Abstract
Nitric oxide (NO) is an important signaling molecule that plays a pivotal role in stress tolerance. To study the role of NO in drought tolerance and elucidate the underlying mechanisms, NO (0 and 100 μM) was applied to drought-treated soybean plants. Drought stress was imposed by PEG (5% (W/V) of PEG 6000. Nitric oxide improved growth of soybean plants under drought as evidenced by enhanced dry weight (30%). Nitric oxide caused a remarkable increase in activities of catalase and superoxide dismutase (SOD) and SOD expression (14.8-fold), which led to a significant decline in malondealdehyde content under drought conditions. Nitric oxide induced proline biosynthesis due to enhancing pyrroline-5- carboxylate synthetase (P5CS) expression (43.66-fold). The growth-promoting effect of NO application in soybean plants was concomitant with change in metabolic profile (phenolic acid and flavonoid compounds). Nitric oxide up-regulated of phenylalanine ammonia-lyase (PAL) expression in drought-treated plants and may influence on the phenylpropanoid production. Nitric oxide increased salicylic acid (SA) content in soybean plants under stress. So, NO and SA are jointly responsible for boosted tolerance to drought stress in soybean plants. The decrease in unsaturated fatty acid through NO application might reflect a reduction in oxidative damage. These results propose a multifaceted contribution of NO through regulation of physiological and metabolic processes in response to drought stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants respond to the drought stress by the adjustment of various physiological and biochemical processes include photosynthesis, water relation, gas exchange, the metabolism of biological compounds, and stabilization of the membrane system (Osakabe et al. 2014). Plants have an operative antioxidant (enzymatic and non-enzymatic) mechanism to cope reactive oxygen species (ROS)-induced injuries. Superoxide dismutase (SOD), catalase (CAT), guaiacol peroxidase (GPX), monodehydroascorbate reductase (MDHAR), ascorbate peroxidase (APX), dehydroascorbate reductase (DHAR), and glutathione reductase (GR) are enzymatic antioxidants. Ascorbate (AsA), glutathione (GSH), carotenoids, tocopherols, and phenolics serve as potent non- enzymatic antioxidants within the cell (Noctor and Foyer 1998; Yang et al. 2021). The involvement of antioxidants—for drought tolerance has been reported in several plants including Mentha pulegium and Brassica napus (Hassanpour et al. 2012; Rezayian et al. 2018a). Plants respond to drought stress at the molecular level mostly by change in gene expression. Various pathways cooperate with each other, which finally cause alteration of target proteins responsible for cellular functions at the physiological and biochemical levels (Shinozaki and Yamaguchi-Shinozaki 2007).
Soybean (Glycine max) is considered as an important leguminous plant due to its application in human nutrition as suitable source for oil and protein (Maltas et al. 2011). This plant requires an appropriate water source during its growth stage to reach high biomass (Buezo et al. 2019). Drought stress severely influences on growth and production of soybean and causes yield loss (Thao and Tran 2012). Isoflavones as secondary metabolites are in soybean seeds and have antifungal and antibacterial properties. Isoflavones have free radical-scavenging properties and their contents are affected by drought stress (Caldwell et al. 2005; Rimbach et al. 2003). Drought stress increased osmolytes content, antioxidant potential, and peroxidation of membrane lipids in soybean plants (Dong et al. 2019).
Tremendous efforts are being carried out to improve strategies to overcome on water stress by increase of drought-tolerant cultivars, modifying the plant calendars, resources conservation etc. and some of these methods are extensive. Application of various compounds is an accepted method for improvement of stress tolerance in plants. Many researches indicated that signaling molecules such as nitric oxide (NO) can enhance drought tolerance in different plants (Cechin et al. 2015; Zhang et al. 2016; Signorelli et al. 2019). Nitric oxide can be biosynthesized through reductive and oxidative ways in plants. The reduction of nitrite, either enzymatically or non-enzymatically, is main pathway for NO biosynthetic pathway in plants. In enzymatic pathway, NO is synthesized by nitrate reductase (NR), which reduces nitrate to NO through nitrite. In non-enzymatic reduction, NO can also be generated by the interaction of two nitrous acid (HNO2) molecules derived from protonated nitrite under low pH, via reduction of NO2 to NO. In oxidative pathway, NO is produced by NO synthase (NOS), which changes l-arginine into l-citrulline in a NADPH-dependent reaction (Kohli et al. 2019). Nitric oxide can cross membranes and considerate such as regulator in plants such as Oryza sativa L. and Poncirus trifoliata in response to stress (Fan et al. 2012; Tian and Lei 2006). Nitric oxide can control stress responses at the genomic, proteomic, and post-proteomic levels. The targets of NO in plants are MAPK, cGMP, cADP ribose and Ca2+ that NO thought these signal transduction pathways influences on different processes (Lau et al. 2021). Although some of studies have specifically associated NO accumulation with stress, there is slight evidence about the particular manner that NO contributes in drought tolerance in plants. Considering the useful role of NO in stress tolerance in plants, this study exhibits how NO regulates the plant responses to drought with an emphasis on evaluating the impacts of exogenous NO on ROS metabolism, fatty acid composition and secondary metabolism during drought stress conditions. Our findings will clear the manner for future investigations that manipulates main stages in these metabolic pathways to prompt tolerance against drought stress.
