Abstract
Drought stress produces many physiological and biochemical changes in plant affecting its life cycle and production. Oxidative damage and antioxidant defense responses are two components of plant to survive under drought stress. Nitric oxide (sodium nitroprusside, SNP) and brassinosteroid (24-epibrassinolide, EBL) were used in this experiment as single and combined application as foliar spray to study the mitigating effect of drought stress in two tomato genotypes EC-625652 (drought susceptible) and EC-620419 (drought tolerant). Drought stress produced harmful effect on number of leaves plant−1, RWCL, fruit set percent, days to first fruit set, number of cluster plant−1, lycopene content, fruit diameter and fruit yield. Plant produces reactive oxygen species (ROS), such as H2O2 in response to drought stress. Exogenous application of SNP and EBL, both in single and combined application, mitigated the deleterious effects of drought and improved drought tolerance by increasing SOD activity, fruit yield, and other physiological processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tomato (Lycopersicon esculentum Mill.) is the second most important vegetable crop in the world after potato. Drought stress is a serious abiotic stress that limits crop production worldwide (Kramer and Boyer 1995). ROS production is the consequence of drought stress. Superoxide (O2 −), singlet oxygen (•O2), hydroxyl ions (OH−), and hydrogen peroxide (H2O2) are accumulated in plant cell during drought stress which have harmful effects on nucleic acids, proteins, and lipids (Smirnoff 1993). Plant has specific antioxidative defense mechanism to combat the effect of these toxic elements by producing antioxidants. Nitric oxide (NO) and brassinosteroid (BR) are known to regulate antioxidant system against abiotic stresses. Accumulation of superoxide dismutase (SOD) scavenges O2 − to H2O2 (Bowler et al. 1992), while peroxidase (POD), catalase (CAT) changes H2O2 to H2O at different cellular locations (Asada 1999). NO is a diffusible gas and a ubiquitous bioactive molecule which plays an important role in signal transduction during stress condition (Lamattina et al. 2003). Braasinosteroid is a plant growth regulator which is involved in many physiological functions, such as stem elongation, leaf bending, pollen tube growth epinasty, proton pump activation, vascular differentiation, and ethylene biosynthesis (Sasse 2003). It also increases resistance level of plants against osmotic stress (Sairam 1994). This paper describes the ameliorating effect of nitric oxide and brassinosteroid on drought stress in tomato plant.
Materials and methods
For the present experiment, tomato genotypes EC-625652 (drought susceptible) and EC-620419 (drought tolerant) were procured from the Indian Institute of Vegetable Research, (IIVR), Varanasi, India. Tomato seeds were germinated in horticulture nursery and transplanted after 1 month (5–6 leaf stages) in 100 pots (20 cm diameter) in net house of the Institute of Agriculture Sciences, Banaras Hindu University, Varanasi. Completely randomised design (CRD) with ten treatments (T0–T9) and five replications of pots were used, where T0 = Control, T1 = drought, T2 = drought + SNP (50 μM), T3 = drought + SNP (100 μM), T4 = drought + EBL (1 μM), T5 = drought + EBL (3 μM), T6 = drought + SNP (100 μM) + EBL (1 μM), T7 = drought + SNP (100 μ M) + EBL (3 μM), T8 = drought + SNP (50 μM) + EBL (1 μM), and T9 = drought + SNP (50 μM) + EBL (3 μM). Each pot has single tomato plant. Drought was induced by withholding water. Water holding was created on 30 days after transplantation at vegetative stage for 7 days. Plants were re-watered when 50% of the treated plants showed the sign of wilting during treatment. Sodium nitroprusside (SNP) was used as NO donor, whereas 24-epibrassinolide ≥85% (EBL) (C28H48O6, M w = 480; Sigma-Aldrich product) was used as brassinosteroid. Both these ameliorative agents were sprayed as foliar application at the stress condition of drought. SNP (50 and 100 μM) and EBL (1 and 3 μM), in single as well as combined form, were used in drought stressed plant as foliar application after 24 h of water holding. Foliar spray was applied on alternate day during treatment. The experiment was done for 2 years of study, i.e., 2013 and 2014.
Volumetric water content (VWC)
In experimental pots, VWC was measured during stress treatment. The time-domain refractometer (TDR300) was used to measure VWC with 3 inch rod in pot soil on alternate days during stress treatment at 2, 5, and 7 days after treatments and expressed as indicated in Table 1. On the basis of VWC (%), it is evident that plant felt water stress under drought treatment T1–T9. Control plant soil showed higher average VWC (68.97%) as compared to drought-induced plant soil average VWC (29.31%).
