Abstract
The present study aimed at investigating the effects of foliar applied nitric oxide (as SNP [sodium nitroprusside]) on sulfur (glutathione reductase, guaiacol peroxidase, and glutathione S-transferase) and nitrate assimilation (nitrite and nitrate reductase) pathway enzymes in maize (Zea mays L.) exposed to water deficit conditions. The seedlings of a drought tolerant (NK8711) and sensitive (P1574) maize hybrid were applied with various SNP doses (0, 50, 100, 150, and 200 µM) under normal and drought stress conditions. Foliar spray of 100 µM markedly improved water status and chlorophyll contents and alleviated drought-induced oxidative damages through increased antioxidant (catalase, ascorbate peroxidase, and superoxide dismutase) activities in both maize hybrids. Moreover, exogenous SNP supply increased nitrite and nitrate reductase activities and upregulated glutathione reductase, glutathione S-transferase, and guaiacol peroxidase compared to no SNP supply. Interestingly, the negative effects of excess NO generation at high SNP doses (150, 200 µM) were more pronounced in P1574 than NK8711 leading to lower biomass accumulation in drought-sensitive hybrid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water scarcity is a major factor that causes extensive losses to agricultural production worldwide (Liu et al. 2017). The scant precipitation and non-uniform distribution of rainfall, particularly in arid–semiarid regions, is supposed to cause more than 30% reduction in global crop production by 2025 (Neufeldt et al. 2013). Plants respond to severe drought through adaptation on morphological, physiological to molecular level (Daryanto et al. 2016; Fahad et al. 2017). Limited water availability triggers generation of toxic reactive oxygen species (ROS) in various cellular compartments (Kaur and Asthir 2017). The development of strategic defense mechanisms, including antioxidant system and diverse stress-responsive signal transduction pathways, has enabled plants to successfully adapt and survive limited water conditions (Forni et al. 2017; Nawaz et al. 2017). Drought stress induces metabolic adjustments and influences gene regulatory network to stimulate production of several antistress-signalling molecules and compounds such as glycinebetaine, abscisic acid, ethylene, and nitric oxide (NO) in plants (Peñuelas et al. 2013). Moreover, plant species or even cultivars of the same species differ in their ability to tolerate drought stress, achieved mainly by activation of antioxidant machinery as reported in tolerant and sensitive maize (Azooz et al. 2009), wheat (Shabbir et al. 2016), and sesame cultivars (Kadkhodaie et al. 2014).
During the past few years, NO-regulated mechanisms have been subject of interest for researchers studying acclimation responses of plants in relation with abiotic stresses including drought (Li et al. 2013; Boogar et al. 2014; Cechin et al. 2015). Several artificial NO donors such as diethylamine (DEA) and sodium nitroprusside (SNP) have been used to study the natural production pathways of NO in plants (Fu et al. 2010; Kaur et al. 2015). All of these NO generators, however, have drawbacks, such as DEA generates a rapid NO burst, which fades out within seconds to minutes. By contrast, SNP dissociation into NO is low but long lasting, which also depends on pH of the medium as well as light intensity and quality (Gupta et al. 2011). Supplemental SNP serves as a stress-signalling molecule that upregulates antioxidant machinery to mitigate the damaging effects of salts (Ahmad et al. 2016), metals (Kharbech et al. 2017), hypoxia (Peng et al. 2016), high (Li et al. 2013) or low temperature (Amooaghaie and Nikzad 2013) and drought stress (Zhang et al. 2016a, b).
Nitrate reductase (NR) pathway is the best-characterized enzymatic pathway for NO production (Gupta et al. 2011), however; reports regarding effects of exogenous SNP (as NO donor) supply on nitrate assimilation pathway under water deficit conditions are scant. Studies involving wheat (Rosales et al. 2011) and Chlamydomonas reinhardtii (Sanz-Luque et al. 2013) showed that SNP application markedly inhibited NR activity in these species. Contrarily, it promoted the enzymatic activity in tomato roots at low levels (Jin et al. 2009), suggesting that it may either act as an antioxidant or may become pro-oxidant at high doses. In soybean, application of high SNP dose (1 mM) markedly decreased cell viability and inhibited root growth (Böhm et al. 2010). Similarly, Tian and Lei (2006) found that high SNP dose (2 mM) promoted lipid peroxidation and increased H2O2 accumulation to inhibit wheat growth. These reports clearly indicate the importance of SNP optimization before application to prevent the toxic effects of NO on plant species.
Considering the previous knowledge on SNP (as NO donor) regulated stress tolerance mechanisms in plants, the aim was to uncover the effects of NO on antioxidant and nitrate assimilation pathway enzymes in maize seedlings exposed to drought stress. Hence, this work was designed with the hypotheses that (1) does exogenous application of NO donor (SNP) successfully mitigates drought-induced oxidative stress in maize? (2) If yes, then how does drought tolerant and susceptible genotypes respond to different levels of SNP? And finally (3) How low or high SNP doses affect the sulfur and nitrate assimilation pathway enzymes under drought stress conditions? In this study, we showed how different doses of exogenous NO source (SNP) influence the enzymatic activities of a drought tolerant (NK8711) and sensitive (P1574) maize hybrids under normal and drought stress conditions.
Materials and methods
Plant material and experimental conditions
Indigenous maize hybrids available from different private seed agencies like Syngenta, Monsanto, and Pioneer were obtained and initially screened out for drought stress tolerance (data not presented). Two maize hybrids viz. NK8711 (Syngenta Pvt. Ltd.) and P1574 (Pioneer Pvt. Ltd.) were identified as the most drought tolerant and sensitive genotypes, respectively, and were selected for the present study. Healthy, physically pure, randomly selected seeds of each hybrid were surface sterilized with 5% sodium hypochlorite solution and grown in plastic pots of 12 kg capacity at 25/16 °C (day and night) with 16-h photoperiod in a growth chamber under semi-controlled environment. Plants were watered with Hoagland nutrient solution at the start of the experiment containing 4.5 mM NH4NO3, 2.5 mM K2HPO4, 1.5 mM K2SO4, 2.5 mM CaCl2·2H2O, 0.25 µM CuSO4·5H2O, 1.0 µM ZnSO4·7H2O, 0.4 µM (NH4)6Mo7O24·4H2O, 1.2 mM MgSO4, and 3.0 µM Fe-EDDHA (Sánchez-Aguayo et al. 2004). The experimental layout was randomized complete block design (RCBD) with three repeats. Each repeat comprised of five seedlings in a pot.
Drought stress and SNP treatments
Initially, all pots were saturated with distilled water and kept for 24 h to drain off the excess water under gravitational effect before sowing. The seeds were allowed to germinate under normal conditions for 1 week. Drought stress was imposed at V2 stage (i.e., 8th day of seed emergence) by withholding water to one set of pots, whereas the other set was regularly watered and served as a control for comparison. Amount of water evaporated was calculated using ML3—ThetaProbe Soil Moisture Sensor (Suppl. Figure 1) and control plants were re-watered accordingly.