Materials and Methods
Plant Materials and Growth Condition
Seeds of soybean (CV. Katoul) were cultivated in Tref peat. One week after sowing, seedlings were transplanted to four plants per containers. Plants were grown in greenhouse and irrigated with half strength Hoagland solution for 2 weeks. The experiment was conducted under greenhouse conditions (16-h light/8-h-dark cycle with a light intensity of 250 μmol−1 m−2 s−1, 27/18 °C ± 1 day/night temperatures and 60% air humidity). For determine the NO optimum concentration, 0, 10, 20, 30, 40, 50, 100, and 200 µM of sodium nitroprusside (SNP) were used. According to growth parameters, 100 µM of SNP was indicated as the suitable concentration for further studies. Drought stress was imposed by PEG (5% (W/V) of PEG 6000, − 0.05 MPa) for three weeks. 10 mL of SNP solution was sprayed uniformly on the plants using an atomizer at alternative days. Three weeks after treatments, the leaves were harvested and stored at − 70 °C and used to analyze the following parameters.
Growth Measurement, H2O2 Content and Lipid Peroxidation
Dry weight of whole plants was determined by keeping them in a hot air oven (60 °C for 72 h) until constant weight was achieved.
Hydrogen peroxide (H2O2) content was estimated via Velikova et al. (2000) method. Leaf tissue (0.5 g) was homogenized in 0.1% TCA then was centrifuged at 11,290 × g for 15 min. Supernatant (0.5 mL) was added to 0.5 mL potassium phosphate buffer (pH 7.0) and 1 mL potassium iodide (1 M) and absorbance was recorded at 390 nm.
Malondialdehyde (MDA) content was determined through Heath and Packer (1968) procedure. Leaf tissue (0.5 g) was homogenized in 0.1% TCA and then was centrifuged at 13,250×g for 10 min. The supernatant (0.5 mL) was mixed with 1 mL of TBA (0.5%) in 20% TCA. The mixture was heated in 95 °C for half-hour and then was centrifuged at 13,250 × g for 15 min. the absorbance of supernatant was recorded at 532 and 600 nm.
Antioxidant Enzymes
Leaf material (0.5 g) was homogenized at 4 ºC with 1 M Tris–HCl (pH 6.8) to estimate different enzymes activities. The homogenate was centrifuged at 13,250 × g for 20 min at 4 ºC and the obtained supernatant was kept at -70 ºC and later used for enzyme assays.
Superoxide dismutase activity was determined spectrophotometrically at 560 nm according to Giannopolitis and Ries (1977) based on the inhibition in the photochemical reduction of nitroblue tetrazolium (NBT). The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.8) with 0.1 mM ethylene diamine tetraacetic acid (EDTA), 75 μM NBT, 13 mM methionine, 2 μM riboflavin, and 100 μL of protein extract. Reactions were carried out for 16 min at a light intensity of 300 μmol−1 m−2 s−1. The non-irradiated reaction mixture served as control and was deducted from absorption at 560 nm. One unit of SOD was defined as the amount of enzyme which caused 50% inhibition of NBT reduction under the assay condition, and the results were expressed as U mg−1 protein.
Catalase activity was assayed by measuring the initial rate of disappearance of H2O2 according to Aebi (1984). The reaction mixture contained 50 mM phosphate buffer (pH 7.0), H2O2 (3%) and 10 µL enzyme extract. The decrease in absorbance was followed for 180 s and CAT activity was expressed as U mg−1 protein.
