Abstract
Seven-day-old maize seedlings were subjected to NaCl (150 mM) without or with the presence of equimolar concentrations of urea, potassium nitrate or ammonium sulfate (at 60 kg N ha−1) for the subsequent 15 days. The results show that NaCl significantly decreased photosynthetic activity parameters (Hill reaction, Pn, Ci and Gs), shoot fresh and dry weights, chlorophylls, protein, GSH and AsA. The activities of ALA-D, Rubisco, PEP-C, MDH, PPDK, SOD, CAT and APX were significantly inhibited. On the contrary, NaCl significantly elevated MDA, O ∙−2 , H2O2, soluble sugars and proline. The application of the nitrogenous sources mostly ameliorated the photosynthetic activity synchronous with counterbalancing the decreases in growth, protein and pigments. The activities of ALA-D, Rubisco, PEP-C, MDH and PPDK enhanced remarkably with N applied under salinity. Furthermore, enhancements were detected in GSH and AsA contents and SOD, CAT and APX activities, which decreased free radicals, soluble sugars and proline. These effects reveal that maize was vulnerable to NaCl stress; however, N overcame the stress status and ameliorated the plant tolerance to salinity concomitant with repairing photosynthetic activity and maintaining higher antioxidants and ROS homeostasis; urea seemed to be the most efficient. Taken together, these findings conclude that N enhanced the capability of maize to tolerate NaCl via protecting photosynthetic apparatus for normal photochemical functioning and improving antioxidants and ROS homeostasis as effective salt tolerance mechanisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salt stress is deleterious for plant physiological and biochemical processes. It is toxic due to ions, oxidative and osmotic stresses, damaging of membranes, water deficiency, oxidative stress, imbalances of nutrients, metabolic dysfunction, denaturing cytosolic enzymes and alteration in growth regulators (Kong et al. 2017). Photosynthesis is the most important metabolic process in plants influenced by salt stress because of limitations in photosynthetic parameters (Pn, Ci and Gs). PSII is considered as crucial in plants photosynthesis (Perreault et al. 2011). In addition to these parameters, photosynthetic pigments and photosynthetic enzymes are fundamental for efficient photosynthesis; however, they are extremely sensitive to stress. Chlorophyll synthesis starts with the combination of two molecules of ALA (Wu et al. 2019) to form porphobilinogen by the action of ALA-D. Of the most important enzymes in C4 photosynthesis are PEP-C, MDH and PPDK besides Rubisco. The key enzyme in photosynthesis is Rubisco (Spreitzer 1993; Nemat Alla et al. 2011). It catalyzes the carboxylation of Ru-1,5-P forming 3-PGA in an irreversible reaction. PEP-C catalyzes the carboxylation of PEP to produce oxaloacetate which is reduced by NADP-MDH to malate with the production of CO2 for Calvin cycle and pyruvate which undergoes conversion to PEP by the action of PPDK, regenerating thus the primary CO2 acceptor.
Plants under salinity stress are affected by osmotic and oxidative stresses. Accumulation of soluble sugars and proline as active metabolites is claimed to be an effective tolerance mechanism to protect plants from osmotic stress by maintaining osmotic balance and increase the stability of macromolecules (Parida and Das 2005; Nemat Alla et al. 2016). Moreover, oxidative stress could result from overgeneration of ROS which leads to disturbances in redox homeostasis (Chakraborty et al. 2019). They indicated that oxidative stress enhances peroxidation of membrane lipid, oxidation of protein, inhibition of enzymes and damage of DNA and RNA. However, a variety of antioxidants are developed in plants to balance the load of cellular ROS. The enzymatic antioxidants such as SOD, CAT and APX are important indices for the reflection of plant resistance to stress (Borella et al. 2019). Non-enzymatic metabolites, i.e., GSH and AsA, are associated with the balance of the cellular redox that could act as signals for the antioxidant mechanism regulation (Nemat Alla and Hassan 2014; Badran et al. 2015; Chakraborty et al. 2019).
Although plants have an antioxidant system to tolerate harsh conditions, this system might be insufficient in sensitive species, so, external supporters are effective in stimulating plant growth and could support plants in tolerating stresses (Hassan et al. 2015; Perveen et al. 2018). N is very important for plant growth and development, so its exogenous application to plants could increase the ability of plants to withstand stress situations of salinity. It is integrated into several N-containing compounds and, moreover, it has important roles in regulating photosynthetic activity. So, the present work aims to investigate the effect of the external application of some nitrogenous sources (urea, potassium nitrate or ammonium sulfate) on modulating photosynthetic activity and ROS homeostasis in maize seedlings to tolerate NaCl stress.
Materials and methods
Conditions of growth
Maize (Zea mays L. Giza 2) grains were sterilized using 3% NaOCl for 10 min. After washing, the seeds were soaked for 8 h in water, then sown in perlite in 50 pots (35 cm in diameter, 10 seeds per pot) and allowed to establish for 7 days. The pots were distributed in five groups, the first to serve as control and the others for NaCl treatments (150 mM) alone or combined with equimolar concentrations of urea (46.5% N), potassium nitrate (13.8% N) or ammonium sulfate (21.2% N), each added at 60 kg N ha−1. The pots were kept at 60% RH, 30 ± 2/15 ± 2 °C day/night, 16 h light and 300 µmol m−2 s−1 PPFD). Water was supplied daily. The experiment was repeated twice (100 pots). The collection of samples was carried out at the time of application (zero time) and at 5, 10 and 15 days after the treatment, washed thoroughly and shoots were separated, weighed then dried for determination of fresh and dry weights, respectively. The second leaves were used for the different analyses, fresh for photosynthetic activity determinations. For the other analyses, samples were frozen using liquid nitrogen.
