic performance (Table 1). In addition, it should be noticed that the ratio between sucrose and starch in the leaves of both cultivars were decreased by silicon addition compared with NaCl treatment alone (Table 2), which is compatible with the hypothesis that the shift from sugar to starch would avoid metabolic alteration (Balibrea et al. 1996, 2000). Rosa et al. (2009) reported that salt stress decreased the starch content in quinoa plants and this decrease was due to the alteration in carbon allocation in order to produce free proline for the alleviation of salinity stress. Recently, Detmann et al. (2012) suggested that in rice, a silicon accumulator, silicon could regulate the nitrogen/carbon balance and amino acid remobilization and led to an improved crop yield. The role of silicon in controlling nitrogen and carbon metabolism in cucumber plants, which accumulate less Si than rice (Wu et al. 2015), needs to be studied in future.
Carbohydrate metabolism in roots
To maintain normal growth under salinity, plants need to regulate carbon allocation, partitioning and its use in the sink organs (Pelleschi et al. 1997; Li et al. 2015c). In Cu-treated cucumber, the decline in photosynthesis was proposed to be the consequence of an altered source-sink relationship, rather than toxic effect of copper on photosynthesis (Vinit-Dunand et al. 2002). The sucrolytic activities have been suggested to be involved in coordinating sink-source relations by maintaining sink demand and sucrose synthesis and transport (Balibrea et al. 2000). Root is a sink tissue and the first part of a plant to be impacted by salinity. However, little is known about the regulative effects of silicon on carbohydrate metabolism in roots. In this study, in the roots of both cultivars, the activities of SPS and enzymes involved in sucrose degradation (Figs. 3f–h and 4f–h) were all increased under salt stress. The increase of sucrose level in the roots might have been due to a higher sucrose synthesis than degradation, and salt stress-induced increase of glucose and fructose levels might be attributed to the increases in activities of SUS, AI and NI (Figs. 3f–h and 4f–h). The effect of silicon on the levels of soluble sugar in roots is cultivar-dependant under salt stress (Table 2): in ‘JinYou 1’, silicon addition resulted in a higher accumulation of soluble sugar; whereas in ‘JinChun 5’, added silicon alleviated the increase. In ‘JinYou 1’, added silicon further increased the invertase activities (Fig. 3g–h), which may have facilitated sucrose degradation and hexose accumulation; however, the sucrose level was not decreased but even increased (Table 2). On the other hand, the SPS and β-amylase activities were enhanced by added silicon under salt stress and there was a positive linear correlation between sucrose level and SPS activity. High β-amylase activity can facilitate the breakdown of starch, which leads to an accumulation of sucrose (Basu et al. 2007). These results suggest that the silicon-mediated sucrose accumulation in the roots of ‘JinYou 1’ might be attributed to the enhancement of SPS and β-amylase activities. Similarly, the decrease of sucrose in the roots of ‘JinChun 5’ mediated by added silicon was due to the inhibition of SPS and β-amylase activities.
Silicon-mediated increase in root soluble sugar level (especially sucrose) in ‘JinYou 1’ may function as an osmoprotectant and stabilize cellular membranes (Rosa et al. 2009; Dong et al. 2011), and may also have partly contributed to the decreased root xylem osmotic potential, which enhanced water uptake ability under salt stress as observed in our previous study (Zhu et al. 2015). Similarly, under water stress, silicon has also been found to enhance osmotic adjustment via active accumulation of some osmolytes including soluble sugar in rice and sorghum (Sonobe et al. 2011; Ming et al. 2012). In this study, the ratio of hexose/sucrose in roots of the two cucumber cultivars was decreased under salt stress, and by and large, silicon did not change it in the two cultivars, except 15 days after salt stress in ‘JinYou 1’ (Table 2). This suggests that silicon addition basically did not affect the sucrose utilization efficiency in roots under salt stress, except in ‘JinYou 1’ at the end of the stress period, but the accumulation of sucrose in this cultivar might have contributed to osmotic adjustment and root water uptake, as discussed above.