Foliar spray treatments (0, 50, 100, 150, and 200 µM) were developed by dissolving sodium nitroprusside dihydrate (Na2 [Fe (CN)5 NO] 2H2O; purity ≥ 98.0%; Mol. wt. 297.95; Sigma-Aldrich Ltd., USA) in distilled water. Water spray was used as a control. All the treatments were added with 0.1% Tween-20 (v/v) to enhance fluid retention on leaf. Foliar spray was carried out at V3 stage (15th day of seed emergence) and repeated after 1 week. The leaf samples were collected after second foliar spray for the estimation of physiological and biochemical attributes. After 4 weeks, at appearance of wilting symptoms in stressed plants, the plants were harvested for the estimation of biomass parameters. Dry matter content was obtained by keeping the harvested seedlings in an oven at 65 °C for 72 h. The observed phenotypes of both maize hybrids supplemented with various SNP doses under drought stress conditions are given in Fig. 1.
Measurement of morphological and physiological indices
The physiological indices corresponding to plant height (PHSI), root length (RLSI), shoot and root fresh weight (SFSI, RFSI), and dry matter (DMSI) were calculated using the following formulae reported by Kausar et al. (2012):
where Ps, Rs, Ss, Fs, and Ds represent plant height, root length, shoot fresh weight, root fresh weight, and dry matter of stressed plants, respectively. Similarly, Pc, Rc, Sc, Fc, and Dc indicate the plant height, root length, shoot fresh weight, root fresh weight, and dry matter of normal or control plants, respectively.
Determination of leaf water status and chlorophyll contents
To estimate leaf relative water content (RWC), the youngest leaf from each treatment was weighed immediately (FW) and then soaked in deionized water at 4 °C for 24 h to record turgid weight (TW). The leaves were later incubated in an oven at 65 °C for 72 h to obtain dry weight (DW). The RWC was estimated using following formula proposed by Mayak et al. (2004):
For determination of excised leaf water loss (ELWL), the fully expanded youngest leaf from each group was immediately weighed (FW), incubated at room temperature for 6 h to record wilted weight (LW), and later oven dried at 65 °C for 72 h to obtain DW as described by Clarke (1987):
A portable chlorophyll meter viz. SPAD-502 (Konica Minolta, Tokyo, Japan) was used to estimate the leaf chlorophyll contents. Three plants in each pot were selected and the values were recorded from the two fully expanded uppermost leaves of each plant. The average of six SPAD values was considered as chlorophyll content of plants in each repeat.
Biochemical assays
Fully expanded, healthy and fresh leaves (second from the top) from each repeat were sampled at V5 stage (i.e., 22nd day after seed germination) to determine the ROS, NO, antioxidant, and nitrate assimilation pathway enzymes. The leaf tissues were immediately frozen in liquid N2 and later kept at − 80 °C until biochemical analyses.
Estimation of H2O2, MDA and NO content
The ability of the leaf samples (crude plant extract) to scavenge H2O2 was assessed by the method of Ruch et al. (1989). The H2O2 content was measured using an extinction coefficient (ε = 0.28 mM−1 cm−1) and expressed as nmol of H2O2 scavenged g−1 DW.
The level of lipid peroxidation was estimated as malondialdehyde (MDA content) following Cakmak and Horst (1991). The MDA content was estimated using its absorption coefficient (ε = 550 mM−1 cm−1) and expressed as nmol g−1 fresh mass according to the following formula:
where V represents the volume of crushing medium, W indicates leaf fresh weight, and A600 and A532 represent the absorbance at 600 and 532 nm wavelength, respectively
Reports published by Hu et al. (2003) and Ding et al. (1998) were used for the determination of NO content. Young leaves weighing 0.5 g were ground and homogenized in 3 ml of cool acetic acid buffer (50 mM), prepared by adding 4% zinc diacetate, with pH 3.6. The homogenate was cold centrifuged (4 °C) at 10,000×g for 15 min and 0.1 g charcoal was added in the supernatant. The filtrate (1 ml) collected after filtration and vortex was mixed with Greiss reagent (1 ml) and incubated at 25 °C for 30 min. Absorbance of solution was read at 540 nm and a standard curve was developed to calculate NO content using NaNO2.
Assessment of antioxidant activities
The antioxidant activities were measured according to Venisse et al. (2001). Leaf samples (1.0 g) were ground and homogenized in a cold room (4 °C) with 10 ml of 50 mM cool sodium phosphate buffer (pH 7.5) containing polyethyleneglycol (1 mM), phenylmethylsulfonyl fluoride (1 mM), polyvinylpyrrolidone (8%), and Triton X-100 (0.01%). The homogenate was cold centrifuged (10,000×g) at 4 °C for 20 min. The supernatant (protein extract) was separated to quantify different enzyme activities at 25 °C using UV plate reader (96-well), Synergy HT, Biotek Instrument, USA.
The enzymatic activity of catalase (CAT) activity was assayed following Chance and Maehly (1955), whereas the reports of Elia et al. (2003) and Nakano and Asada (1981) were used to estimate ascorbate peroxidase (APX) and guaiacol peroxidase (GPX) activities expressed as µmol ascorbate min−1 mg−1 protein and µmol guaiacol min−1 mg−1 protein, respectively. The procedure reported by Ekler et al. (1993) was followed to record superoxide dismutase (SOD) activity measured as SOD mediated inhibition of photochemical reduction of nitro blue tetrazolium. The lipoxygenase (LOX) activity was determined as proposed by Anthon and Barrett (2001), whereas enzymatic activities of glutathione S-transferase (GST) and glutathione reductase (GR) were estimated according to the methods of Foyer and Halliwell (1976) and Habig et al. (1974), respectively.
Determination of NR and NiR activity
The enzymatic activity of nitrate reductase (NR) in leaf samples was measured according to the procedure of Sym (1984) using KNO3 as a substrate, whereas the method reported by Ramarao et al. (1983) was used to determine nitrite reductase (NiR) activity using NaNO2 as a substrate. The NR and NiR activities were expressed as µmol NO2 g−1 DW min−1.
Statistical analysis
The data collected consisted of the mean values obtained from experiment repeated three times. The statistical analysis was performed by STATISTICA Computer Program (Version 8.1) using ANOVA (analysis of variance) technique and mean values were compared by post-hoc Tukey test at 5% probability level.
Results
Effect on morphological and physiological indices
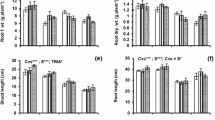
Foliar SNP spray significantly affected (P ≤ 0.05) the morphological and physiological indices of maize seedlings; however, both maize genotypes showed differential response to exogenous NO supply (Suppl. Table 1). Drought-tolerant hybrid NK8711 maintained significantly (P ≤ 0.05) higher SFSI (22%), RFSI (54%), RLSI (19%), and DMSI (23%) than drought-sensitive P1574 hybrid (Fig. 2b–e). Compared with the control (water spray), foliar treatment of SNP at 100 µM gave significantly higher PHSI (24%), SFSI (69%), RFSI (65%), and DMSI (26%), whereas the seedlings sprayed with SNP at 150 µM exhibited maximum increase (34%) in RLSI (Fig. 2a–e).