Determination of photosynthetic activity
PSII activity was measured by the photoreduction rate of 2,6-DCPIP (Trebst 1972). Leaves were homogenized in Na-tricine (50 mM, pH 7.8) containing sucrose and magnesium chloride (300 and 3 mM, respectively). The pellets were collected by centrifugation and resuspended using Na tricine (1 mM, pH 7.8) containing NaCI and magnesium chloride (10 mM each) and absorbance was monitored at 600 nm. Photosynthetic gas-exchange variables (Pn, Ci and Gs) were determined using InfraRed Gas Analyzer in fully expanded leaves between 10 and 12 am. The conditions were set for determinations for CO2 concentration (at 360 ± 20 ppm), temperature (at 30 ± 2 °C), and relative humidity (at 60 ± 4%).
Determination of protein and pigments
Protein was extracted from fresh tissue with chilled acetone. After filtration, residues were dried and the acetone powders were obtained (Harborne 1984). Extraction was completed from aliquots of powders using Tris–HCl (pH 9, 50 mM) and then centrifuged at 48,200×g for 15 min at 4 °C. Determination of protein content was carried out according to Bradford (1976). Chlorophyll contents were determined according to Metzner et al. (1965).
Determination of soluble sugars and proline
The extraction of soluble sugars was performed in 80% ethanol. An aliquot was mixed, reacted with anthrone reagent (8.6 mM anthrone in 80% H2SO4), heated, cooled in ice bath and the absorbance was measured at 623 nm according to Schlüter and Crawford (2001). The extraction of proline was carried out in 3% sulfosalicylic acid, then aliquots of the extracts were reacted with glacial acetic acid and acid ninhydrin and then absorbance was read at 520 nm according to Bates et al. (1973).
Determination of GSH and AsA
TCA (5%, w/v) containing 10 mM EDTA was used for the extraction of GSH. After centrifugation at 12,000×g for 15 min, aliquots were used for the assay of GSH using phosphate buffer (pH 6.8, 100 mM) with 10 mM EDTA, 1 mM CDNB and 1.0 U glutathione-S-transferase and incubated at 35 °C for 30 min, then absorbance was measured at 340 nm as described by Anderson and Gronwalds (1991). Phosphoric acid (62.5 mM) was used for the extraction of AsA. The extracts were centrifuged at 12,000×g for 20 min and then eluted with 4.5 mM H2SO4 using ion exclusion column. Folin–Ciocalteu reagent and 10% TCA were used for the assay of AsA (Mukherjee and Choudhuri 1983) and absorbance was measured at 760 nm.
Determination of MDA, O ∙−2 and H2O2
TCA (10%, w/v) was used for the extraction of MDA. After being centrifuged, MDA content was measured using 10% TCA containing 0.5% thiobarbituric acid and absorbance was read at 450, 532 and 600 nm. MDA content was determined from the equation: 6.45(A532–A600) − 0.56A450 (Sun et al. 2010). The extraction of O ∙−2 was carried out in 200 mM phosphate buffer (pH 7.2) with 1 mM diethyl dithiocarbamate, 3 mM potassium cyanide and 5 mM H2O2. O ∙−2 was measured by determining the nitroblue tetrazolium chloride reduction at 540 nm as described by Chaitanya and Naithani (1994). H2O2 was extracted in 0.1% TCA and the assay was performed in potassium phosphate buffer containing KI and absorbance was measured at 390 nm (Okuda et al. 1991).
Enzyme extraction and assays
The extraction of ALA-D was carried out with Tris–HCl (0.05 M, pH 9). The assay was conducted using Tris–HCl (50 mM, pH 7.0) and ALA (5 mg ml−1). The quantity of porphobilinogen formed was measured at 555 nm as described by Mauzerall and Granick (1956) using the modified Ehrlich’s reagent. Rubisco was extracted with Tris–HCl (20 mM, pH 8.0) containing 10 mM magnesium chloride, 10 mM sodium bicarbonate, 5 mM DTT, 1 mM EDTA, 0.002% Hibitane, and 1% PVP (Keys and Parry 1990). Hepes–KOH (50 mM, pH 7.5) with 10 mM DTT was used to extract PEPC, MDH and PPDK (Ashton et al. 1990). Bovine serum albumin, magnesium chloride and potassium phosphate were added during the extraction of PEP-C, while potassium phosphate was used for PPDK extraction. Rubisco activity was assayed in Hepes (50 mM, pH 7.8) containing 10 mM sodium bicarbonate, 0.66 mM Ru-1,5-P, 5 mM ATP, 20 mM magnesium chloride, 0.2 mM NADPH, 5 mM CP, 2.0 U CPase, 2.8 U 3-GAldDH and 2.0 U PGK kinase. Tris–HCl (25 mM, pH 8.0) was used for PEP-C activity assay in the presence of 5 mM magnesium chloride, 1 mM potassium bicarbonate, 2 mM DTT, 5 mM G-6-P, 5 mM PEP, 0.2 mM NADH and 2 U of MDH. Tricine-KOH (25 mM, pH 8.3) was used for MDH activity assay in the presence of 70 mM KCl, 1 mM EDTA, 1 mM DTT, 1 mM OAA and 0.2 mM NADPH. Hepes–KOH (25 mM, pH 8.0) was used for PPDK activity assay in the presence of 8 mM magnesium sulfate, 10 mM sodium bicarbonate, 5 mM ammonium sulfate, 10 mM DTT, 2 mM pyruvate, 1 mM G-6-P, 1 mM ATP, 2.5 mM potassium phosphate, 0.2 mM NADH, 0.5 U PEP-C and 2 U of MDH. The absorbance for all enzymes was read at 340 nm. The enzymatic antioxidants (SOD, CAT and APX) were extracted with 50 mM sodium phosphate buffer (pH 7.0) with 1 mM EDTANa2 and 0.5% PVPP, then centrifuged at 13,000×g. SOD activity was evaluated by measuring the inhibition of nitroblue tetrazolium photochemical reduction (Dhindsa et al. 1981). CAT activity was assayed as described by Beer and Sizer (1952) through the absorbance reduction of H2O2 at 240 nm. The activity of APX was assayed Nakano and Asada (1981) from recording absorption decrement at 290 nm due to ascorbate oxidation.