Determination of Proline Content
Leaf tissue (0.1 g) was homogenized in 5 mL sulfosalicylic acid (3%) and then was centrifuged at 13,250×g for 20 min. Proline content was measured according to Bates et al. (1973). Two mLof supernatant was mixed with acid ninhydrin (2 mL) and acetic acid glacial (2 mL) and then was boiled at 100 °C for one hour. The reaction mixture was extracted with 4 mL toluene and the absorbance was recorded at 520 nm.
Measurement of Total Phenol and Flavonoid Content
In order to preparation of methanolic extract, 0.1 g of dry tissue was homogenized in 5 mL methanol 80% and then was centrifuged at 5000×g for 20 min. For the total phenol content measurement, 0.1 mL methanolic extract was mixed with 2.5 mL Folin–Ciocalteu reagent 10%. The mixtures were neutralized by sodium bicarbonate 7% and then absorbance was recorded at 765 nm (Conde et al. 1995).
Content of flavonoid was measured by Aluminum chloride method (Chang et al. 2002). In this method, 0.1 g of plant material was homogenized in 2 mL of methanol 80%. Methanolic extract (0.5 mL) was mixed with 1.5 mL of methanol, 0.1 mL of AlCl3 (10%), 0.1 mL of potassium acetate (1 M), and 2.8 mL of distilled water and the absorbance was measured at 415 nm after 30 min.
Phenylalanine Ammonia-Lyase Activity
In order to Phenylalanine ammonia-lyase (PAL) activity measurement, 20 μL enzyme extract was mixed with 500 μL boric acid buffer (pH 8) and absorbance was recorded at 290 nm after 30 min. Standard curve was prepared by different concentrations of E-cinnamic acid (Berner et al., 2006).
Analysis of Phenolic Acids and Flavonoids Using HPLC
Dry leaf (1 g) was extracted with 4 mL methanol. The extracts were pooled; the residue was suspended in 4 mL acetonitrile, and to remove lipid components, the extract was treated with 2 mL hexane, and then evaporated again (Owen et al. 2003). For phenolic acid determination, the residue was suspended in 500 μL methanol and phenolic acids were separated by Agilent 1260 infinity high performance liquid chromatography (HPLC; Agilent Technologies, Santa Clara, CA). A 5-μm 250–4.6-mm C18 column (MZ Analysentechnik, Mainz, Germany) was used as the stationary phase. Solvent A was water containing 2% (v/v) acetic acid and solvent B was methanol (Owen et al. 2003). The gradient system used included: 5% B (0–2 min), 25% B (2–10 min), 40% B (10–20 min), 50% B (20–30 min), 100% B (30–40 min), and 5% B (40–50 min). The photodiode array detection (DAD; Agilent Technologies) system was used for detection of phenolic acids set at 278, 300, and 340 nm with a flow rate of 1 mL min−1. The amounts of phenolic acids in samples were calculated from standard curves of authentic standards.
The extraction of flavonoid compounds was done according to the method of Keinänen et al. (2001). The dry leaf (1 g) was extracted with 1.5 mL of 0.5% (v/v) acetic acid in 40% (v/v) aqueous methanol, and then shaken for 3 h. The samples were centrifuged for 10 min at 15,000 × g, and the supernatant was used for HPLC analysis. The flavonoids were separated using the method of Gudej and Tomczyk (2004). The mobile phase (A solvent) consisted of deionized water with 0.01% (v/v) phosphoric acid and (B solvent) consisted of acetonitrile. The gradient system consisted of 18% B (0–30 min), 67% B (30–60 min), and 18% B (60–65 min). The flow rate was 0.8 mL min−1 at 25 °C. The UV detector settings were 254, 280, 300, and 350 nm. The content of flavonoid compounds in the extracts was calculated using standard curves.
Determination of Fatty Acids
Approximately 0.1 g of dry leaf was placed in test tubes. One milliliter of transesterification reagent (methanol/acetyl chloride, 20:1 v/v) was added to each tube. The tubes were heated at 100 °C for 1 h for the transmethylation, being shaken every 10–15 min. The mixture was cooled to room temperature, and 1 mL each of water and hexane were added. The tubes were shaken and centrifuged. The upper one (hexane) was transferred to another tube (Rodriguez-Ruiz et al. 1998) and fatty acid analyzed by gas liquid chromatography.