Morphological and physiological parameters
Numbers of leaves of tagged plants was observed using random sample technique at 20, 40, and 60 days after treatment (DAT). The number of leaves of tagged plants was counted. Relative water content of leaf was measured by the method of Barrs and Weatherley (1962). Hundred mg leaves of tomato were taken and kept in Petridish already filled with double distilled water for 2 h. The turgid weights of the leaf sample were measured, and the same leaf samples were dried in oven at 65 °C for 24 h to record dry weight. The RWCL was calculated using the formula:
H2O2 content (µ mol g−1 FW)
Estimation of H2O2 was done following method of Mukherjee and Choudhari (1983). Leaf sample (0.1 g) was homogenized in 10 ml cold acetone and centrifuged at 10,000 rpm. Four ml of titanium reagent was added followed by 5 ml of concentrated ammonium solution to precipitate peroxide-titanium complex. The contents were centrifuged for 5 min at 10,000 rpm and precipitate dissolved in 10 ml 2 N H2SO4. The residue was centrifuged again to remove undissolved material, and absorbance was recorded at 415 nm against blank in spectrophotometer (Model, SpectraMax M2). Concentration of H2O2 was determined using the standard curve plotted with known concentration of H2O2.
SOD activity (unit g−1 FW min−1)
SOD activity was measured by the method of Dhindsa et al. (1981). Hundred mg leaf sample was grinded with 10 mL of extraction buffer (0.1 M phosphate buffer, pH 7.5 containing 0.5 mM EDTA) and centrifuged at 10,000×g for 10 min. Reaction mixture (3 mL) contained 0.1 mL of 1.5 M sodium carbonate, 0.2 mL of 200 mM methionine, 0.1 mL of 2.25 mM NBT, 0.1 mL of 3 mM EDTA, 1.5 mL of 100 mM potassium phosphate buffer, 1 mL of distilled water, and 0.1 mL of enzyme extract. This reaction mixture was taken in test tubes in duplicate for each enzyme sample. Control was used without enzyme extract. Riboflavin 0.1 mL (60 µM) was added for starting the reaction, and the tubes were kept below a light source of two 15 W florescent lamps for 15 min. Reaction was stopped by switching off the light. Tubes without enzyme extract developed maximum colour. A non-irradiated complete mixture that did not develop colour served as blank. Absorbance was recorded at 560 nm:
where (−) = without enzyme, (+) = with enzyme.
Lycopene content was determined by the method of Sadasivam and Manickam (1992). Five grams of fruit sample was crushed with acetone and extract transferred to separating funnel containing 15 mL of petroleum ether and mixed gently. Five mL sodium sulphate (5%) was added and mixed thoroughly by shaking and then transferred to 25 mL volumetric flask and diluted with petroleum ether for colour measurement. Absorbance of the extract was measured at 503 nm using spectrophotometer (Model, SpectraMax M2) with petroleum ether as a blank. Lycopene content of the sample was calculated using the following formula:
Analysis for reproductive parameters was done using the conventional methods.
Statistical analysis
Completely randomised design (CRD) with three replicates was used for this experiment. Data were statistically analysed by one-way analysis of variance (ANOVA), using SPSS (version 16.0). Data were presented in the form of mean ± standard error mean (SEM). The same letters within the columns are not significant. Duncan’s multiple range test (DMRT) at P ≤ 0.05 probability level was used for separation of means (Duncan 1955).
Results
The results for each parameter are presented for both the years of study, i.e., 2013 and 2014.
Number of leaves
In drought susceptible genotype EC-625652, drought stress (T1) reduced average leaf number (46%) as compared to control (T0) (Fig. 1). Combined treatment of SNP (100 µM) and EBL (3 µM) in T7 showed maximum mitigating effect by increasing leaf number (57.63%) as compared to drought stress, T1 followed by T9 (54.83%), T8 (51.83%). EBL (3 µM), when used singly (T5), showed more leaf number (47.67%) as compared to T1 followed by T4 (42.68%). Single use of SNP (100 µM; T3) was found to be more effective by increasing leaf number (42.51%) as compared to T1 followed by T2 (26.41%), with the treatment of SNP (50 µM).
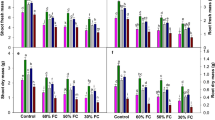
1–14 Effect of nitric oxide and brassinosteroids on number of leaves (Figs. 1, 2), relative water content of leaf (Figs. 3, 4), hydrogen peroxide (H2O2) content (Figs. 5, 6), superoxide dismutase (SOD) activity (Figs. 7, 8), days to first fruit set (Fig. 9), fruit set percent (Fig. 10), number of flower cluster plant−1 (Fig. 11), lycopene content (Fig. 12), equatorial fruit diameter (Fig. 13), fruit yield (Fig. 14), in tomato genotypes EC-625652 (susceptible) and EC-620419 (tolerant) under drought stress, where T0 = Control, T1 = drought, T2 = drought + SNP (50 µM), T3 = drought + SNP (100 µM), T4 = drought + EBL (1 µM), T5 = drought + EBL (3 µM), T6 = drought + SNP (100 µM) + EBL (1 µM), T7 = drought + SNP (100 µM) + EBL (3 µM), T8 = drought + SNP (50 µM) + EBL (1 µM), and T9 = drought + SNP (50 µM) + EBL (3 µM). Data are in the form of mean ± SEM and means followed by the same letters within the columns are not significantly different at P ≤ 0.05 using Duncan’s multiple range test
Relative water content of leaf (RWCL)
RWCL is the physical indicator of plant affected by drought stress. It was observed during both years 2013 and 2014, in both the genotypes under drought stress vis-a-vis the effect of nitric oxide, brassinosteroid and their combination at 20, 40, and 60 DAT (Fig. 1). During 2013, in the drought susceptible genotype EC-625652, drought stress (T1) reduced RWCL (27.62%) significantly as compared to control (T0). Combined treatment of SNP (100 µM) and EBL (3 µM) in T7 showed maximum mitigating effect by increasing average RWCL (33.36%) as compared to drought stress, T1 followed by T9 (30.64%) and T8 (29.24%). EBL (3 µM), T5 used singly, showed more average RWCL (21.66%) as compared to T1 followed by T4 (15.83%). Single application of SNP (100 µM; T3) was found more effective by increasing RWCL (19.10%) as compared to T1 followed by T2 (16.48%).