Statistical analysis
The pots of this work were arranged in a complete randomized block. The design consisted of 100 pots from duplicate repetition of the experiment, five different treatments and 10 replications for each treatment. The values were calculated from six replicates as mean ± SD. ANOVA was applied to full data, followed by LSD at P ≤ 0.05.
Results
Photosynthetic activity and growth parameters
The changes in photosynthetic activity parameters (Hill reaction, Pn, Ci, Gs) in 7-day-old maize seedlings throughout 15 days from application of 150 mM NaCl are depicted in Fig. 1. NaCl significantly declined the photosynthetic activity parameters in maize leaves throughout the experimental period in comparison with controls; an augmented decline was detected with the elapse of time. Due to NaCl treatment, the reduction was noted on the 15th day following treatment as 37% in Hill reaction, 54% in net photosynthesis rate, 64% in CO2 concentration and 42% in stomatal conductance. Nonetheless, the application of equimolar concentrations of the nitrogenous sources (urea, potassium nitrate or ammonium sulfate) to salinity-treated seedlings greatly counterbalanced the NaCl-induced reductions in photosynthetic parameters; urea was in particular the most efficient. By the 15th day following treatment, the induced reductions in all parameters of the photosynthetic activity by NaCl were highly withdrawn following N application to become only 3–6% by urea, 3–13% by potassium nitrate or 7–15% by ammonium sulfate.
Interaction of equimolar concentrations of the nitrogenous sources (urea, potassium nitrate or ammonium sulfate each added at 60 kg N ha−1) on the parameters of photosynthetic activity (Hill reaction, PSII as µM DCPIP mg−1 chlorophyll h−1 and Pn, Ci, and Gs as mol m−2 s−1) in shoots of 7-day-old maize seedlings treated with 150 mM NaCl during the following 15 days. Mean values (± SD) were of six replicates, and LSD (vertical bars) was calculated at P ≤ 0.05
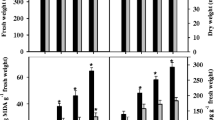
Fresh weight, dry weight and protein contents in maize shoots were also significantly decreased by NaCl treatment, and the magnitude of decrease by NaCl was augmented with the elapse of time (Fig. 2). Fresh weight was more affected on the 15th day from treatment than dry weight (48 and 15%, respectively), while the reduction in protein content was about 34%. However, the application of the nitrogenous sources induced retractions in the effects of NaCl; urea was more efficient than potassium nitrate or ammonium sulfate. NaCl-induced decreases in fresh weight and dry weight were retracted on the 15th day from application of NaCl to become, respectively, 5 and 9% by urea, 11 and 12% by potassium nitrate or 15 and 12% by ammonium sulfate; however, slight increases were detected in the protein content of NaCl-treated seedlings by urea, potassium nitrate or ammonium sulfate (10, 4 and 3%, respectively).
Interaction of equimolar concentrations of the nitrogenous sources (urea, potassium nitrate or ammonium sulfate each added at 60 kg N ha−1) on fresh and dry weights (g per plant) and protein content (mg g−1 DW) in shoots of 7-day-old maize seedlings treated with 150 mM NaCl during the following 15 days. Mean values (± SD) were of six replicates, and LSD (vertical bars) was calculated at P ≤ 0.05
Chlorophyll and photosynthetic enzymes
Similarly, NaCl treatment led to significant reductions in chlorophyll contents in relation to the untreated controls, and the magnitude of reductions became about 25 and 21% in chlorophyll a and chlorophyll b, respectively, by the 15th day following treatment (Fig. 3). The ratio of chlorophyll a/b exhibited the lowest values in NaCl-treated seedlings all over the experiment, but the highest ones were detected in the untreated control. At the same time, significant inhibitions were detected in ALA-D activity in maize leaves by NaCl treatment throughout the entire experimental period, and the magnitudes of inhibition was 44% on the 15th day of treatment. Nevertheless, these decreases were nullified upon the application of urea, potassium nitrate or ammonium sulfate. Moreover, the application of the nitrogenous sources resulted in moderate values of chlorophyll a/b ratios between NaCl-treated and the untreated control. The nitrogenous sources lowered the effects of NaCl to become on the 15th day following treatment only 6, 10 and 11%, respectively, in chlorophyll a, 5, 7 and 8% in chlorophyll b or 4, 11 and 12% in ALA-D activity.