In this study, the starch contents in the roots were decreased during late period of salt stress (Table 2). As previously discussed, salt stress might induce a decrease in the capacity of assimilates transport from source leaves to roots (sink), which may result in the consumption of starch to product energy (Mittler 2006), as can be seen from the salt-stress-induced increase of β-amylase activities (Fig. 6b, d). Silicon-mediated changes of β-amylase activities were different in both cucumber cultivars (Fig. 6b, d), but the root starch contents were increased by added silicon in the stressed plants of both cultivars. The discrepancy between silicon-mediated changes of starch content and β-amylase activity in ‘JinYou 1’ may suggest the involvement of other enzymes in starch metabolism, which remains to be investigated. In this study, during late period of salt stress, added silicon obviously increased the ratio of carbohydrate content in roots to that in leaves (Table 3). This may suggest that added silicon promoted assimilate export from the source leaves, therefore enhanced starch accumulation in the roots. Under stress conditions, an increase of starch content in roots is beneficial for plant growth (Dong et al. 2011). Silicon-mediated increase in the root starch content (Table 2) may have provided more energy storage and enhanced stress tolerance in cucumber plants.
Action mode of silicon?
It is suggested that silicon deposited in cell walls can form amorphous colloidal complexes with organic macromolecules, which increases the absorption surfaces and can thus affect water and solute transport in plants (Gao et al. 2004). Diogo and Wydra (2007) also found that the increase of resistance to bacterial wilt mediated by silicon in tomato was associated with its induced changes in polysaccharide structure of xylems vessel walls. Isa et al. (2010) found that silicon enhanced the growth of low-silica rice mutant independent of silica deposition, and suggested that the accumulated silicon in cell walls might play an important physiological role. Ghareeb et al. (2011) suggested that Si induced bacterial wilt resistance in tomato by priming plants, and they also suggested that the priming effects were mediated via ethylene, jasmonic acid and/or reactive oxygen species. These studies imply that silicon deposited in cell walls may regulate solute transport through physical or physiological mechanism(s). In this study, there is a possibility that silicon affected the cell wall properties and thus regulated the assimilate transportation, while a direct involvement of silicon in regulating assimilate transport and its physiological metabolism can not be excluded either. However, before a conclusion can be drawn, further studies are needed to explore the action mode of silicon in plants.
Conclusions
Salt stress caused the accumulation of photosynthetic assimilates in the leaves and decreased assimilate export to the roots. These caused the inhibition of photosynthetic performance and induced ROS accumulation, and therefore reduced the growth of cucumber plants. Silicon improved the growth of cucumber plants under salt stress by regulating assimilate transport and allocation: silicon-mediated decrease of assimilate accumulation in leaves may contribute to the alleviation of photosynthetic feedback repression; while silicon-enhanced assimilate transport provides more energy storage in the roots, which is beneficial for plant growth and stress tolerance under stress conditions. Our results may help to understand the mechanism for silicon-mediated salt tolerance in plants.