Hydrogen peroxide (H2O2, µ mol g−1 FW)
In genotype EC-625652, drought stress (T1) showed significant increase (203.72%) as compared to control (T0) (Fig. 1). Combined treatment (T7) of SNP (100 µM) and EBL (3 µM) performed best by decreasing H2O2 content (37.77%) as compared to drought stress (T1) followed by T9 (31.52%), T8 (31.39%), and T6 (26.68%). EBL (3 µM; T5), when used singly, showed a better effect by decreasing H2O2 content (31.31%) as compared to T1 followed by T4 (23.99%). Single application of SNP, T3 (100 µM) showed good effect by decreasing H2O2 content (29.46%) as compared to T1 followed by T2 (23.37%) having 50 µM SNP.
Superoxide dismutase activity (SOD, unit g−1 FW min−1)
Drought stress (T1) showed a significant increase (45.15%) in SOD activity as compared to control (T0) (Fig. 1). Combined treatment of SNP (100 µM) and EBL (3 µM) in T7 showed the best effect by increasing SOD content (68.63%) as compared to drought stress (T1) followed by T9 (51.58%), T8 (49.51%), and T6 (43.55%). EBL (3 µM; T5) alone showed a better effect by increasing SOD activity (34.20%) as compared to T1 followed by T4 (22.82%), having 1 µM EBL. Single application of SNP (100 µM; T3) showed a good effect by increasing SOD activity (30.73%) as compared to T1 followed by T2 (20.97%).
Days to first fruit set
Drought stress (T1) caused days to first fruit set to significantly decrease (26.90%) as compared to control (T0) (Fig. 1). Combined treatment of SNP (100 µM) and EBL (3 µM) has shown best effect by increasing average days to first fruit set (22.10%) as compared to drought stress (T1) followed by T9 (21.10%), T8 (21.10%), and T6(20.50%). Single foliar application of EBL (3 µM; T5) produced a better effect by increasing days to first fruit set (14.20%) as compared to T1 followed by T4 (13.20%). Single application of SNP (100 µM), T3, showed good effect by increasing days to first fruit set (17.90%) as compared to T1 followed by T2 (7.40%) with the treatment of SNP (50 µM).
Fruit set percent
Water stress (T1) caused fruit set percent to significantly decrease (25.56%) as compared to control (T0) (Fig. 1). Combined treatment of SNP (100 µM) and EBL (3 µM) in T7 has shown good effect by increasing average fruit set percent (25.54%) as compared to drought stress (T1) followed by T6 (24.36%), T9 (22.08%), and T8 (21.19%). In case of single application of EBL, T5 (3 µM) showed a better effect by increasing fruit set percent (18.45%) as compared to T1 followed by T4 (11.55%) with the treatment of EBL (1 µM). In case of single application of SNP (100 µM), T3, showed the best effect by increasing fruit set percent (11.55%) as compared to T1 followed by T2 (7.12%) with the treatment of SNP (50 µM).
Number of flower clusters plant−1
Figure 1 shows the effect of nitric oxide, brassinosteroid, and their combination on number of flower clusters plant−1 under drought stress in two tomato genotypes. During 2013, in the drought susceptible genotype EC-625652, under drought stress (T1), the number of flower clusters plant−1 significantly decreased (42.86%) as compared to control (T0). Combined treatment of SNP (100 µM) and EBL (3 µM) produced the best effect by increasing average number of flower clusters plant−1 (37.46%) as compared to drought stress, followed by T9 (34.33%), T8 (34.33%), and T6 (21.84%). EBL (3 µM; T5), when applied alone, showed a better effect by an increasing number of flower clusters plant−1 (24.96%) as compared to T1 followed by T4 (18.17%) with the treatment of EBL (1 µM). Single application of SNP (50 µM), T2, showed a good effect by an increasing number of flower clusters plant−1 (18.71%) as compared to T1 followed by T3 (6.22%).