Effect of exogenous SNP (as NO donor) foliar spray on Plant height stress tolerance index (a), root length stress tolerance index (b), shoot fresh weight stress tolerance index (c), root fresh weight stress tolerance index (d), and dry matter stress tolerance index (e) of 4-week-old seedlings of drought tolerant (NK8711) and sensitive (P1574) maize (Zea mays L.) hybrids under normal and drought stress conditions. Capped bars above means represent standard error values (± SE) of three repeats. Small alphabets above means represent significant differences (P ≤ 0.05) among treatments
Effect on leaf water status and chlorophyll contents
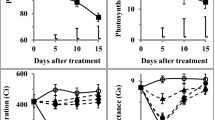
The leaf chlorophyll and water contents decreased significantly (P ≤ 0.01) in plants exposed to drought stress (Suppl. Table 2). Foliar SNP treatment at 100 µM considerably (P ≤ 0.001) increased (20%) leaf RWC; however, leaf water contents were markedly reduced in seedlings sprayed with 200 µM SNP (Fig. 3a). A marked reduction (P ≤ 0.01) in ELWL (80%) was recorded in seedlings sprayed with SNP at 100 µM as compared to water spray (Fig. 3b). Exogenous NO supply significantly improved leaf chlorophyll contents (13%) in seedlings sprayed with SNP at 100 µM; however, chlorophyll contents remain unchanged at higher or lower SNP doses as compared to control (Fig. 3c). Drought-tolerant hybrid NK8711 showed significantly higher chlorophyll contents (7%) and lower ELWL (8%) than drought-sensitive P1574 hybrid (Fig. 3b, c). Non-significant interaction was noted among water stress (W), NO foliar spray (N), and maize hybrids (G) for RWC and chlorophyll content (Suppl. Table 2).
Effect of exogenous SNP (as NO donor) foliar spray on leaf relative water contents (a), excised leaf water loss (b), and chlorophyll contents (c) of 4-week-old seedlings of drought tolerant (NK8711) and sensitive (P1574) maize (Zea mays L.) hybrids under normal and drought stress conditions. Capped bars above means represent standard error values (± SE) of three repeats. Small alphabets above means represent significant differences (P ≤ 0.05) among treatments
Effect on lipid peroxidation, H2O2, NO, and LOX
Drought stress triggered lipid peroxidation (57%) and strongly increased the H2O2 production (156%) in both maize hybrids. Application of SNP considerably reduced (P ≤ 0.01) ROS generation by decreasing MDA (54%) and H2O2 (88%) content in water-stressed seedlings supplemented with SNP at 100 µM compared to control (Fig. 4a, b). H2O2 production was the highest (17.87 nmol g−1 FW) in P1574 seedlings supplemented with 200 µM; however, both maize hybrids differed non-significantly for MDA content at various SNP concentrations (Fig. 4b).
Effect of exogenous SNP (as NO donor) foliar spray on lipid peroxidation (a), H2O2 (b), NO contents (c), and lipoxygenase activity (d) of 4-week-old seedlings of drought tolerant (NK8711) and sensitive (P1574) maize (Zea mays L.) hybrids under normal and drought stress conditions. Capped bars above means represent standard error values (± SE) of three repeats. Small alphabets above means represent significant differences (P ≤ 0.05) among treatments
Accumulation of NO and activities of LOX were markedly (P ≤ 0.001) increased in both maize hybrids under drought stress (Suppl. Table 2). Exposure to drought stress significantly enhanced NO concentration (108%) and LOX activity (75%) in the leaves of maize seedlings with respect to normal conditions (Fig. 4c, d). An increasing trend in leaf NO content and LOX activity was observed with increasing SNP levels. Maximum NO synthesis was recorded (71.96 and 69.55 nmol g−1 DW) in P1574 plants supplemented with SNP at 150 and 200 µM, respectively, under drought stress conditions (Fig. 4c). Similarly, the highest LOX activity (52.67 µmol min−1 mg−1 protein) was recorded in P1574 seedlings sprayed with SNP at 200 µM. Foliar SNP spray at 100 µM resulted in the lowest (33%) enzymatic activity of LOX in both maize hybrids with respect to no SNP supply (Fig. 4d).
Effect on enzymatic activities
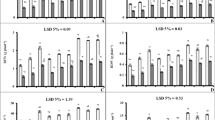
Drought stress markedly influenced the enzymatic activities of antioxidants in maize seedlings (Suppl. Table 3). Compared with normal seedlings, activities of CAT, GPX, SOD, and APX were considerably increased by 137, 148, 131, and 346%, respectively, in maize seedlings exposed to drought stress. The seedlings treated with SNP at 100 µM showed further increase (P ≤ 0.001) in CAT (45%), GPX (47%), SOD (53%), and APX (111%) activity, whereas treatment with the highest SNP level (200 µM) reduced CAT, SOD, and APX activities by 25, 47, and 48%, respectively, as compared to control (water spray). Interestingly, GPX activity remained unchanged at higher SNP doses of 150 and 200 µM (Fig. 5a–d). Sharp increases in GR (148%) and GST (315%) content were recorded in water-stressed maize seedlings with respect to normal ones. Foliar SNP application at 100 µM significantly increased GR (55%) and GST (98%) activity compared to control, i.e., no SNP application under drought stress (Fig. 5e, f).
Effect of exogenous SNP (as NO donor) foliar spray on activities of antioxidative and sulfur assimilation pathway enzymes viz. catalase (a), guaiacol peroxidase (b), superoxide dismutase (c), ascorbate peroxidase (d), glutathione reductase (e), and glutathione S-transferase (f) in 4-week-old seedlings of drought tolerant (NK8711) and sensitive (P1574) maize (Zea mays L.) hybrids under normal and drought stress conditions. Capped bars above means represent standard error values (± SE) of three repeats. Small alphabets above means represent significant differences (P ≤ 0.05) among treatments
Enzymatic activities of NR and NiR were markedly reduced (P ≤ 0.01) under drought stress. Exposure to drought stress incurred a marked decrease in NR (54%) and NiR (55%) activity compared to well-watered control. Maximum increase in NR (4.65 µmol NO2 g−1 FW min−1) and NiR (4.65 and 7.09 µmol NO2 g−1 FW min−1) activities was observed in P1574 seedlings foliar applied with 200 µM SNP under water deficit conditions (Fig. 6a, b). Drought-sensitive hybrid P1574 maintained noticeably higher CAT, APX, SOD, GR, and NiR activities than NK8711, but both hybrids differed non-significantly in terms of GPX, GST, and NR activities (Suppl. Table 3).