References
Alaoui-Sossé B, Genet P, Vinit-Dunand F, Toussaint M, Epron D, Badot P (2004) Effect of copper on growth in cucumber plants (Cucumis sativus) and its relationships with carbohydrate accumulation and changes in ion contents. Plant Sci 166:1213–1218
Ashraf M, Rahmatullah AM, Ahmad R, Mujeeb F, Sarwar A, Ali L (2010) Alleviation of detrimental effects of NaCl by silicon nutrition in salt-sensitive and salt-tolerant genotypes of sugarcane (Saccharum officinarum L.). Plant Soil 326:381–391
Baker NR (2008) Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113
Balibrea ME, Dell’Amico J, Bolarín MC, Pérez-Alfocea F (2000) Carbon partitioning and sucrose metabolism in tomato plants growing under salinity. Physiol Plant 110:503–511
Balibrea ME, Santa Cruz AM, Bolarín MC, Pérez-Alfocea F (1996) Sucrolytic activities in relation to sink strength and carbohydrate composition in tomato fruit growing under salinity. Plant Sci 118:47–55
Ballicora MA, Iglesias AA, Preiss J (2004) ADP-Glucose pyrophosphorylase: a regulatory enzyme for plant starch synthesis. Photosynth Res 79:1–24
Basu PS, Ali M, Chaturvedi SK (2007) Osmotic adjustment increases water uptake, remobilization of assimilates and maintains photosynthesis in chickpea under drought. Indian J Exp Biol 45:261–267
Bhatia S, Asthir B (2014) Calcium mitigates heat stress effect in wheat seeding growth by altering carbohydrate metabolism. Indian J Plant Physiol 19:138–143
Blumwald E (2000) Sodium transport and salt tolerance in plants. Curr Opin Cell Biol 12:431–434
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cardemil L, Varner JE (1985) Starch degradation metabolism towards sucrose synthesis in germinating Araucaria araucana seeds. Plant Physiol 76:1047–1054
Chen D, Yin L, Deng X, Wang S (2014) Silicon increases salt tolerance by influencing the two-phase growth response to salinity in wheat (Triticum aestivum L.). Acta Physiol Plant 36:2531–2535
Chen W, Yao X, Cai K, Chen J (2011) Silicon alleviates drought stress of rice plants by improving plant water status, photosynthesis and mineral nutrient absorption. Biol Trace Elem Res 142:67–76
Chinnusamy V, Jagendorf A, Zhu JK (2005) Understanding and improving salt tolerance in plants. Crop Sci 45:437–448
Choudhury B, Mitra S, Biswas AK (2010) Regulation of sugar metabolism in rice (Oryza sativa L.) seedlings under arsenate toxicity and its improvement by phosphate. Physiol Mol Biol Plants 16:59–68
Datta R, Selvi MT, Seetharama N, Sharma R (1999) Stress-mediated enhancement of β-amylase activity in pearl millet and maize leaves is dependent on light. J Plant Physiol 154:657–664
Detmann KC, Araujo WL, Martins SC, Sanglard LM, Reis JV, Detmann E, Rodrigues FA, Nunes-Nesi A, Fernie AR, DaMatta FM (2012) Silicon nutrition increases grain yield, which, in turn, exerts a feed-forward stimulation of photosynthetic rates via enhanced mesophyll conductance and alters primary metabolism in rice. New Phytol 196:752–762
Diogo RVC, Wydra K (2007) Silicon-induced basal resistance in tomato against Ralstonia solanacearum is related to modification of pectic cell wall polysaccharide structure. Physiol Mol Plant 70:120–129
Dong C, Wang X, Shang Q (2011) Salicylic acid regulates sugar metabolism that confers tolerance to salinity stress in cucumber seedlings. Sci Hortic 129:629–636
Duffus C, Rosie R (1973) Starch hydrolysing enzymes in developing barley grain. Planta 109:153–160
Esmaeili S, Salehi H, Eshghi S (2015) Silicon ameliorates the adverse effects of salinity on turfgrass growth and development. J Plant Nutr 38:1885–1901