Lycopene content (mg 100 g−1)
Drought stress (T1) led to significant increase in lycopene content (23.46%) in the drought susceptible genotype EC-625652 as compared to control (T0) (Fig. 1). Combined treatment of SNP (100 µM) and EBL (3 µM) in T7 showed the best effect by an increasing average lycopene content (22.80%) as compared to drought stress, T1, followed by T9 (19.89%), T8 (18.57%), and T6 (16.40%). A single application of EBL (3 µM) showed a better effect by increasing lycopene content (12.81%) as compared to T1 followed by T4 (6.62%), with the treatment of EBL (1 µM). In a single application, SNP (100 µM) showed a good effect by an increasing lycopene content (8.69%) as compared to T1 followed by T2 (5.75%) having 50 µM SNP.
Equatorial fruit diameter (mm)
In the drought susceptible genotype EC-625652, drought stress (T1) led fruit diameter (width) to decrease significantly (45.53%) as compared to control (T0) (Fig. 1). Combined treatment of SNP (100 µM) and EBL (3 µM, T7) showed the best effect by an increasing average fruit diameter (width) (59.22%) as compared to drought stress, T1 followed by T9 (55.31%), T8 (53.31%), and T6 (48.96%). EBL (3 µM), T5 when applied singly, increased fruit diameter (width) (50.20%) as compared to T1 followed by T4 (36.07%) with the treatment of EBL (1 µM). A single application of SNP (100 µM) and T3 showed a good effect by an increasing fruit diameter (width) (47.81%) as compared to T1 followed by T2 (40.81%), with the treatment of SNP (50 µM).
Fruit yield plant−1 (kg)
In the drought susceptible genotype EC-625652, drought stress (T1) led fruit yield plant−1 to significantly decrease (53.52%) as compared to control (T0) (Fig. 1). Combined treatment of SNP (100 µM) and EBL (3 µM) in T7 produced the best effect by an increasing average fruit yield plant−1 (88.21%) as compared to drought stress, T1 followed by T9 (80.51%), T8 (75.38%), and T6 (73.33%). EBL, when applied singly (T5, 3 µM), showed a better effect by an increasing fruit yield plant−1 (66.15%) as compared to T1 followed by T4 (53.13%) with the treatment of EBL (1 µM). In a single application of SNP (T3, 100 µM), a good effect was observed with increased fruit yield plant−1 (45.64%) as compared to T1 followed by T2 (34.67%) having 50 µM SNP.
Drought tolerant genotype EC-620419 also showed almost similar trend under drought stress and with treatments of SNP and EBL, for all these parameters. Similar trend was observed during investigation made during 2014.
Discussion
Drought stress affects leaf turgor and assimilation supply for growth which causes reduction in number of leaf (Reddy et al. 2003; Seng 2014). In the present study, the number of leaves was reduced significantly under the influence of drought stress. Similar results were found in the previous studies of Hussain et al. (2008), Bhatt and Srinivasa Rao (2005) and Sankar et al. (2007). Treatments with SNP and EBR, both singly and in combined form, increased number of leaves in the present study. These results are in conformity with the earlier findings of Shi et al. (2014) and Cechin et al. (2015). Relative water content of leaf was reduced in consequence of water stress in T1 as compared to control, T0. This result is in agreement with Hayat et al. (2008), Yuan et al. (2010) and Calcagno et al. (2011). 24-EBL significantly increased RWCL in tomato (Yuan et al. 2010). Exogenous application of SNP and EBL significantly increased RWCL under drought stress in the present study, similar to those reported by Hayat et al. (2010). SNP treatment in wheat seedlings increased drought tolerance by maintaining higher water in tissues as compared to stressed plant (Garcia-Mata and Lamattina 2001). SNP treatment is reported to increase RWCL under drought stress (Cechin et al. 2006). Drought stress is closely related to oxidative stress in which H2O2 and other active oxygen species (AOS) are produced in plant cell (Cassells and Curry 2001; Lamattina et al. 2003; Konieczny et al. 2008). Stress condition produces reactive oxygen species (ROS) which acts as toxic elements in higher concentration and work as signalling molecules in low concentration and produces antioxidant system (Gill and Tuteja 2010). Drought stress resulted in a significant increase in H2O2 content (Zhang et al. 2010). Exogenous application of BRs increases the antioxidant defense mechanism of plants under stress conditions (Yardanova et al. 2004). Application of EBL showed less amount of H2O2 as compared to drought stressed tomato plant (Behnamnia et al. 2009a; Yuan et al. 2010). NO (SNP) decreased hydrogen peroxide content by scavenging H2O2 under water stress. This result is in conformity with the earlier findings of Tian and Lei (2006), Sang et al. (2008), Siddiqui et al. (2010) and Kavas et al. (2013). Drought stress produces oxidative stress which disturbs pro-oxidant antioxidant balance in the cell (Reddy et al. 2005). Several antioxidant enzymes, such as SOD, CAT and peroxidase, increased in cell in response to drought stress (Reddy et al. 2005). Antioxidant capacity of plants has been improved by the treatment with BRs under stress conditions (Yin et al. 2008). Exogenous application of BRs significantly increased the activity of SOD under drought stress in the present finding (Vardhini et al. 2011; Yuan et al. 2010; Behnamnia et al. 2009b), and also NO treatment increased SOD activity under drought stress (Siddiqui et al. 2010; Cechin et al. 2015). Higher SOD activity improves the ROS scavenging system and controls