Effect of exogenous SNP (as NO donor) foliar spray on activities of nitrate assimilation pathway enzymes viz. nitrate reductase (a) and nitrite reductase (b) in 4-week-old seedlings of drought tolerant (NK8711) and sensitive (P1574) maize (Zea mays L.) hybrids under normal and drought stress conditions. Capped bars above means represent standard error values (± SE) of three repeats. Small alphabets above means represent significant differences (P ≤ 0.05) among treatments
Discussion
Harmful effects of drought stress on growth and dry mass accumulation in maize have been extensively studied in recent past (Quiroga et al. 2017; Anjum et al. 2017). Drought-induced production of toxic ROS results in progressive oxidative stress and damages cellular compartments (Signorelli et al. 2013). NO-mediated post-translational modification of antioxidative enzymes is considered critical to scavenge ROS in plants exposed to water deficit conditions (Begara-Morales et al. 2016). Research on NO donors has identified their positive role on germination, photosynthesis, and antioxidant activities in different stressed plants (Boogar et al. 2014; Wu et al. 2017). In this study, we highlight the differential response of maize hybrids to various SNP (used as NO donor) levels under water deficit conditions. It particularly focused the interplay between antioxidant and nitrate assimilation enzymes at various SNP levels.
Drought-induced reduction in growth of maize seedlings may be the result of reduced cell expansion and enlargement due to loss in turgor (Yagmur and Kaydan 2008). The dehydration of protoplasm (Shabbir et al. 2016) or changes in cell-wall permeability due to lipid peroxidation (Cechin et al. 2015) result in reduced plant height (measured in terms of PHSI in the present study) under water deficit conditions (Fig. 2a). In plants, loss of turgor due to stomatal closure (Nawaz et al. 2015) restricts partitioning and translocation of photosynthates (Miao et al. 2006; Hussain et al. 2016) that consequently decreases dry matter as evident by reduced DMSI. The positive effects of NO on SFSI, RFSI, DMSI, and RLSI indicate that it can stimulate root growth and seedling establishment (Yu et al. 2014; Hu et al. 2016). NO-stimulated increase in RLSI (Fig. 2b) might be the consequence of increased NR activity (as observed in present study), thereby further supporting the evidence that NR-derived NO actively participates in signalling and root tissue development (Pető et al. 2013). These data support the suggestion that NO participates in key processes responsible for primary root elongation, root hair differentiation, and development of lateral and adventitious roots (Yu et al. 2014). However, high SNP doses inhibit root elongation and stimulate lateral root formation (Correa-Aragunde et al. 2004).
The findings that higher doses (150 and 200 µM) of SNP reduced root length and seedling growth corroborate other studies that low SNP doses increase biomass and promote hypocotyl elongation during the vegetative growth phase of plants (Hebelstrup et al. 2013; Hu et al. 2016). It is noteworthy that NO effects on plant growth are concentration dependent, so care must be taken to optimize NO doses before exogenous application to plant species (Cechin et al. 2015). These findings are concurrent with the previous reports related to the dose-dependent effect of SNP (as a source of NO) in Solanum tuberosum (Beligni and Lamattina 1999), A. thaliana (He et al. 2004), Triticum aestivum (Tian and Lei 2006), Pisum sativum (Leshem 1996), and Zea mays (An et al. 2005). Maize hybrids show different degrees of response to various abiotic stresses (Fu et al. 2017; Quiroga et al. 2017). Higher accumulation of biomass in drought-tolerant (NK8711) than sensitive hybrid (P1574) suggests that variations exists among crop species or even within the species of the same crop in their response to exogenous NO supply (Lin et al. 2013).
Increasing evidence suggests that plants tend to maintain high water potential in their protoplasts to support growth and function under drought stress (Nawaz et al. 2015; Shabbir et al. 2016). The data clearly suggest that drought stress markedly reduced leaf RWC (Fig. 3a) that may have decreased leaf water potential, since there exists a positive correlation between RWC and leaf water status under limited water conditions (Askari and Ehsanzadeh 2015). Foliar SNP spray helped to achieve membrane stability due to reduced MDA content as reported earlier in T. aestivum (Bavita et al. 2012), Helianthus annuus (Cechin et al. 2015), and Oryza sativa (He et al. 2014). NO acts like a hormone and employs key physiological processes to regulate water homeostasis through osmotic adjustment under water limited environment (Misra et al. 2011; Habib et al. 2013). Exogenous SNP supply decreased ELWL (Fig. 3b), which could be explained by reduced transpiration as a consequence of ABA mediated stomatal closure. Being a stress-signalling molecule, NO regulates ABA synthesis and influences protein S-nitrosylation and Ca2+-sensitive ion channels to induce stomatal closure under water deficit conditions (Garcia-Mata et al. 2003; Sokolovski and Blatt 2004).
Foliar SNP spray (100 µM) markedly increased the chlorophyll contents of maize seedlings (Fig. 3c). These results suggest that optimum NO supply stimulates chlorophyll biosynthesis and chloroplast differentiation by increasing iron availability in guard cells (Zhang et al. 2006). Previously, SNP application was observed to protect the photosynthetic apparatus of water-stressed Poncirus trifoliata (Fan and Liu 2012) and T. aestivum (Alavi et al. 2014) seedlings. The improvement in photosynthetic performance was ascribed to NO ability to detoxify ROS that degrade pigments and cause instability of chlorophyll complexes (Simaei et al. 2012). Contrarily, Cechin et al. (2015) reported that SNP treatment failed to influence photosynthetic pigments of H. annuus seedlings. The inconsistent findings about effects of exogenous NO supply on chlorophyll contents may be related to variation in doses and time of application as well as differences in sensitivity among various crop species (Santisree et al. 2015).
Abiotic stresses including drought trigger ROS production that induces molecular damage in crop plants (Askari and Ehsanzadeh 2015; Fu et al. 2017). Drought stress disrupts cellular homeostasis and increases lipid peroxidation leading to oxidative stress in plants (Quiroga et al. 2017). In this study, increased damage to membrane stability was evident by higher levels of MDA in drought stressed than normal seedlings (Fig. 4a). Drought-induced lipid peroxidation influences the normal functioning of membranes and alters the lipid composition that negatively impacts physiological activities linked to plasma membrane in maize (Fu et al. 2017). Exogenous SNP supply helped to reduce lipid peroxidation as indicated by the low MDA contents and LOX activity in NO treated (100 µM SNP) seedlings under drought stress. It highlights the importance of NO to scavenge free radicals (Dwivedi et al. 2016), linked to lipid peroxidation, thereby increasing antioxidative ability to enhance drought tolerance in plants (Izabela et al. 2013). There was a significant reduction in LOX activity (33%) in seedlings foliar applied with 100 µM SNP in contrast to higher SNP doses (150 and 200 µM) (Fig. 4d). These results provide further evidence that high SNP doses aggravate lipid peroxidation and H2O2 production under oxidative stress conditions (Böhm et al. 2010; Lin et al. 2013). Indirect cellular damages by ROS could also be manifested by increased H2O2 (highly reactive to biomolecules or membranes and leads to OH− production) or NO production (as observed in present study) under oxidative stress conditions (Yildiztugay et al. 2014). Increased SOD activity, as a result of exogenous SNP supply, might also be the outcome of more H2O2 or NO production (Fig. 4b, c) and high LOX activity (Zhao et al. 2008), since SOD is a key player in conversion of toxic O2·− to less harmful H2O2 under water deficit conditions (Askari and Ehsanzadeh 2015). It has been shown that exogenous SNP supply enhances SOD activities that promote the conversion of O2·− to H2O2 under oxidative stress (Yildiztugay et al. 2014). Suppression of SOD activity at high doses (150 and 200 µM) could be explained by the direct scavenging of O2·− by NO (Bavita et al. 2012).