Gao X, Zou C, Wang L, Zhang F (2004) Silicon improves water use efficiency in maize plants. J Plant Nutr 27:1457–1470
Geigenberger P, Stitt M (1993) Sucrose synthase catalyses a readily reversible reaction in vivo in developing potato tubers and other plant tissues. Planta 189:329–339
Ghareeb H, Bozsó Z, Ott PG, Repenning C, Stahl F, Wydra K (2011) Transcriptome of silicon-induced resistance against Ralstonia solanacearum in the silicon non-accumulator tomato implicates priming effect. Physiol Mol Plant Pathol 75:83–89
Gil R, Boscaiu M, Lull C, Bautista I, Lid NA, Vicente O (2013) Are soluble carbohydrates ecologically relevant for salt tolerance in halophytes? Funct Plant Biol 40:805–808
Goldschmidt EE, Huber SC (1992) Regulation of photosynthesis by end-product accumulation in leaves of plants storing starch, sucrose, and hexose sugars. Plant Physiol 99:1443–1448
Golldack D, Lüking I, Yang O (2011) Plant tolerance to drought and salinity: stress regulating transcription factors and their functional significance in the cellular transcriptional network. Plant Cell Rep 30:1383–1391
Gong H, Randall DP, Flowers TJ (2006) Silicon deposition in the root reduces sodium uptake in rice (Oryza sativa L.) seedlings by reducing bypass flow. Plant Cell Environ 29:1970–1979
Gong H, Zhu X, Chen K, Wang S, Zhang C (2005) Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci 169:313–321
Gorbe E, Calatayud A (2012) Applications of chlorophyll fluorescence imaging technique in horticultural research: a review. Sci Hortic 138:24–35
Gunes A, Pilbeam DJ, Inal A, Bagci EG, Coban S (2007) Influence of silicon on antioxidant mechanisms and lipid peroxidation in chickpea (Cicer arietinum L.) cultivars under drought stress. J Plant Interact 2:105–113
Guntzer F, Keller C, Meunier J (2012) Benefits of plant silicon for crops: a review. Agron Sustain Dev 32:201–213
Gurmani AR, Bano A, Ullah N, Khan H, Jahangir M, Flowers TJ (2013) Exogenous abscisic acid (ABA) and silicon (Si) promote salinity tolerance by reducing sodium (Na+) transport and bypass flow in rice (Oryza sativa indica). Aust J Crop Sci 7:1219–1226
Han S, Chen L, Jiang H, Smith BR, Yang L, Xie C (2008) Boron deficiency decreases growth and photosynthesis, and increases starch and hexoses in leaves of citrus seedlings. J Plant Physiol 165:1331–1341
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circular. California Agricultural Experiment Station, Berkeley, 347
Hu L, Xiang L, Zhang L, Zhou X, Zou Z, Hu X (2014) The photoprotective role of spermidine in tomato seedlings under salinity-alkalinity stress. PLoS One 9:110855–110855
Huang Y, Bie ZL, Liu ZX, Zhen A, Wang WJ (2009) Protective role of proline against salt stress is partially related to the improvement of water status and peroxidase enzyme activity in cucumber. Soil Sci Plant Nutr 55:698–704
Hubbard NL, Huber SC, Pharr DM (1989) Sucrose phosphate synthase and acid invertase as determinants of sucrose concentration in developing muskmelon (Cucumis melo L.) fruits. Plant Physiol 91:1527–1534
Huber SC, Huber JL (1996) Role and regulation of sucrose-phosphate synthase in higher plants. Ann Rev Plant Physiol Mol Bio 47:431–444
Hütsch BW, Jung S, Schubert S (2015) Comparison of salt and drought - stress effects on maize growth and yield formation with regard to acid invertase activity in the kernels. J Agron Crop Sci 201:353–367