Analysis of Gene Expression
To recognize the effect of NO and drought on the expression of genes, quantitative real-time PCR technique was used for the determination of expression level of SOD, PAL, and P5CS using rRNA (as an internal control). Appropriate primers (Table 1) were designed using PRIMER EXPRESS software (Applied Biosystems). Total RNA was extracted from leaf using RiboEx solution (GenALL, Seoul, Korea), according to Babaei et al. (2021). The isolated RNAs were treated with DNase I (Fermentas, Lithuania) for 20 min at 37 °C to eliminate any genomic DNA contamination. RNA concentration and integrity were examined by spectrophotometry and 1% agarose electrophoresis gel, respectively. The reverse transcription (RT) was performed for gene expression analysis using 2 μg of total RNA, 200 units of M-MulV reverse transcriptase (Thermo scientific, USA), Oligo (dT), and random hexamer primers (MWG, Germany) in a total volume of 20 μL of reaction mixture, according to the manufacturer’s instruction. An initial denaturation at 95 °C for 5–7 min, followed by 35 cycles of denaturation at 95 °C for 10 s, annealing at 60 °C for 30 s and extension at 72 °C for 30 s. All reactions were tested at least in duplicate and specificity of qPCR products were verified by polyacrylamide gel electrophoresis and melt curve analysis. The mean of ΔCT after treatment and the control condition was calculated and finally the expression of the gene was estimated by the ratio formula (ratio = 2− ΔΔCT).
Statistical Analysis
Each experiment was repeated three times and the data were analyzed by using one-way analysis of variance (ANOVA) using SPSS (version 21) and means were compared by Duncan’s test at the 0.05 level of confidence. HCA (Hierarchical cluster analysis) was used for evaluating correlation between each pair of variables and performed using online CIMminner software.
Results
Growth, H2O2 and MDA Content
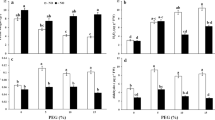
In order to evaluate whether NO could induce the growth; dry weight was measured in soybean plants. Dry weight significantly diminished (27.7%) in stressed plants as compared to well watered plants. NO in combination with drought enhanced (30%) dry weight in soybean plants (Fig. 1a).
Effect of drought and NO treatment on dry weight (a), H2O2 (b), MDA (c) and CAT activity (d) in soybean plants (50 days). Different letters above columns indicated a significant difference at p < 0.05 using Duncan multiple range test. The CAT activity was defined as 1 μmol of H2O2 decomposed per min. Hydrogen peroxide: H2O2; malondialdehyde: MDA; catalase: CAT
H2O2 content enhanced (2.35-fold) in drought conditions. NO treatment could not inhibit of H2O2 production in the drought-stressed and unstressed plants (Fig. 1b). Drought stress caused a considerable rise in MDA content by 1.72-fold compared with those in the unstressed plants. NO treatment induced mitigation of lipid peroxidation (47.42%) in soybean plants under drought stress (Fig. 1c).
Enzymatic Antioxidants
Catalase activity enhanced under drought stress. Nitric oxide treatment of soybean plants led to marked increase in control and stress conditions. Nitric oxide treatment induced a 5.82-fold increase in CAT activity (Fig. 1d). Superoxide dismutase activity showed non-significant change in soybean under stress condition. Exogenous application of NO led to a significant increase in SOD activity and expression of SOD by 4.05- and 14.8-fold, respectively as compared without NO. These parameters in drought and NO-treated group was significantly higher than that of drought or NO-treated group, which suggested synergism between NO and drought (Fig. 2a,b).
Change in SOD activity (a), SOD expression (b), proline (c) and P5CS expression (d) in soybean plants (50 days) under drought stress and NO treatment. Different letters above columns indicated a significant difference at p < 0.05 using Duncan multiple range test. One unit of SOD activity was defined as the amount of enzyme that caused 50% inhibition in NBT reduction. Superoxide dismutase: SOD; pyrroline-5- carboxylate synthetase: P5CS; nitroblue tetrazolium: NBT.
Proline Accumulation and P5CS Expression
The soybean plants exposed to drought stress showed increased proline content by 61.93% relative to control. Foliar spray with NO alone or in combination with stressed plants was significantly increased proline accumulation. Proline content was remarkably induced by 2.24-fold by NO application (Fig. 2c). NO treatment in stressed plants led 43.66-flod increase in P5CS expression relative to their corresponding drought-alone treatment (Fig. 2d). The synergism between NO and drought in soybean plants regarding proline content and P5CS expression was also observed.
Phenolic Acid and Flavonoid Metabolism
Water stress caused significant rise in total phenol content as compared with well watered plants. Nitric oxide treatment was not effective in improving total phenol in the control and stressed plants (Fig. 3a). Drought stress improved flavonoid content than that of the control plants. The treatment of NO significantly caused a further increase this content in the leaves of soybean plants under drought stress (Fig. 3b).