The detoxification of harmful H2O2 to H2O is accelerated by upregulation of antioxidative machinery in plant cells (Nawaz et al. 2015). CAT, GPX, APX, and GR are the most prevalent antioxidants produced in water-stressed plants (Mittler 2002). It was noted that NO donor (SNP) upregulated enzymatic activity of antioxidants supporting the evidence that NO stimulates activity of iron containing enzymes (Wang et al. 2004; Zhang et al. 2016a, b). NO-mediated alleviation of oxidative damage was evident by reduced H2O2 levels in water-stressed maize seedlings treated with 100 µM SNP. CAT activity is thought to be not induced by water stress (Smirnoff 1993), as it is located in peroxisomes and other related organelles (Wang et al. 2011). Hence, increased CAT activity (Fig. 5a) might be attributed to its ability to detoxify H2O2 in water-stressed seedlings (Wang et al. 2011). Contrarily, foliar SNP spray did not affect GPX activity in Cicer arietinum (Sheokand et al. 2010) and Z. mays (Yildiztugay et al. 2014) seedlings subjected to salinity and drought stress, respectively. This conflicting result might be related to the use of very high SNP doses (300 µM and 1 mM) by these researchers as high SNP supply may also inhibit the enzymatic activities of antioxidants (Lin et al. 2013). Foliar SNP spray (100 µM) increased GST activity (Fig. 5f), which is another important H2O2 scavenger involved in decomposition of organic hydroperoxides under oxidative stress conditions. Application of NO has been found to increase GST activity in T. aestivum (Hasanuzzaman et al. 2012) and Glycine max (Dinler et al. 2014) that might be ascribed to increased glutathione synthesis and regeneration to prevent H2O2 mediated membrane degradation.
NR is an important enzyme of nitrate assimilation pathway primarily involved in NO generation (Gupta and Kaiser 2010) due to nitrite accumulation in cells of plants exposed to various biotic and abiotic factors such as fungal attack (Srivastava et al. 2009), aphids infestation (Sytykiewicz 2014, 2016), hypoxia (Benamar et al. 2008), floral transition (Seligman et al. 2008), salinity (Hayat et al. 2012), and drought stress (Sang et al. 2008). Compared to no SNP supply, H2O2-induced enzymatic activities of antioxidants (SOD, APX, CAT, GR, and GPX) were markedly increased in maize seedlings treated with SNP (100 µM) under water deficit conditions (Fig. 5). This finding implies that drought-induced H2O2 accumulation mediates NO production and, in turn, activates protein kinases to stimulate antioxidant activity (Du et al. 2008; Wu et al. 2017). NR-mediated NO oscillation was observed to facilitate antioxidant enzymes in Ulva compressa (González et al. 2012), T. aestivum (Sun et al. 2014), and Zea mays (Zhang et al. 2007). Interestingly, high SNP dose (200 µM) markedly increased the activity of nitrite pathway enzymes (Fig. 6). These findings imply that high SNP doses facilitate endogenous NO production (as observed in present study, Fig. 4c), which in turn, stimulate the post-translational regulatory pathways of NR. An increase in NiR by SNP application (Fig. 6b) might be associated with NR catalyzed nitrite reduction due to enhancement in NR activity and nitrite production. Contrarily, Jin et al. (2009) observed that low SNP supply promoted NR activity in Solanum lycocarpum, which was significantly inhibited at high SNP doses. Likewise, Rosales et al. (2011) reported a marked decrease in NR activity of T. aestivum leaves treated with SNP dose of 500 µM. These contrasting reports suggest that the factors such as crop species, SNP levels, and environmental conditions influence NO-mediated NR activity in plants.
Concluding remarks
The studies investigating the effects of low or high SNP (as NO donor) doses on sulfur and nitrate assimilation pathway enzymes are scant. Our findings highlight the importance of NO (as SNP)-regulated enzymatic processes in improving drought tolerance in maize at early growth stages. Drought-mediated oxidative stress, evident by increased H2O2 and MDA content, markedly reduced seedling growth properties of maize. Foliar SNP treatment at 100 µM triggered sulfur and nitrate assimilation pathway enzymes to effectively ameliorate drastic effects of drought stress in maize seedlings. Interestingly, higher SNP doses (150 and 200 µM) aggravated the toxic effects of oxidative stress by increased MDA, H2O2, and NO content and enhanced the activity of LOX that inhibited the enzymatic activities of antioxidants. Differential response of maize hybrids to SNP supply supports our hypothesis and suggests that NO-mediated stress tolerance mechanisms may vary even within species of same crop and influence regulatory processes in a dose-dependent manner.
Author contribution statement
SM carried out the experimental work and performed all laboratory analyses. FN conceived the study and wrote the first draft of manuscript. MN supervised the laboratory experiments. MYA coordinated and supervised laboratory analyses. All authors contributed to the study and gave final approval to publish the manuscript in its current form.