Isa M, Bai S, Yokoyama T, Ma JF, Ishibashi Y, Yuasa T, Iwaya-Inoue M (2010) Silicon enhances growth independent of silica deposition in a low-silica rice mutant, lsi1. Plant Soil 331:361–375
Jouyban Z (2012) The effects of salt stress on plant growth. Tech J Engin App Sci 2:7–10
Khelil A, Menu T, Ricard B (2007) Adaptive response to salt involving carbohydrate metabolism in leaves of a salt-sensitive tomato cultivar. Plant Physiol Biochem 45:551–559
Kingston-Smith AH, Walker RP, Pollock CJ (1999) Invertase in leaves: conundrum or control point? J Exp Bot 50:735–743
Li H, Zhu Y, Hu Y, Han W, Gong H (2015a) Beneficial effects of silicon in alleviating salinity stress of tomato seedlings grown under sand culture. Acta Physiol Plant. doi:10.1007/s11738-015-1818-7
Li T, Heuvelink E, Marcelis LFM (2015b) Quantifying the source-sink balance and carbohydrate content in three tomato cultivars. Front Plant Sci 6:416
Li Z, Jing W, Peng Y, Zhang XQ, Ma X, Huang LK, Yan Y (2015c) Spermine alleviates drought stress in white clover with different resistance by influencing carbohydrate metabolism and dehydrins synthesis. PLoS One 10:e0120708
Liang YC (1998) Effects of Si on leaf ultrastructure, chlorophyll content and photosynthetic activity in barley under salt stress. Pedosphere 8:289–296
Liang Y, Sun W, Zhu Y, Christie P (2007) Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: A review. Environ Pollut 147:422–428
Liu P, Yin L, Wang S, Zhang M, Deng X, Zhang S, Tanaka K (2015) Enhanced root hydraulic conductance by aquaporin regulation accounts for silicon alleviated salt-induced osmotic stress in Sorghum bicolor L. Environ Exp Bot 111:42–51
Ma JF (2004) Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci Plant Nutr 50:11–18
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence - a practical guide. J Exp Bot 51:659–668
Ming DF, Pei ZF, Naeem MS, Gong HJ, Zhou WJ (2012) Silicon alleviates PEG-induced water-deficit stress in upland rice seedlings by enhancing osmotic adjustment. J Agron Crop Sci 198:14–26
Miron D, Schaffer AA (1991) Sucrose phosphate synthase, sucrose synthase, and invertase activities in developing fruit of Lycopersicon esculentum Mill. and the sucrose accumulating Lycopersicon hirsutum Humb. and Bonpl. Plant Physiol 95:623–627
Mittler R (2006) Abiotic stress, the field environment and stress combination. Trends Plant Sci 11:15–19
Muller-Rober BT, Kossmann J, Hannah LC, Willmitzer L, Sonnewald U (1990) One of two different ADP-glucose pyrophosphorylase genes from potato responds strongly to elevated levels of sucrose. Mol Gen Genet 224:136–146
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60:324–349
Parvaiz A, Satyawati S (2008) Salt stress and phyto-biochemical responses of plants-a review. Plant Soil Environ 54:89–99
Pei ZF, Ming DF, Liu D, Wan GL, Geng XX, Gong HJ, Zhou WJ (2010) Silicon improves the tolerance to water-deficit stress induced by polyethylene glycol in wheat (Triticum aestivum L.) seedlings. J Plant Growth Regul 29:106–115
Pelleschi S, Rocher JP, Prioul JL (1997) Effect of water restriction on carbohydrate metabolism and photosynthesis in mature maize leaves. Plant Cell Environ 20:493–503
Ranwala AP, Miller WB (1998) Sucrose-cleaving enzymes and carbohydrate pools in Lilium longiflorum floral organs. Physiol Plant 103:541–550
Richter JA, Erban A, Kopka J, Zörb C (2015) Metabolic contribution to salt stress in two maize hybrids with contrasting resistance. Plant Sci 233:107–115
Riesmeier JW, Willmitzer L, Frommer WB (1994) Evidence for an essential role of the sucrose transporter in phloem loading and assimilate partitioning. EMBO J 13:1–7