Interaction of equimolar concentrations of the nitrogenous sources (urea, potassium nitrate or ammonium sulfate each added at 60 kg N ha−1) on chlorophyll a and b contents (mg g−1 DW), their ratio and ALA-D activity (mg porphobilinogen released g−1 DW h−1) in shoots of 7-day-old maize seedlings treated with 150 mM NaCl during the following 15 days. Mean values (± SD) were of six replicates, and LSD (vertical bars) was calculated at P ≤ 0.05
Figure 4 shows that NaCl treatment significantly inhibited the activities of Rubisco, PEP-C, MDH and PPDK throughout the entire experiment as compared to controls: the effect was fast and high for Rubisco and PEP-C, but steady for MDH and PPDK; the magnitudes of inhibition in Rubisco, PEP-C, MDH and PPDK on the 15th day from treatment was noted as 60, 54, 48 and 35%, respectively. The application of the nitrogenous sources, particularly urea, greatly counterbalanced the NaCl-induced inhibitions. By the 15th day following treatment, the inhibitions in the enzyme activities by NaCl were highly withdrawn by urea, potassium nitrate or ammonium sulfate to become only 11, 26 and 20% in Rubisco, 27, 22 and 23% in PEP-C, 7, 16 and 17% in MDH and 7, 18 and 16% in PPDK.
Interaction of equimolar concentrations of the nitrogenous sources (urea, potassium nitrate or ammonium sulfate each added at 60 kg N ha−1) on Rubisco, PEP-C, MDH and PPDK activities (decrease in absorbance mg−1 protein min−1) in 7-day-old maize shoots treated with 150 mM NaCl during the following 15 days. Mean values (± SD) were of six replicates, and LSD (vertical bars) was calculated at P ≤ 0.05
Osmoprotectants, antioxidants and ROS
Soluble sugars and proline contents were significantly elevated by NaCl during the whole experiment as compared to controls; however, GSH and AsA contents were significantly decreased (Fig. 5). The magnitude of elevation of soluble sugars and proline reached about 22 and 40%, respectively, by the 15th day from treatment, while the drop of GSH or AsA reached about 51 or 45%, respectively. Anyway, the use of the nitrogenous sources greatly alleviated the rises in soluble sugars and proline as well as the decrease in GSH and AsA such that on the 15th day from treatment, the increases in soluble sugars retracted to only 9, 5 and 3% by urea, potassium nitrate or ammonium sulfate, respectively, while the increases in proline retracted to 16, 6 and 4%. However, the decreases in GSH were 5, 11 and 10%, while under the same conditions the decreases in AsA were 7, 12 and 13%, respectively.
Interaction of equimolar concentrations of the nitrogenous sources (urea, potassium nitrate or ammonium sulfate each added at 60 kg N ha−1) on soluble sugars, proline contents (mg g−1 dry weight), GSH and AsA contents (μg g−1 dry weight) in shoots of 7-day-old maize seedlings treated with 150 mM NaCl during the following 15 days. Mean values (± SD) were of six replicates, and LSD (vertical bars) was calculated at P ≤ 0.05
The activities of the antioxidant enzymes were remarkably inhibited following NaCl treatment; these inhibitions reached about 58, 45 and 43% in SOD, CAT and APX, respectively, by the 15th day of NaCl application (Fig. 6). These inhibitions in activities were markedly retracted upon the application of urea, potassium nitrate or ammonium sulfate to become, respectively, on the 15th day of treatment only 15, 18 and 21%, respectively. in SOD, 8, 10 and 19% in CAT or 4, 14 and 16% in APX.
Interaction of equimolar concentrations of the nitrogenous sources (urea, potassium nitrate or ammonium sulfate each added at 60 kg N ha−1) on SOD, CAT and APX activities (Units mg−1 protein) in 7-day-old maize shoots treated with 150 mM NaCl during the following 15 days. Mean values (± SD) were of six replicates, and LSD (vertical bars) was calculated at P ≤ 0.05
On the contrary, the application of NaCl induced significant elevation in MDA, O .−2 and H2O2 contents throughout the experimental period (Fig. 7). The elevation was greater in MDA and H2O2 (146 and 130%, respectively, on the 15th day following treatment) relative to O .−2 (87%). The increments of these contents were greatly lowered upon the application of the nitrogenous sources such that the magnitude of increases became by urea, potassium nitrate ammonium only 28, 34 and 54% in MDA, 13, 23 and 36% in O .−2 or 17, 22 and 34% in H2O2.