References
Ahmad P, Latef AA, Hashem A, Abd-Allah EF, Gucel S, Tran LSP (2016) Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front Plant Sci 7:1–11
Alavi SM, Arvin MJ, Manoochehri-Kalantari K (2014) Salicylic acid and nitric oxide alleviate osmotic stress in wheat (Triticum aestivum L.) seedlings. J Plant Int 9:683–688
Amooaghaie R, Nikzad K (2013) The role of nitric oxide in priming-induced low-temperature tolerance in two genotypes of tomato. Seed Sci Res 23:123–131
An L, Liu Y, Zhang M, Chen T, Wang X (2005) Effects of nitric oxide on growth of maize seedling leaves in the presence or absence of ultraviolet-B radiation. J Plant Physiol 162:317–326
Anjum SA, Ashraf U, Tanveer M, Khan I, Hussain S, Shahzad B, Zohaib A, Abbas F, Saleem MF, Ali I, Wang LC (2017) Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front Plant Sci 8:1–12
Anthon GE, Barrett DM (2001) Colorimetric method for the determination of lipoxygenase activity. J Agric Food Chem 49:32–37
Askari E, Ehsanzadeh P (2015) Drought stress mitigation by foliar application of salicylic acid and their interactive effects on physiological characteristics of fennel (Foeniculum vulgare Mill.) genotypes. Acta Physiol Plant 37:1–14
Azooz MM, Ismail AM, Elhamd MA (2009) Growth, lipid peroxidation and antioxidant enzyme activities as a selection criterion for the salt tolerance of maize cultivars grown under salinity stress. Int J Agric Biol 11:21–26
Bavita A, Shashi B, Navtej SB (2012) Nitric oxide alleviates oxidative damage induced by high temperature stress in wheat. Indian J Exp Biol 50:372–378
Begara-Morales JC, Sánchez-Calvo B, Chaki M, Valderrama R, Mata-Pérez C, Padilla MN, Corpas FJ, Barroso JB (2016) Antioxidant systems are regulated by nitric oxide-mediated post-translational modifications (NO-PTMs). Front Plant Sci 7:152
Beligni MV, Lamattina L (1999) Nitric oxide counteracts cytotoxic processes mediated by reactive oxygen species in plant tissues. Planta 208:337–344
Benamar A, Rolletschek H, Borisjuk L, Avelange-Macherel MH, Curien G, Mostefai HA, Andriantsitohaina R, Macherel D (2008) Nitrite–nitric oxide control of mitochondrial respiration at the frontier of anoxia. Biochim Biophys Acta (BBA) Bioenerg 1777:1268–1275
Böhm FMLZ, Ferrarese MDLL, Zanardo DIL, Magalhaes JR, Ferrarese-Filho O (2010) Nitric oxide affecting root growth, lignification and related enzymes in soybean seedlings. Acta Physiol Plant 32:1039–1046
Boogar AR, Salehi H, Jowkar A (2014) Exogenous nitric oxide alleviates oxidative damage in turfgrasses under drought stress. S Afr J Bot 92:78–82
Cakmak I, Horst WJ (1991) Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83:463–468
Cechin I, Cardoso GS, Fumis TDF, Corniani N (2015) Nitric oxide reduces oxidative damage induced by water stress in sunflower plants. Bragantia 74:200–206
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. Methods Enzymol 2:764–775
Clarke JM (1987) Use of physiological and morphological traits in breeding programmes to improve drought resistance of cereals. Chapter 14. In Improving winter cereals for moisture-limiting areas, Capri (Italy), 27–31 Oct 1985. Wiley
Correa-Aragunde N, Graziano M, Lamattina L (2004) Nitric oxide plays a central role in determining lateral root development in tomato. Planta 218:900–905
Daryanto S, Wang L, Jacinthe PA (2016) Global synthesis of drought effects on maize and wheat production. PLoS One 11:e0156362. https://doi.org/10.1371/journal.pone.015636
Ding AH, Nathan CF, Stuehr DJ (1998) Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. The J Immunol 141:2407–2412
Dinler BS, Antoniou C, Fotopoulos V (2014) Interplay between GST and nitric oxide in the early response of soybean (Glycine max L.) plants to salinity stress. J Plant Physiol 171:1740–1747
Du S, Zhang Y, Lin X, Wang Y, Tang C (2008) Regulation of nitrate reductase by nitric oxide in Chinese cabbage pakchoi (Brassica chinensis L.). Plant Cell Environ 31:195–204
Dwivedi SL, Ceccarelli S, Blair MW, Upadhyaya HD, Are AK, Ortiz R (2016) Landrace germplasm for improving yield and abiotic stress adaptation. Trends Plant Sci 21:31–42
Ekler Z, Dutka F, Stephenson GR (1993) Safener effects on acetochlor toxicity, uptake, metabolism and glutathione S-transferase activity in maize. Weed Res 33:311–318
Elia AC, Galarini R, Taticchi MI, Dörr AJM, Mantilacci L (2003) Antioxidant responses and bioaccumulation in Ictalurus melas under mercury exposure. Ecotoxicol Environ Saf 55:162–167
Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, Ihsan MZ (2017) Crop production under drought and heat stress: plant responses and management options. Front Plant Sci 8:1147
Fan QJ, Liu JH (2012) Nitric oxide is involved in dehydration/drought tolerance in Poncirus trifoliata seedlings through regulation of antioxidant systems and stomatal response. Plant Cell Rep 31:145–154
Forni C, Duca D, Glick BR (2017) Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil 410:335–356
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Fu LJ, Shi K, Gu M, Zhou YH, Dong DK, Liang WS, Song FM, Yu JQ (2010) Systemic induction and role of mitochondrial alternative oxidase and nitric oxide in a compatible tomato–tobacco mosaic virus interaction. Mol Plant Microbe Interact 23:39–48
Fu J, Liu Z, Li Z, Wang Y, Yang K (2017) Alleviation of the effects of saline-alkaline stress on maize seedlings by regulation of active oxygen metabolism by Trichoderma asperellum. PLoS One 12:e0179617
Garcia-Mata C, Gay R, Sokolovski S, Hills A, Lamattina L, Blatt MR (2003) Nitric oxide regulates K+ and Cl-channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proc Nat Acad Sci 100:11116–11121
González A, de los Ángeles Cabrera M, Henríquez MJ, Contreras RA, Morales B, Moenne A (2012) Cross talk among calcium, hydrogen peroxide, and nitric oxide and activation of gene expression involving calmodulins and calcium-dependent protein kinases in Ulva compressa exposed to copper excess. Plant Physiol 158:1451–1462
Gupta KJ, Kaiser WM (2010) Production and scavenging of nitric oxide by barley root mitochondria. Plant Cell Physiol 51:576–584
Gupta KJ, Fernie AR, Kaiser WM, van Dongen JT (2011) On the origins of nitric oxide. Trends Plant Sci 16:160–168
Habib N, Ashraf M, Shahbaz M (2013) Effect of exogenously applied nitric oxide on some key physiological attributes of rice (Oryza sativa L.) plants under salt stress. Pak J Bot 45:1563–1569