Rizwan M, Ali S, Ibrahim M, Farid M, Adrees M, Bharwana SA, Zia-ur-Rehman M, Qayyum MF, Abbas F (2015) Mechanisms of silicon-mediated alleviation of drought and salt stress in plants: a review. Environ Sci Pollut R 22:1–16
Rohanipoor A, Norouzi M, Moezzi A, Hassibi P (2013) Effect of silicon on some physiological properties of maize (Zea mays) under salt stress. J Biol Environ Sci 7:71–79
Romero-Aranda MR, Jurado O, Cuartero J (2006) Silicon alleviates the deleterious salt effect on tomato plant growth by improving plant water status. J Plant Physiol 163:847–855
Rosa M, Hilal M, González JA, Prado FE (2009) Low-temperature effect on enzyme activities involved in sucrose–starch partitioning in salt-stressed and salt-acclimated cotyledons of quinoa (Chenopodium quinoa Willd.) seedlings. Plant Physiol Biochem 47:300–307
Rosales MA, Rubio-Wilhelmi MM, Castellano R, Castilla N, Ruiz JM, Romero L (2007) Sucrolytic activities in cherry tomato fruits in relation to temperature and solar radiation. Sci Hortic 113:244–249
Rossel JB, Wilson IW, Pogson BJ (2002) Global changes in gene expression in response to high light in Arabidopsis. Plant Physiol 130:1109–1120
Savvas D, Giotis D, Chatzieustratiou E, Bakea M, Patakioutas G (2009) Silicon supply in soilless cultivations of zucchini alleviates stress induced by salinity and powdery mildew infections. Environ Exp Bot 65:11–17
Shi Y, Wang Y, Flowers TJ, Gong H (2013) Silicon decreases chloride transport in rice (Oryza sativa L.) in saline conditions. J Plant Physiol 170:847–853
Shi Y, Zhang Y, Yao H, Wu J, Sun H, Gong H (2014) Silicon improves seed germination and alleviates oxidative stress of bud seedlings in tomato under water deficit stress. Plant Physiol Biochem 78:27–36
Shu S, Yuan L, Guo S, Sun J, Yuan Y (2013) Effects of exogenous spermine on chlorophyll fluorescence, antioxidant system and ultrastructure of chloroplasts in Cucumis sativus L. under salt stress. Plant Physiol Biochem 63:209–216
Sonobe K, Hattori T, An P, Tsuji W, Eneji AE, Kobayashi S, Kawamura Y, Tanaka K, Inanaga S (2011) Effect of silicon application on sorghum root responses to water stress. J Plant Nutr 34:71–82
Soylemezoglu G, Demir K, Inal A, Gunes A (2009) Effect of silicon on antioxidant and stomatal response of two grapevine (Vitis vinifera L.) rootstocks grown in boron toxic, saline and boron toxic-saline soil. Sci Hortic 123:240–246
Stitt M (1991) Rising CO2 levels and their potential significance for carbon flow in photosynthetic cells. Plant Cell Environ 14:741–762
Tetlow IJ (2004) Recent developments in understanding the regulation of starch metabolism in higher plants. J Exp Bot 55:2131–2145
Todaka D, Matsushima H, Morohashi Y (2000) Water stress enhances beta-amylase activity in cucumber cotyledons. J Exp Bot 51:739–745
Tuna AL, Kaya C, Higgs D, Murillo-Amador B, Aydemir S, Girgin AR (2008) Silicon improves salinity tolerance in wheat plants. Environ Exp Bot 62:10–16
Tymowska-Lalanne Z, Kreis M (1998) Expression of the Arabidopsis thaliana invertase gene family. Planta 207:259–265
Vaculík M, Pavlovič A, Lux A (2015) Silicon alleviates cadmium toxicity by enhanced photosynthetic rate and modified bundle sheath’s cell chloroplasts ultrastructure in maize. Ecotoxicol Environ Saf 120:66–73
Vinit-Dunand F, Epron D, Alaoui-Sossé B, Badot P (2002) Effects of copper on growth and on photosynthesis of mature and expanding leaves in cucumber plants. Plant Sci 163:53–58