Interaction of equimolar concentrations of the nitrogenous sources (urea, potassium nitrate or ammonium sulfate each added at 60 kg N ha−1) on the contents of MDA (absorbance decrease min−1 mg−1 protein), O .−2 (Δ540 g−1 DW min−1) and H2O2 (μ mol g−1 DW) in shoots of 7-day-old maize seedlings treated with 150 mM NaCl during the following 15 days. Mean values (± SD) were of six replicates, and LSD (vertical bars) was calculated at P ≤ 0.05
Discussion
Photosynthetic activity and growth parameters
Salinity affects plants by various effects, including growth reductions, ion toxicity, energy waste, reduced carbon fixation and shortage of photosynthetic activities (Kong et al. 2017). Under salinity stress, salts can reach excessive levels in the apoplast that would lead to cell dehydration, they may accumulate in the cytoplasm with a consequent inhibition in enzymes or may be increased in the chloroplast that causes toxicity to photosynthesis (Munns and Tester 2008). The present study showed that the decrease detected in photosynthetic activity, growth parameters and protein of NaCl-treated seedling may result from the deleterious influences of ions and to the N-metabolism reduction. Nonetheless, these effects were mostly counterbalanced by the application of N sources, most effectively urea since N has important roles under saline conditions both in nutrition and in osmosis.
The significant reduction in photosynthesis induced by salinity might be due to stomatal and/or non-stomatal factors. Stomatal factors such as stomatal closure could limit gas-exchange parameters in leaves, whereas non-stomatal factors are mostly cell membrane damage and inhibition in the activity of Rubisco (Lang et al. 2018). Such response in the efficacy of photosynthesis toward salinity was, indeed, detected in maize in the present results. In fact, NaCl depressed Hill reaction, Pn, Ci and Gs in maize leaves. So, stomatal resistance became higher and gas exchange became lower than normal with consequences of retardations in the photosynthetic machinery. Jedmowski et al. (2013) indicated that stomatal closure could raise CO2 diffusion resistance, which could depress the photosynthetic rate with a consequent drop in photosynthesis. So, the decreased intercellular CO2 concentration and stomatal conductance, in the present study, indicate that maize leaves underwent stomatal closure by NaCl with a consequent reduction in the CO2 concentration diffusing into leaves causing reduction in photosynthetic rate.
The impacts on photosynthetic parameters induced in NaCl-stressed maize seedlings were reduced with the application of the nitrogenous sources. As compared to the stressed seedlings, urea in the same conditions elevated Hill reaction, net photosynthesis rate, CO2 concentration and stomatal conductance to more than 1.5-, 2.0-, 2.5- and 1.6-fold. Similar elevation—but to a lower extent—were also detected by potassium nitrate or ammonium sulfate. These findings conclude that N supplementation protected the photosynthetic apparatus and mitigated the salinity-induced damage. In general, N supplies nutrition for plant growth and so could minimize the salinity-induced nutrient deficiency. As shown in the results, urea was the most efficient.
In fact, urea is an important N form, because it is considered as the major fertilizer in addition to its importance among the plant metabolites. So, appropriate levels could ameliorate several reactions in the physiology and biochemistry of plants under stress conditions, maintain relative water content and alleviate stress-induced damage. The changes in photosynthetic parameters by NaCl and the recovery by N application were in conformity with growth parameter changes. So, N as an exogenous supporter was needed for maize to withstand salinity stress. Tolerance is a highly desirable trait in crops; the salt tolerance/resistance is, however, slight in these crops. Plants have an antioxidant system to tolerate harsh conditions; nonetheless, this system might be insufficient in sensitive species under abiotic stresses. So, several strategies have been performed to alleviate plant growth reduction through mitigating the adverse effects of stress (Wu et al. 2019).
Chlorophyll and photosynthetic enzymes
The efficiency of photosynthesis depends, besides the photosynthetic parameters, on the photosynthetic pigments and enzymes; they could be decreased by salinity and would decline the photosynthetic activities. Chlorophylls are essential for photosynthesis and, moreover, they are markers for plant photosynthetic efficiency at varied situations. ALA-D enzyme is the key for the biosynthesis of chlorophylls. The present results show that NaCl significantly decreased chlorophylls and ALA-D activity. In confirmation, the degradation of pigments by salt stress reflected vulnerability of photosystems to salt stress (Mazumdar et al. 2019). However, application of nitrogenous sources increased protein for enzyme activities and stimulated ALA-D for chlorophyll biosynthesis. This improvement would compensate for the influence of PSII by the effects of excessive energy of excitation as indicated by Gururani et al. (2015). The enhancement was most pronounced with urea. In addition, the ratio of chlorophyll a/b was negatively affected by NaCl, indicating the stress impact on the photoinhibition risks of photosystem composition; however, N rendered these values close to normal. As indicated, N is essential for the structure of chlorophylls, so an exogenous supply would provide a sufficient amount of N to promote chlorophyll biosynthesis. In this account, Kumawat and Mahla (2015) concluded that urea provided more appropriate substrates for pigment biosynthesis and stimulated photosynthesis, enhanced leaf dry matter production and promoted plant growth. Therefore, urea could enhance photosynthetic pigment contents which exhibit a positive relationship with photosynthetic parameters. So, the inefficient photosynthesis in salt-stressed plants could be regarded mainly due to the reduced pigment contents; however, N induced amelioration in photosynthesis possibly due to enhancing pigment biosynthesis and/or to an elevation in the rigidity of cell wall.