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hasanuzzaman M, Nahar K, Alam MM, Fujita M (2012) Exogenous nitric oxide alleviates high temperature induced oxidative stress in wheat (Triticum aestivum L.) seedlings by modulating the antioxidant defense and glyoxalase system. Aust J Crop Sci 6:1314
Hayat S, Yadav S, Wani AS, Irfan M, Alyemini MN, Ahmad A (2012) Impact of sodium nitroprusside on nitrate reductase, proline content, and antioxidant system in tomato under salinity stress. Hortic Environ Biotechnol 53:362–367
He YK, Tang RH, Hao Y, Stevens RD, Cook CW, Am SM, Jing LF, Yang ZG, Chen LG, Guo FQ, Fiorani F, Jackson RB, Crawford NM, Pei ZM (2004) Nitric oxide represses the Arabidopsis floral transition. Science 305:1968–1971
He J, Ren Y, Chen X, Chen H (2014) Protective roles of nitric oxide on seed germination and seedling growth of rice (Oryza sativa L.) under cadmium stress. Ecotoxicol Environ Saf 108:114–119
Hebelstrup KH, Shah JK, Igamberdiev AU (2013) The role of nitric oxide and hemoglobin in plant development and morphogenesis. Physiol Plant 148:457–469
Hu X, Fang J, Cai W, Tang Z (2003) NO-mediated hypersensitive responses of rice suspension cultures induced by incompatible elicitor. Chin Sci Bull 48:358–363
Hu H, Zhou Z, Sun X, Zhang Z, Meng Q (2016) Protective effect of nitric oxide (NO) against oxidative damage in Larix gmelinii seedlings under ultraviolet-B irradiation. Forests 7:251
Hussain RA, Ahmad R, Nawaz F, Ashraf MY, Warraich EA (2016) Foliar NK application mitigates drought effects in sunflower (Helianthus annuus L.). Acta Physiol Plant 38:83. https://doi.org/10.1007/s11738-016-2104-z
Izabela M, Ilona CM, Edyta S, Maria F, Stanisław G, Maciej TG (2013) Impact of osmotic stress on physiological and biochemical characteristics in drought susceptible and drought-resistant wheat genotypes. Acta Physiol Plant 35:451–461
Jin CW, Du ST, Zhang YS, Lin XY, Tang CX (2009) Differential regulatory role of nitric oxide in mediating nitrate reductase activity in roots of tomato (Solanum lycocarpum). Ann Bot 104:9–17
Kadkhodaie A, Razmjoo J, Zahedi M, Pessarakli M (2014) Selecting sesame genotypes for drought tolerance based on some physiochemical traits. J Agron 106:111–118
Kaur G, Asthir B (2017) Molecular responses to drought stress in plants. Biol Plant 61:201–209
Kaur G, Singh HP, Batish DR, Mahajan P, Kohli RK, Rishi V (2015) Exogenous nitric oxide (NO) interferes with lead (Pb)-induced toxicity by detoxifying reactive oxygen species in hydroponically grown wheat (Triticum aestivum) roots. PLoS One 10:1–18
Kausar A, Ashraf MY, Ali I, Niaz M, Abbass QA (2012) Evaluation of sorghum varieties/lines for salt tolerance using physiological indices as screening tool. Pak J Bot 44:47–52
Kharbech O, Houmani H, Chaoui A, Corpas FJ (2017) Alleviation of Cr (VI)-induced oxidative stress in maize (Zea mays L.) seedlings by NO and H2S donors through differential organ-dependent regulation of ROS and NADPH-recycling metabolisms. J Plant Physiol 219:71–80
Leshem YY (1996) Nitric oxide in biological systems. Plant Growth Regul 18:155–159
Li ZG, Yang SZ, Long WB, Yang GX, Shen ZZ (2013) Hydrogen sulphide may be a novel downstream signal molecule in nitric oxide-induced heat tolerance of maize (Zea mays L.) seedlings. Plant Cell Environ 36:1564–1572
Lin Y, Yang L, Paul M, Zu Y, Tang Z (2013) Ethylene promotes germination of Arabidopsis seed under salinity by decreasing reactive oxygen species: evidence for the involvement of nitric oxide simulated by sodium nitroprusside. Plant Physiol Biochem 73:211–218
Liu Y, Li P, Xu GC, Xiao L, Ren ZP, Li ZB (2017) Growth, morphological, and physiological responses to drought stress in Bothriochloa ischaemum. Front Plant Sci 8:1–11
Mayak S, Tirosh T, Glick BR (2004) Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem 42:565–572
Miao Y, Lv D, Wang P, Wang XC, Chen J, Miao C, Song CP (2006) An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell 18:2749–2766
Misra AN, Misra M, Singh R (2011) Nitric oxide ameliorates stress responses in plants. Plant Soil Environ 57:95–100
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nawaz F, Ashraf MY, Ahmad R, Waraich EA, Shabbir RN, Bukhari MA (2015) Supplemental selenium improves wheat grain yield and quality through alterations in biochemical processes under normal and water deficit conditions. Food Chem 175:350–357
Nawaz F, Shabbir RN, Shahbaz M, Majeed S, Raheel M, Hassan W, Sohail MA (2017) Cross talk between nitric oxide and phytohormones regulate plant development during abiotic stresses. In: El-Esawi M (ed) Phytohormones-signaling mechanisms and crosstalk in plant development and stress responses. InTech, pp 117–141. https://doi.org/10.5772/intechopen.69812
Neufeldt H, Jahn M, Campbell BM, Beddington JR, DeClerck F, De Pinto A, Gulledge J, Hellin J, Herrero M, Jarvis A, LeZaks D (2013) Beyond climate-smart agriculture: toward safe operating spaces for global food systems. Agric Food Secur 2:1–6
Peng R, Bian Z, Zhou L, Cheng W, Hai N, Yang C, Yang T, Wang X, Wang C (2016) Hydrogen sulfide enhances nitric oxide-induced tolerance of hypoxia in maize (Zea mays L.). Plant Cell Rep 35:2325–2340
Peñuelas J, Sardans J, Estiarte M, Ogaya R, Carnicer J, Coll M, Filella I (2013) Evidence of current impact of climate change on life: a walk from genes to the biosphere. Glob Change Biol 19:2303–2338
Pető A, Lehotai N, Feigl G, Tugyi N, Ördög A, Gémes K, Tari I, Erdei L, Kolbert Z (2013) Nitric oxide contributes to copper tolerance by influencing ROS metabolism in Arabidopsis. Plant Cell Rep 32:1913–1923
Quiroga G, Erice G, Aroca R, Chaumont F, Ruiz-Lozano JM (2017) Enhanced drought stress tolerance by the arbuscular mycorrhizal symbiosis in a drought-sensitive maize cultivar is related to a broader and differential regulation of host plant aquaporins than in a drought-tolerant cultivar. Front Plant Sci 8:1056
Ramarao CS, Paul VH, Dhak BD, Kadrekar SB (1983) A simple in vivo method for determination of nitrite reductase activity in rice root. Z Z Pflanzenphysiol 109:81–85
Rosales EP, Iannone MF, Groppa MD, Benavides MP (2011) Nitric oxide inhibits nitrate reductase activity in wheat leaves. Plant Physiol Biochem 49:124–130
Ruch RJ, Cheng SJ, Klaunig JE (1989) Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 10:1003–1008
Sánchez-Aguayo I, Rodríguez-Galán JM, García R, Torreblanca J, Pardo JM (2004) Salt stress enhances xylem development and expression of S-adenosyl-l-methionine synthase in lignifying tissues of tomato plants. Planta 220:278–285