the level of ROS leading to increased drought tolerance capacity (Ghahfarokhi et al. 2015). Treatment with NO significantly reduced the superoxide anion level in the microsomes isolated from soybean embryonic axes (Caro and Puntarulo 1998). Increased SOD activity was correlated with increased drought tolerance (Asada 1999). Increased SOD activity in drought stress has also been correlated with enhancement of catalase activity (Bin et al. 2010), because it is well understood by the previous research that SOD detoxifies superoxide anion free radicals (O2 −) by forming H2O2, and then H2O2 can be eliminated by catalase (Hasheminasab et al. 2012). Days to flowering and fruiting are accelerated in plants by stress condition (Singh et al. 2007). Drought stress affects reproductive phase by delaying or inhibiting flowering, and it accelerates days to flowering (Wudiri and Henderson 1985; Fang et al. 2009). NO has an important role in floral regulation (He et al. 2004). According to Desclaux and Roumet (1996), plant developmental phase is stimulated to turn from vegetative to reproductive phase by the indication of drought stress. Reproductive phase is the most susceptible to drought stress in tomato (Wahid et al. 2007; Preedy and Watson 2008). Drought stress can delay or inhibit flowering and accelerate days to fruiting (Wudiri and Henderson 1985). Low water availability at the stage of vegetative growth, flowering and fruiting affects physiological processes in plant (Rad and Vijaya 1991). Reproductive stages in tomato, such as flower and fruit setting, are most sensitive to drought stress (Salter 1954). Drought stress reduced flower and fruit set percent in chick pea (Fang et al. 2009). According to Horchani et al. (2008), there were two possible reasons for tomato flower and fruit abscission under drought stress. First, there might be stress-induced ethylene accumulation in the above ground organs. Second, the carbohydrate supply to the flowers and fruits might be restricted because of a limitation in photosynthetic activity. Treatments with SNP and EBL, in single and combined form, regulated days taken for fruiting, in the present study. Drought stress reduced number of flower plant−1 in T1 as compared to control T0. The results are similar to the study of Subramanian et al. (2006) and Fang et al. (2009). Number of flower clusters−1 reduced under drought stress (Veershetty 2004). Treatments with SNP and EBL increased number of flower clusters−1 by improved reproductive growth with reduction of water loss and accumulation of higher water in plant tissues. It has been reported in the previous finding that BRs actively participate in floral development and pollen tube growth. NO shows the effect on reproductive growth and flowering (Simpson 2005). Nitric oxide mitigates the impact of drought stress and regulates flowering in plants (Corpas et al. 2011).
Lycopene is a key quality parameter in tomato which plays an important role in biosynthesis of carotenoids. It is responsible for red colour in tomato and processed products. Lycopene acts as an antioxidant having specific role in defense mechanism against environmental stresses by scavenging peroxyl radicals and quenching singlet oxygen. An experiment done by Giannakoula and Ilias (2013) estimated quality parameter in tomato genotypes under drought stress condition, and there was a significant increase in lycopene content during water and salinity stress. Lycopene content in tomato fruits increased up to 32% under drought stress. Treatments with SNP and EBL increased lycopene content in the present study (Ali and Ismail 2014). Low water availability reduced proper growth and development of fruit. Phloem translocation and assimilation of photosynthetic material are also affected by drought stress. It is reported in the previous studies that fruit dimension gets reduced under drought stress in tomato (Molla et al. 2003; Chavan et al. 2010), in brinjal (Subramanian et al. 1993 and Halil et al. 2001). According to Giardini et al. (1988), under low water condition, tomato plant has reduced yield and fruit size. Higher water accumulation and reduction in water loss by the exogenous application of SNP and EBL improved growth and development process in reproductive phase of plant. BR application increased yield by increasing fruit size in tomato (Nuñez 2000). Treatments with SNP and EBL increased fruit length (Ali and Ismail 2014). Average fruit yield plant−1 (kg) was reduced in drought stress treatment (T1) in both genotypes EC-625652 and EC-620419 as compared to control (T0). Similar result was reported by others (Rana and Kalloo 1989; Chavan et al. 2010). Drought stress is a serious environmental stress which affects agriculture productivity and yield. It is an important factor which harms more than 50% of crop yield worldwide (Bray et al. 2000; Wang et al. 2003). According to Kramer (1969), drought stress affects physiological process of plant at different stages and reduces the quality and yield. A major impact of drought stress is the reduction in photosynthesis by decreasing leaf area, impairs photosynthetic system, and premature leaf senescence; finally, it is associated with reduction in food production. Application of EBL and SNP increased yield under drought stress in the present finding. BRs’ application increases tomato yield (Vardhini and Rao 2001) by increasing fruit size. SNP is involved in increased photosynthesis and final yield under drought stress (Santisree et al. 2015).