In integration with photosynthetic capacity in maize, the photosynthetic pathway in C4 plants involved some additional enzymatic reactions that precede the Calvin cycle. Therefore, Rubisco catalyzes the carboxylation of Ru-1,5-P in the Calvin cycle for maintaining the rate of carbon fixation. However, these additional special reactions convert PEP to OAA by a carboxylation reaction stimulated by PEP-C, reducing OAA to malate and then pyruvate by MDH, and phosphorylating pyruvate to PEP by PPDK for the generation of the first acceptor of CO2 in C4. The activities of these enzymes in addition to Rubisco, in the present results, were significantly inhibited by NaCl, causing further retardation of the photosynthetic machinery. Due to the stomatal closure by NaCl, the inhibition in PEP-C would retard OAA formation for MDH with a consequent decline both in CO2 release for Rubisco and Calvin cycle and in pyruvate for PPDK. In confirmation, significant inhibitions were also detected in PPDK, PEP-C and MDH in maize due to different stress conditions (Nemat Alla and Hassan 1998; Hassan et al. 2008). So, an inhibition in these enzymatic activities could explicate the decreased rate of photosynthetic activity. However, these inhibitions were markedly alleviated by N sources, concomitant with a repair in the photosynthetic process. The enzyme activity enhancement was synchronized with the improvement in CO2 concentration and stomatal conductance that would subsequently ameliorate photosynthetic rate. The results clearly indicate the coincidence of the changes in enzyme activities with the changes in photosynthetic parameters and also with chlorophyll and growth parameters.
Osmoprotectants, antioxidants and ROS
Under abiotic stress conditions, plants accumulate considerable organic solutes to protect the functioning of normal metabolic processes, and also other processes for the metabolism of adaptation to depress ROS extra production. Soluble sugars and proline are low-molecular-weight compatible solutes that avert the harmful effects of stress. Hayat et al. (2012) indicated that these osmoprotectants lower inhibitions induced in enzyme activities by ions, raise the enzyme thermal stability and foreclose enzyme complex dissociation. On the other hand, Szabados and Savouré (2010) reported that proline could regulate the function of mitochondria; moreover, it acts in the redox balancing modulation. Koenigshofer and Loeppert (2019) concluded that plants produce proline to protect the different meristematic tissues. The present results are in conformity with these findings; there were increased accumulations in soluble sugars and proline in maize shoots by NaCl, while the presence of nitrogenous sources, particularly urea, overcame these accumulations. These dramatic changes could be due to changes in their metabolic processes and to disorder in their transportation. Soluble sugars and proline act likely in counterbalancing salinity-induced rises of osmotic potential and protecting cell components through elimination of ROS. However, the retractions in their values by N application indicate that N relieved maize from salinity stress, and the relief was most evident with urea.
On the other hand, the overproduction of MDA, H2O2 and O .−2 induces oxidative damage in plant cells and disturbs redox homeostasis (Nemat Alla et al. 2007; Chakraborty et al. 2019). ROS are generated in plant seedlings comprising important requirements in signaling; however, their ranges should remain suitable, and salinity adversely affected this scenario. Plants have to overcome these imbalances in metabolism through the ROS detoxification machinery. In the present results, the increments of ROS contents in NaCl-treated maize seedlings were greatly lowered upon the application of the nitrogenous sources, indicating the induction of NaCl-induced oxidative stress in treated samples while N stimulated the relief. On the contrary, NaCl greatly dropped SOD, CAT and APX activities; a similar drop was also detected for GSH and AsA contents; however, more inductions were detected following N supplementation. These results affirm that N application highly counterbalanced the diminutions in antioxidants and the elevations in ROS confirming that N enforced maize to enhance the enzymatic and nonenzymatic antioxidants to tackle with oxidative damages. The ameliorations in these antioxidants along with the retraction in the oxidative stress parameters could conclude that N, particularly urea, improved antioxidants and ROS homeostasis as effective salt tolerance mechanisms. Urea could increase antioxidase activities by adjusting osmosis and activating the antioxidants for alleviation of oxidative nuisance and scavenging of ROS (Kaya et al. 2015). Moreover, Zhang et al. (2015) concluded that urea remarkably elevated N and nitric oxide which could reduce the generation of ROS, stimulate the SOD, CAT and POD activities, and enhance photosynthetic pigments. So, improving antioxidants, ROS homeostasis and osmoregulation were generally synchronized with the ameliorations in photosynthetic activity and growth parameters. These findings confirm that N could increase maize tolerance to NaCl through effectors of stress adaptation that mediate osmoregulation, antioxidants, ROS homeostasis and photosynthetic activity.
Conclusion
The significant decrease in photosynthetic activity parameters (Hill reaction, Pn, Ci and Gs) of maize seedlings by NaCl was synchronized with remarkable decreases in fresh and dry weights, protein, chlorophylls, GSH and AsA, along with great depressions in ALA-D, Rubisco, PEP-C, MDH, PPDK, SOD, CAT and APX activities; however, there were significant stimulations in MDA, O .−2 , H2O2, soluble sugars and proline. The reduced growth might be due to low availability of N under adverse conditions, since vital processes in plants need N for biological systems. The depressions in photosynthetic activity and enzymes and in antioxidants concomitantly with the accumulated ROS could reveal that maize was vulnerable to suffer from NaCl stress. On the contrary, this study clearly showed that N effectively mitigated salt stress impacts. N stimulated growth and reduced NaCl negative effects on the photosynthetic apparatus and on the antioxidant system and ROS homeostasis. These improvements indicate a repair in photosynthetic activity and antioxidant system by N; urea seemed the most efficient. So, the present findings conclude that N increased maize tolerance to NaCl through protecting the photosynthetic apparatus and improving antioxidants and ROS homeostasis.