Sang J, Jiang M, Lin F, Xu S, Zhang A, Tan M (2008) Nitric oxide reduces hydrogen peroxide accumulation involved in water stress-induced subcellular anti-oxidant defense in maize plants. J Integr Plant Biol 50:231–243
Santisree P, Bhatnagar-Mathur P, Sharma KK (2015) NO to drought-multifunctional role of nitric oxide in plant drought: do we have all the answers? Plant Sci 239:44–55
Sanz-Luque E, Ocaña-Calahorro F, Llamas A, Galvan A, Fernandez E (2013) Nitric oxide controls nitrate and ammonium assimilation in Chlamydomonas reinhardtii. J Exp Bot 64:3373–3383
Seligman K, Saviani EE, Oliveira HC, Pinto-Maglio CAF, Salgado I (2008) Floral transition and nitric oxide emission during flower development in Arabidopsis thaliana is affected in nitrate reductase-deficient plants. Plant Cell Physiol 49:1112–1121
Shabbir RN, Waraich EA, Ali H, Nawaz F, Ashraf MY, Ahmad R, Awan MI, Ahmad S, Irfan M, Hussain S, Ahmad Z (2016) Supplemental exogenous NPK application alters biochemical processes to improve yield and drought tolerance in wheat (Triticum aestivum L.). Environ Sci Pollut Res 23:2651–2662
Sheokand S, Bhankar V, Sawhney V (2010) Ameliorative effect of exogenous nitric oxide on oxidative metabolism in NaCl treated chickpea plants. Braz J Plant Physiol 22:81–90
Signorelli S, Corpas FJ, Borsani O, Barroso JB, Monza J (2013) Water stress induces a differential and spatially distributed nitro-oxidative stress response in roots and leaves of Lotus japonicus. Plant Sci 201:137–146
Simaei M, Khavari-Nejad RA, Bernard F (2012) Exogenous application of salicylic acid and nitric oxide on the ionic contents and enzymatic activities in NaCl-stressed soybean plants. Am J Plant Sci 3:1495–1503
Smirnoff N (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125:27–58
Sokolovski S, Blatt MR (2004) Nitric oxide block of outward-rectifying K+ channels indicates direct control by protein nitrosylation in guard cells. Plant Physiol 136:4275–4284
Srivastava N, Gonugunta VK, Puli MR, Raghavendra AS (2009) Nitric oxide production occurs downstream of reactive oxygen species in guard cells during stomatal closure induced by chitosan in abaxial epidermis of Pisum sativum. Planta 229:757–765
Sun C, Lu L, Liu L, Liu W, Yu Y, Liu X, Lin X (2014) Nitrate reductase-mediated early nitric oxide burst alleviates oxidative damage induced by aluminum through enhancement of antioxidant defenses in roots of wheat (Triticum aestivum). New Phytol 201:1240–1250
Sym GJ (1984) Optimisation of the in-vivo assay conditions for nitrate reductase in barley (Hordeum vulgare L. cv. Igri). J Sci Food Agric 35:725–730
Sytykiewicz H (2014) Differential expression of superoxide dismutase genes in aphid-stressed maize (Zea mays L.) seedlings. PLoS One 9:e94847
Sytykiewicz H (2016) Deciphering the role of NADPH oxidase in complex interactions between maize (Zea mays L.) genotypes and cereal aphids. Biochem Biophys Res Commun 476:90–95
Tian X, Lei Y (2006) Nitric oxide treatment alleviates drought stress in wheat seedlings. Biol Plant 50:775–778
Venisse JS, Gullner G, Brisset MN (2001) Evidence for the involvement of an oxidative stress in the initiation of infection of pear by Erwinia amylovora. Plant Physiol 125:2164–2172
Wang Q, Rowan MJ, Anwyl R (2004) β-amyloid-mediated inhibition of NMDA receptor-dependent long-term potentiation induction involves activation of microglia and stimulation of inducible nitric oxide synthase and superoxide. J Neurosci 24:6049–6056
Wang Y, Suo B, Zhao T, Qu X, Yuan L, Zhao X, Zhao H (2011) Effect of nitric oxide treatment on antioxidant responses and psbA gene expression in two wheat cultivars during grain filling stage under drought stress and rewatering. Acta Physiol Plant 33:1923–1932
Wu S, Hu C, Tan Q, Xu S, Sun X (2017) Nitric oxide mediates molybdenum-induced antioxidant defense in wheat under drought stress. Front Plant Sci 8:1085
Yagmur M, Kaydan D (2008) Alleviation of osmotic stress of water and salt in germination and seedling growth of triticale with seed priming treatments. Afr J Biotechnol 7:2156–2162
Yildiztugay E, Ozfidan-Konakci C, Kucukoduk M (2014) Exogenous nitric oxide (as sodium nitroprusside) ameliorates polyethylene glycol-induced osmotic stress in hydroponically grown maize roots. J Plant Growth Regul 33:683–696
Yu M, Lamattina L, Spoel SH, Loake GJ (2014) Nitric oxide function in plant biology: a redox cue in deconvolution. New Phytol 202:1142–1156
Zhang Y, Wang L, Liu Y, Zhang Q, Wei Q, Zhang W (2006) Nitric oxide enhances salt tolerance in maize seedlings through increasing activities of proton-pump and Na+/H+ antiport in the tonoplast. Planta 224:545–555
Zhang AY, Jiang MY, Zhang JH, Ding H, Xu S, Hu X, Tan M (2007) Nitric oxide induced by hydrogen peroxide mediates abscisic acid-induced activation of the mitogen-activated protein kinase cascade involved in antioxidant defense in maize leaves. New Phytologist 175:36–50
Zhang S, Melzer MM, Sen SN, Çelebi-Ölçüm N, Warren TH (2016a) A motif for reversible nitric oxide interactions in metalloenzymes. Nat Chem 8:663–669
Zhang L, Li X, Li X, Wei Z, Han M, Zhang L, Li B (2016b) Exogenous nitric oxide protects against drought-induced oxidativestress in Malus rootstocks. Turk J Bot 40:17–27
Zhao L, He J, Wang X, Zhang L (2008) Nitric oxide protects against polyethylene glycol-induced oxidative damage in two ecotypes of reed suspension cultures. J Plant Physiol 165:182–191
Acknowledgements
The present work is a part of doctoral research studies of Ms. Sadia Majeed, an HEC (Higher Education Commission of Pakistan) scholar at the Department of Agronomy, The Islamia University of Bahawalpur (IUB), Pakistan. The doctoral studies of Ms. Sadia Majeed are financially supported by HEC under Grant no. 213-60216-2AV2-112 awarded to Ms. Sadia Majeed. The authors appreciate and acknowledge the valued assistance offered by Dr, Samina Ijaz, Department of Biochemistry and Biotechnology, IUB, to successfully complete the designed analytical work. We would also like to extend our sincere thanks to Ms. Saba Tauseef, Ms. Kinza Mehmood and Ms. Iqra Khalid for their technical assistance in analytical work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. A. Kleczkowski.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Majeed, S., Nawaz, F., Naeem, M. et al. Effect of exogenous nitric oxide on sulfur and nitrate assimilation pathway enzymes in maize (Zea mays L.) under drought stress. Acta Physiol Plant 40, 206 (2018). https://doi.org/10.1007/s11738-018-2780-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-018-2780-y