Conclusion
Drought stress significantly reduced morphological parameters, such as leaf area and relative leaf water content (RLWC) as a result of low water availability in both genotypes. Low water availability induced phenological changes in plant as days to first fruit set was hastened in both genotypes. Lycopene content was slightly increased with drought stress, but it was accelerated by the application of SNP and EBL. Biochemical parameters, such as H2O2 content and SOD activity, significantly increased with the effect of drought stress. Number of flower clusters plant−1, equatorial diameter of fruit and fruit yield plant−1 (kg) significantly declined with the effect of drought stressed plant as compared to control. Drought susceptible genotype EC-625652 was more affected with the deleterious effect of drought stress as compared to resistant genotype EC-620419. Exogenous applications of SNP and EBL in different concentrations, in both single and combined treatments, ameliorated the effect of drought stress by improving drought resistance capacity of plant in various physiological and biochemical parameters studied. The combined treatment of SNP @ 100 µM and EBL @ 3 µM (T7) showed the best results in various parameters followed by SNP @ 50 µM + EBL @ 3 µM (T9), SNP @ 50 µM + EBL @ 1 µM (T8), and SNP @ 100 µM + EBL @ 1 µM (T6). Single application of SNP and EBL also showed good results. Application of EBL was more effective than SNP in single treatment. Single application of EBL @ 3 µM was more effective than EBL @ 1 µM, while SNP @ 100 µM was more effective than SNP @ 50 µM.
Author contribution statement
KKJ and PD have contributed equally.
Abbreviations
- SNP:
-
Sodium nitroprusside
- EBL:
-
24-Epibrassinolide
- ROS:
-
Reactive oxygen species
- RWCL:
-
Relative water content of leaf
- H2O2 :
-
Hydrogen peroxide
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
References
Ali HEM, Ismail GSM (2014) Tomato fruit quality as influenced by salinity and nitric oxide. Turk J Bot 38:122–129
Asada K (1999) The water–water cycle in chloroplasts, scavenging of active oxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Barrs HD, Weatherley PE (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Bio Sci 15:413–428
Behnamnia M, Kalantari KM, Ziaie J (2009a) The effects of brassinosteroid on the induction of biochemical changes in Lycopersicon esculentum under drought stress. Turk J Bot 33:417–428
Behnamnia M, Kalantari KM, Rezanejad F (2009b) Exogenous application of brassinosteroid alleviates drought-induced oxidativestress in Lycopersicon esculentum L. Gen App Plant Physiol 35(1–2):22–34
Bhatt RM, Srinivasa Rao NK (2005) Influence of pod load on response of okra to water stress. Indian J Plant Physiol 10:54–59
Bin T, Sang-Zhong X, Xi-Ling Z, Young-Lian Z, Fa-Zhan Q (2010) Changes of antioxidative enzymes and lipid peroxidation in leaves and roots of water logging sensitive maize genotypes seedling stage. Agric Sci China 9(5):651–661
Bowler C, Montagu MV, Inze D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 43(1):83–116
Bray EA, Bailey SJ, Weretilnyk E (2000) Responses to abiotic stresses. In: Gruissem W, Buchannan B, Jones R (eds) Biochemistry and molecular biology of plants. Ameri Soc Plant Physiol, Rockville, pp 1158–1249
Calcagno AM, Rivas M, Castrillo M (2011) Structural, physiological and metabolic integrated responses of two tomato (Solanum lycopersicum L.) cultivars during leaf rehydration. Aust J Crop Sci 5:695–701
Caro A, Puntarulo S (1998) Nitric oxide decreases superoxide anion generation by microsomes from soybean embryonic axes. Physiol Plant 104:357–364
Cassells AC, Curry RF (2001) Oxidative stress and physiological, epigenetic and genetic variability in plant tissue culture, implications for micropropagators and genetic engineers. Plant Cell Tiss Org Cult 64:145–157
Cechin I, Rossi SC, Oliveira VC, Fumis TF (2006) Photosynthetic responses and proline content of mature and young leaves of sunflower plants under water deficit. Photosynthetica 44(1):143–146
Cechin I, Cardoso GS, Fumis TF, Corniani N (2015) Nitric oxide reduces oxidative damage induced by water stress in sunflower plants. Bragantia Campinas 74(2):200–206
Chavan ML, Janagoudar BS, Dharmatti PR, Koti RV (2010) Effect of drought on growth attributes of tomato genotypes. Indian J Plant Physiol 15(1):11–18
Corpas FJ, Leterrier M, Valderrama R, Airaki M, Chaki M, Palma JM, Barroso JB (2011) Nitric oxide imbalance provokes a nitrosative response in plants under abiotic stress. Plant Sci 181(5):604–611
Desclaux D, Roumet P (1996) Impact of drought stress on the phenology of two soybean (Glycine max L. Merr) cultivars. Field Crops Res 46:61–70