Author contribution statement
MMN and NMH have the same contribution toward the experiment design, attainment of data, analysis and performance of the experiment and prearranging of the manuscript. The ultimate manuscript was read and approved by MMN and NMH.
Abbreviations
- 2,6-DCPIP:
-
2,6-Dichlorophenolindophenol
- 3-PGA:
-
3-Phosphoglyceric acid
- ALA:
-
δ-Aminolevulinate
- ALA-D:
-
δ-Aminolevulinate dehydratase
- ANOVA:
-
Analysis of variance
- APX:
-
Ascorbate peroxidase
- AsA:
-
Ascorbic acid
- CAT:
-
Catalase
- CDNB:
-
1-Chloro-2,4-dinitrobenzene
- Ci:
-
Sub-stomatal CO2 concentration
- CP:
-
Creatine phosphate
- CPase:
-
Creatine phosphokinase
- DTT:
-
Dithiothreitol
- Gs:
-
Stomatal conductance
- GSH:
-
Glutathione
- Hibitane:
-
Chlorohexidine diacetate
- LSD:
-
Least significant differences
- MDA:
-
Malondialdehyde
- MDH:
-
Malate dehydrogenase
- Na-tricine:
-
N-tris hydroxymethyl methyl glycine
- O ∙−2 :
-
Superoxide radical
- OAA:
-
Oxaloacetate
- PEP:
-
Phosphoenolpyruvate
- PEP-C:
-
Phosphoenolpyruvate carboxylase
- PGAldDH:
-
Glyceraldehydes-3-phosphate dehydrogenase
- PGK:
-
Phosphoglycerate kinase
- Pn:
-
Net photosynthetic rate
- PPDK:
-
Pyruvate phosphate dikinase
- PVP:
-
Polyvinyl pyrrolidone
- PVPP:
-
Polyvinyl polypyrrolidone
- ROS:
-
Reactive oxygen species
- Ru-1,5-P:
-
Ribulose-1,5-bisphosphate
- Rubisco:
-
Ru-1,5-P carboxylase/oxygenase
- SOD:
-
Superoxide dismutase
- TCA:
-
Trichloroacetic acid
References
Anderson MP, Gronwalds JW (1991) Atrazine resistance in velvetleaf (Abutilon theophrasti) biotype due to enhanced glutathione S-transferase activity. Plant Physiol 96:107–109
Ashton AR, Burnell JN, Furbank RT, Jenkins CL, Hatch MD (1990) Enzymes of C4 Photosynthesis. In: Dey PM, Harborne JB (eds) Methods in plant biochemistry. Enzymes of primary metabolism, vol 3. Academic Press, London, pp 39–72
Badran EG, Abogadallah GM, Nada RM, Nemat Alla MM (2015) Role of glycine in improving the ionic and ROS homeostasis during NaCl stress in wheat. Protoplasma 252:835–844
Bates LE, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Beer RF Jr, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Borella J, Becker R, Lima MC, de Oliveira DS, Braga EJ, de Oliveira AC, do Amarante L (2019) Nitrogen source influences the antioxidative system of soybean plants under hypoxia and re-oxygenation. Sci Agric 76:51–62
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chaitanya KS, Naithani SC (1994) Role of superoxide, lipid peroxidation and superoxide dismutase in membrane perturbation during loss of viability in seeds of Shorea robusta Gaertn. f. New Phytol 126:623–627
Chakraborty K, Bishi SK, Goswami N, Singh AL, Bhaduri D, Zala PV (2019) Salinity-induced changes in seed germination and the expression profile of antioxidant enzymes in peanut as early and late responses in emerging radicles. Acta Physiol Plant 41:134–149
Dhindsa RS, Plumb-Dhindsa P, Throne TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–110
Gururani MA, Venkatesh J, Tran LSP (2015) Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol Plant 8:1304–1320
Harborne JB (1984) Macromolecules. In: Harborne JB (ed) Phytochemical methods. Chapman and Hall, London, p 243
Hassan NM, Serag MS, El-Feky FM, Nemat Alla MM (2008) In vitro selection of mung bean and tomato for improving tolerance to NaCl. Ann Appl Biol 152:319–330
Hassan NM, El-Bastawisy ZM, El-Sayed AK, Ebeed HT, Nemat Alla MM (2015) Roles of dehydrin genes in wheat tolerance to drought stress. J Adv Res 6:179–188
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments. Plant Signal Behav 7:1456–1466
Jedmowski C, Ashoub A, Brüggemann W (2013) Reactions of Egyptian landraces of Hordeum vulgare and Sorghum bicolor to drought stress, evaluated by the OJIP fuorescence transient analysis. Acta Physiol Plant 35:345–354
Kaya C, Sonmez O, Ashraf M, Polat T, Tuna L, Aydemir S (2015) Exogenous application of nitric oxide and thiourea regulates on growth and some key physiological processes in maize (Zea mays L.) plants under saline stress. Soil Water J S:61–66
Keys AJ, Parry MA (1990) Ribulose bisphosphate carboylase/oxygenase and carbonic anhydrase. In: Dey PM, Harborne JB (eds) Methods in plant biochemistry. Enzymes of primary metabolism, vol 3. Academic Press, London, pp 1–14
Koenigshofer H, Loeppert H (2019) The up-regulation of proline synthesis in the meristematic tissues of wheat seedlings upon short-term exposure to osmotic stress. J Plant Physiol 237:21–29