Vu JCV, Niedz RP, Yelenosky G (1993) Glycerol stimulation of chlorophyll synthesis, embryogenesis, and carboxylation and sucrose metabolism enzymes in nucellar callus of ‘Hamlin’ sweet orange. Plant Cell Tiss Org 33:75–80
Wang B, Ma M, Lu H, Meng Q, Li G, Yang X (2015b) Photosynthesis, sucrose metabolism, and starch accumulation in two NILs of winter wheat. Photosynth Res 126:363–373
Wang S, Liu P, Chen D, Yin L, Li H, Deng X (2015a) Silicon enhanced salt tolerance by improving the root water uptake and decreasing the ion toxicity in cucumber. Front Plant Sci 6:759
Wei W, Li QT, Chu YN, Reiter RJ, Yu XM, Zhu DH, Zhang WK, Ma B, Lin Q, Zhang JS, Chen SY (2015) Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J Exp Bot 66:695–707
Winter H, Huber SC (2000) Regulation of sucrose metabolism in higher plants: localization and regulation of activity of key enzymes. Crit Rev Biochem Mol Biol 35:253–289
Wu J, Guo J, Hu Y, Gong H (2015) Distinct physiological responses of tomato and cucumber plants in silicon-mediated alleviation of cadmium stress. Front Plant Sci 6:453
Xu CX, Ma YP, Liu YL (2015) Effects of silicon (Si) on growth, quality and ionic homeostasis of aloe under salt stress. S Afr J Bot 98:26–36
Yang J, Zhang J, Wang Z, Zhu Q (2001) Activities of starch hydrolytic enzymes and sucrose-phosphate synthase in the stems of rice subjected to water stress during grain filling. J Exp Bot 52:2169–2179
Yin L, Wang S, Li J, Tanaka K, Oka M (2013) Application of silicon improves salt tolerance through ameliorating osmotic and ionic stresses in the seedling of Sorghum bicolor. Acta Physiol Plant 35:3099–3107
Yin L, Wang S, Tanaka K, Fujihara S, Itai A, Den X, Zhang S (2016) Silicon-mediated changes in polyamines participate in silicon-induced salt tolerance in Sorghum bicolor L. Plant Cell Environ 39:245–258
Yuan Y, Zhong M, Shu S, Du N, He L, Yuan L, Sun J, Guo S (2015) Effects of exogenous putrescine on leaf anatomy and carbohydrate metabolism in cucumber (Cucumis sativus L.) under salt stress. J Plant Growth Regul 34:451–464
Zhang JY, Wu FZ (2009) Effects of salt stress on chlorophyll content and chloroplast ultrastructure of different salt-tolerant cucumber varieties. China Vegetables 10:13–16 (in Chinese with English abstract)
Zhang RH, Li J, Guo SR, Tezuka T (2009) Effects of exogenous putrescine on gas-exchange characteristics and chlorophyll fluorescence of NaCl-stressed cucumber seedlings. Photosynth Res 100:155–162
Zheng X, Tian S (2006) Effect of oxalic acid on control of postharvest browning of litchi fruit. Food Chem 96:519–523
Zhu Y, Gong H (2014) Beneficial effects of silicon on salt and drought tolerance in plants. Agron Sustain Dev 34:455–472
Zhu Y, Xu X, Hu Y, Han W, Yin J, Li H, Gong H (2015) Silicon improves salt tolerance by increasing root water uptake in Cucumis sativus L. Plant Cell Rep 34:1629–1646
Zhu ZJ, Wei GQ, Li J, Qian QQ, Yu JP (2004) Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci 167:527–533
Acknowledgments
This study was supported by the National Natural Science Foundation of China (31471866, 31272152, 31501751).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jian Feng Ma.
Y. Zhu and J. Guo contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhu, Y., Guo, J., Feng, R. et al. The regulatory role of silicon on carbohydrate metabolism in Cucumis sativus L. under salt stress. Plant Soil 406, 231–249 (2016). https://doi.org/10.1007/s11104-016-2877-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-2877-2