Dhindsa RS, Plump-Dhindsa P, Thorpe TA (1981) Leaf senescence, correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Duncan BD (1955) Multiple range and multiple F-test. Biometrics 11:1–42
Fang X, Turner NC, Yan G, Li F, Siddique KHM (2009) Flower numbers, pod production, pollen viability, and pistil function are reduced and flower and pod abortion increased in chickpea (Cicer arietinum L.) under terminal drought. J Exp Bot 61:335–345
Garcia-Mata C, Lamattina L (2001) Nitric oxide induces stomatal closure and enhances the adaptive plant responses against drought stress. Plant Physiol 126:1196–1204
Ghahfarokhi MG, Mansurifar S, Taghizadeh-Mehrjardi R, Saeidi M, Jamshidi AM, Ghasemi E (2015) Effects of drought stress and rewatering on antioxidant systems and relative water content in different growth stages of maize (Zea mays L.) hybrids. Arch Agron Soil Sci 61:493–506
Giannakoula AE, Ilias IF (2013) The effect of water stress and salinity on growth and physiology of tomato (lycopersicon esculentum mill.). Arch Biol Sci Belgrade 65(2):611–620
Giardini L, Giovanardi R, Borin M (1988) Water consumption and yield response of tomato in relation to water availability at different soil depth. Acta Hortic 228:119–126
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plant. Plant Physiol Biochem 48(12):909–930
Halil K, Cengiz K, Ismail TAS, David H (2001) The influence of water deficit on vegetative growth, physiology, fruit yield and quality of egg plants. Bulg J Plant Physiol 27(3–4):34–46
Hasheminasab H, Assad MT, Aliakbari A, Sahhafi S (2012) Influence of drought stress on oxidative damage and antioxidant defense systems in tolerant and susceptible wheat genotypes. J Agric Sci 4(8):20–29
Hayat S, Hasana SA, Fariduddina Q, Ahmad A (2008) Growth of tomato (Lycopersicon esculentum) in response to salicylic acid under water stress. J Plant Interact 3(4):297–304
Hayat S, Yadav S, Ali B, Ahmad A (2010) Interactive effect of nitric oxide and brassinosteroids on photosynthesis and the antioxidant system of Lycopersicon esculentum. Russ J Plant Physiol 57(2):212–221
He YK, Tang RH, Yi H, Stevens RD, Cook CW, Ahn SM, Jing L, Yang Z, Chen L, Guo F, Fiorani F, Jackson RB, Crawford NM, Pei ZM (2004) Nitric oxide represses the Arabidopsis floral transition. Science 305:1968–1971
Horchani F, Aloui A, Brouquisse R, Aschi-Smiti S (2008) Physiological responses of tomato plants (Solanum lycopersicum) as affected by root hypoxia. J Agron Crop Sci 194:297–303
Hussain M, Malik MA, Farooq M, Ashraf MY, Cheema MA (2008) Improving drought tolerance by exogenous application of glycinebetaine and salicylic acid in sunflower. J Agron Crop Sci 194:193–199
Kavas M, Baloğlu MC, Akça O, Köse FS, Gökçay D (2013) Effect of drought stress on oxidative damage and antioxidant enzyme activity in melon seedlings. Turk J Biol 37:491–498
Konieczny R, Libik M, Tuleja M (2008) Oxidative events during in vitro regeneration of sunflower. Acta Physiol Plant 30:71–79
Kramer PJ (1969) Plant and soil water relationships, a modern synthesis. McGraw-Hill Series in Organismic Biology, New York, p 482
Kramer PJ, Boyer JS (1995) Water relations of plants and soils. Academic Press, San Diego
Lamattina L, García-Mata C, Graziano M, Pagnussat G (2003) Nitric oxide, the versatility of an extensive signal molecule. Annu Rev Plant Biol 54:109–136
Molla MdN, Chandra AM, Georges TD (2003) Effect of water stress at different growth stages on greenhouse tomato yield and quality. Hortic Sci 38(7):1389–1393
Mukherjee SP, Choudhari MA (1983) Implications of water stress induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Planta 58:116–170
Nuñez M (2000) Análogos de brasinoesteroides cubanos como biorreguladores en la agricultura. Informe final de Proyectos de Investigaciones. Código 00300047. PNCT. Biotecnología Agrícola. CITMA. La Habana
Preedy VR, Watson RR (2008) Tomatoes and tomato products, Nutritional, medicinal and therapeutic properties, Science Publishers, pp 664
Rad DVR, Vijaya S (1991) Effect of induced moisture stress at different phenological stages on growth and yield of tomato cultivars. South Indian Hortic 39(2):81–87
Rana MK, Kalloo G (1989) Morphological attributes associated with the adaptation under water deficit condition in tomato. Veg Sci 16(1):32–38
Reddy TY, Reddy VR, AnbumozhI V (2003) Physiological responses of groundnut (Arachis hypogaea L.) to drought stress and its amelioration: a critical review. Plant Growth Regul 41:75–78
Reddy AM, Kumar SG, Jyothsnakumari G, Thimmanaik S, Sudhakar C (2005) Lead induced changes in antioxidant metabolism of horsegram (Macrotyloma uniflorum (Lam.) Verdc.) and bengalgram (Cicer arietinum L.). Chemosphere 60:97–104