Kong X, Luo Z, Dong H, Li W, Chen Y (2017) Non-uniform salinity in the root zone alleviates salt damage by increasing sodium, water and nutrient transport genes expression in cotton. Sci Rep 7:2879
Kumawat RN, Mahla HR (2015) Effect of foliar applied urea and planting pattern on the leaf pigments and yield of cluster bean (Cyamopsis tetragonoloba L.) grown in low rainfall areas of western India. Legum Res 38:96–100
Lang Y, Wang M, Xia JB, Zhao QK (2018) Efects of soil drought stress on photosynthetic gas exchange traits and chlorophyll fuorescence in Forsythia suspensa. J For Res 29:45–53
Mauzerall D, Granick S (1956) The occurrence and determination of 5-aminolevulinic acid and porphobilinogen in urea. J Biol Chem 219:435–446
Mazumdar P, Lau S, Singh P, Takhtgahi HM, Harikrishna JA (2019) Impact of sea-salt on morpho-physiological and biochemical responses in banana (Musa acuminata cv. Berangan). Physiol Mol Biol Plants 25:713–726
Metzner H, Rau H, Singer H (1965) Untersuchungen zur synchronisierbarkeit einzelner pigment mangel mutanten von Chlorella. Planta 65:186–194
Mukherjee SP, Choudhuri MA (1983) Implications of water stress induced changes in the levels of endogenous ascorbic acid and H2O2 in Vigna seedlings. Plant Physiol 58:166–170
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxide in spinach chloroplast. Plant Cell Physiol 22:867–880
Nemat Alla MM, Hassan NM (1998) Efficacy of exogenous GA3 and herbicide safeners in protection of Zea mays from metolachlor toxicity. Plant Physiol Biochem 36:809–815
Nemat Alla MM, Hassan NM (2014) Alleviation of isoproturon toxicity to wheat by exogenous application of glutathione. Pestic Biochem Physiol 112:56–62
Nemat Alla MM, Badawi AM, Hassan NM, El-Bastawisy ZM, Badran EG (2007) Induction of glutathione and glutathione-associated enzymes in butachlor-tolerant plant species. Am J Plant Physiol 2:195–205
Nemat Alla MM, Khedr AA, Serag MM, Abu-Alnaga AZ, Nada RM (2011) Physiological aspects of tolerance in Atriplex halimus L. to NaCl and drought. Acta Physiol Plant 33:547–557
Nemat Alla MM, Abogadallah GM, Badran EG, Nada RM, Hassan NM (2016) Role of CaCl2 in osmoregulation and up-regulation of the salt stress related genes, NHX1 and SOS1 in wheat during NaCl stress. Agrochimica 60:29–42
Okuda T, Masuda Y, Yamanaka A, Sagisaka S (1991) Abrupt increase in the level of hydrogen peroxide in leaves of winter wheat is caused by cold treatment. Plant Physiol 97:1265–1267
Parida AK, Das AB (2005) Salt tolerance and salinity effect on plants: a review. Ecotoxicol Environ Saf 60:324–349
Perreault F, Dionne J, Didur O, Juneau P, Popovic R (2011) Efect of cadmium on photosystem II activity in Chlamydomonas reinhardtii: alteration of O-J-I-P fuorescence transients indicating the change of apparent activation energies within photosystem II. Photosynth Res 107:151–157
Perveen S, Iqbal N, Saeed M, Zafar S, Arshad Z (2018) Role of foliar application of sulfur-containing compounds on maize (Zea mays L. var. Malka and hybrid DTC) under salt stress. Braz J Bot 41:805–815
Schlüter U, Crawford RM (2001) Long-term anoxia tolerance in leaves of Acorus calamus L. and Iris pseudacorus L. J Exp Bot 52:2213–2225
Spreitzer RL (1993) Genetic dissection of Rubisco structure and function. Annu Rev Plant Physiol Plant Mol Biol 44:411–434
Sun CQ, Chen FD, Teng NJ, Liu ZL, Fang WM, Hou XL (2010) Interspecifc hybrids between Chrysanthemum grandiforum (Ramat.) Kitamura and C. indicum (L.) Des Moul. and their drought tolerance evaluation. Euphytica 174:51–60
Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Trebst A (1972) Measurement of Hill reaction and photoreduction. In: Pietro SA (ed) Methods in enzymology. Academic Press, New York, pp 146–165
Wu Y, Liao W, Dawuda MM, Hu L, Yu J (2019) 5-Aminolevulinic acid (ALA) biosynthetic and metabolic pathways and its role in higher plants. Plant Growth Regul 87:357–374
Zhang LX, Zheng P, Ruan Z, Tian L, Ashraf M (2015) Nitric oxide accumulation and glycinebetaine metabolism in two osmotically stressed maize cultivars supplied with different nitrogen forms. Biol Plant 59:183–186
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by P. Wojtaszek.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nemat Alla, M.M., Hassan, N.M. Nitrogen alleviates NaCl toxicity in maize seedlings by regulating photosynthetic activity and ROS homeostasis. Acta Physiol Plant 42, 93 (2020). https://doi.org/10.1007/s11738-020-03080-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-020-03080-6