Sadasivam S, Manickam A (1992) Biochemical Methods for Agricultural Sciences. Wiley Eastern Ltd, Madras, p 246
Sairam RK (1994) Effect of homobrassinolide application on plant metabolism and grain yield under irrigated and moisture stress condition of two wheat varieties. Plant Growth Regul 14:173–181
Salter PJ (1954) The effects of different water regimes on the growth of plants under class. 1. Experiments with tomatoes (Lycopersicon esculentum Mill.). J Hortic Sci 29:258–268
Sang J, Jiang M, Lin F, Xu S, Zhang A, Tan M (2008) Nitric oxide reduces hydrogen peroxide accumulation involved in water stress-induced subcellular anti-oxidant defense in maize plants. J Integr Plant Biol 50:231–243
Sankar B, Jaleel CA, Manivannan P, Kishorekumar A, Somasundaram R, Panneerselvam R (2007) drought-induced biochemical modifications and proline metabolism in Abelmoschus esculentus (L.) Moench. Acta Bot Croat 66(1):43–56
Santisree P, Bhatnagar P, Sharma KK (2015) NO to drought-multifunctional role of nitric oxide in plant drought: do we have all the answers? Plant Sci 239:44–55
Sasse JM (2003) Physiological actions of brassinosteroids, an update. J Plant Growth Regul 22:276–288
Seng KH (2014) The effects of drought, waterlogging and heat stress on tomatoes (Solanum lycopersicon L), Ph.D. Thesis, Publisher, Lincoln University. http://hdl.handle.net/10182/6432
Shi H, Ye T, Zhu JK, Chan Z (2014) Constitutive production of nitric oxide leads to enhanced drought stress resistance and extensive transcriptional reprogramming in Arabidopsis. J Exp Bot 65(15):4119–4131
Siddiqui MH, AL-Whaaibi MH, Basalah MO (2010) Role of nitric oxide in tolerance of plants to abiotic stress. Protoplasma 248:447–455
Simpson GG (2005) NO flowering. Bioessays 27:239–241
Singh JP, Prasad S, Singh KN, Singh R (2007) Screening of heat tolerant wheat varieties by membrane thermo stability index in relation to yield and yield attributing traits. Int J Plant Sci 2(2):159–165
Smirnoff N (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125:27–58
Subramanian KS, Sevaraj KV, Selvakumari G, Shanmugasundaram VS (1993) Influence of moisture stress regimes and nitrogen on growth and yield of brinjal (Solanum melongena L). South Ind Hort 41(1):16–21
Subramanian KS, Santhanakrishnan P, Balasubramanian P (2006) Responses of field grown tomato plants to arbuscular mycorrhizal fungal colonization under varying intensities of drought stress. Sci Hortic 107:245–253
Tian X, Lei Y (2006) Nitric oxide treatment alleviates drought stress in wheat seedlings. Biol Plant 50:775–778
Vardhini BV, Rao SSR (2001) Effect of brassinosteroids on growth and yield of tomato (Lycopersicon esculentum Mill.) under field conditions. I J Plant Physiol 6:326–328
Vardhini BV, Sujatha E, Rao SSR (2011) Brassinosteroids, alleviation of water stress in certain enzymes of sorghum seedlings. J Phytol 3(10):38–43
Veershetty (2004) Studies on variability, character association and genetic diversity in tomato tomato (Lycopersicon esculentum Mill.). M.Sc.(Agri.) Thesis, Univ Agric Sci Dharwad (India)
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61(3):199–223
Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures, towards genetic engineering for stress tolerance. Planta 218:1–14
Wudiri BB, Henderson DW (1985) Effects of water stress on flowing and fruit set inprocessing tomatoes. Sci Hortic 27(3):189–198
Yardanova RY, Christov KN, Popova LP (2004) Antioxidative enzymes in barley plants subjected to soil flooding. Environ Exp Bot 51:93–101
Yin H, Chen Q, Yi M (2008) Effects of short-term heat stress on oxidative damage and responses of antioxidant system in Lilium longiflorum. Plant Growth Regul 54:45–54
Yuan GF, Jia CG, Li Z, Sun B, Zhang LP, Wang QM (2010) Effect of brassinosteroids on drought resistance and abscisic acid concentration in tomato under water stress. Sci Hortic 126(2):103–108
Zhang S, Su-ying H, Wen-hua Y, Hua-li W, Zhang M, Li-wang Q (2010) Changes in H2O2 content and antioxidant enzyme gene expression during the somatic embryogenesis of larix laptolapies. Plant Cell Tiss Organ Cult 100:21–29
Acknowledgements
The authors would like to thank UGC, New Delhi for fellowship to the first author, and IIVR, Varanasi for providing tomato genotypes.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest with this publication.
Additional information
Communicated by T. K. Mondal.
Rights and permissions
About this article
Cite this article
Jangid, K.K., Dwivedi, P. Physiological and biochemical changes by nitric oxide and brassinosteroid in tomato (Lycopersicon esculentum Mill.) under drought stress. Acta Physiol Plant 39, 73 (2017). https://doi.org/10.1007/s11738-017-2373-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-017-2373-1