Phenylalanine ammonia-lyase activity significantly enhanced in soybean plants subjected to stress condition. Exogenous application of NO alone or in combination with stressed plants caused significantly increased in PAL activity of soybean plants. Nitric oxide treatment induced a 63.93% increase of PAL activity in stressed plants. Drought stress did not influence on PAL expression, NO application markedly improved (5.10-flod) this parameter in soybean plants under drought stress (Fig. 3c,d).
There were significant differences in phenolic compounds among all treatments. Drought stress significantly enhanced caffeic acid, cinnamic acid, coumaric acid, ferulic acid, gallic acid and salicylic acid (SA) by 85.71, 93.75, 97.22, 94.44, 64.51, and 95.61% as compared to control, respectively. Among phenolic acid compounds, coumaric acid showed maximum increase under stress condition. Exception to coumaric acid, all phenolic acid compounds remarkably improved by exogenous application of NO and this effect was more pronounced (6.25-flod) in cinnamic acid (Fig. 4).
The alternations in the content of flavonoid compounds showed different trends under drought stress and NO treatments. Our results showed that drought stress significantly augmented luteolin content (88.29%) of soybean plants but did not affect catechin, daidzein, diosmin and myrecetin. Supplementation with NO decreased daidzein and diosmin content relative to their corresponding drought-alone treatment. Nitric oxide application caused significant increase in catechin, luteolin and myricetin content and the maximum induction (3.18-flod) was detected in myricetin content (Fig. 5).
Fatty Acids Profile
Drought stress significantly induced palmitic acid (C16), linolenic acid (C18:3), behinic acid (C22), nervonic acid (C24:1), saturated fatty acid (SFA) and polyunsaturated fatty acid (PUFA), but palmitoleic acid (C16:1), stearic acid (C18), eicosenoic acid (C20:1), erucic acid (C22:1), and monounsaturated fatty acid (MUFA) declined under stress. Linoleic acid (C18:2) and lignoceric acid (C24) were unchanged by drought stress. Nitric oxide treatment improved palmitoleic acid, linoleic acid, eicosenoic acid, erucic acid, lignoceric acid, nervonic acid and MUFA in the plants exposed to drought and the improvement effect of NO treatment on fatty acids was prominent in linoleic acid (7.27-flod). Nitric oxide treatment in combination with drought led significant decrease in PUFA (Table 2).
Correlation Among the Studied Parameters
The HCA analysis provided an overview of the biochemical and physiological traits in response to drought stress and NO application Fig. 6. This analysis organized the studied parameters into the two main groups, which were then divided into multiple minor groups. Plant growth positively corrected with SOD activity, SOD expression, CAT activity, P5CS expression, PAL activity, PAL expression, cinnamic acid, ferulic acid, catechin, and myricetin. Phenylalanine ammonia-lyase activity showed a positive correlation with phenolic acid and flavonoid compounds. Salicylic acid such as signaling molecule positively correlated SOD activity, CAT activity, proline, P5CS expression, PAL activity, phenolic acid, and flavonoid compounds. Plant growth exhibited a negative relationship with stress markers (H2O2 and MDA).
Discussion
In the present study, the drought stress decreased dry weight, which is a typical drought response as stated in other plants such as Mentha pulegium and Brassica napus (Hassanpour et al. 2012; Rezayian et al. 2018b). A noticeable decline in growth of soybean plants may be due to destructive effects of drought on plant physiological processes. Drought stress leads reduction in water content and turgor pressure in Capsicum annuum, thereby prevents of enlargement and cell division and decreases plant growth (Delfine et al. 2002). The present work presented that the application of NO significantly moderated drought-induced inhibition in soybean plant growth. A similar result was reported in rice plants using exogenous application of NO (Mostofa et al. 2015). Improvement of growth by NO was confirmed by promoting of physiological and biochemical responses in soybean plants under drought condition. Kaya et al. (2015) indicated the growth-improving effect of NO application in maize plants under salt stress.
The H2O2 production corresponded with the lipid peroxidation (MDA content) and proposes drought-mediated oxidative stress. Supplementation with NO in drought-stressed soybean plants significantly decreased lipid peroxidation content which might be correlated with the induction of antioxidant potential. Redox imbalance happened in plants under drought, so sustaining redox homeostasis is a key mechanism for plant tolerance against stress (Noctor et al. 2014). In view of these data, it is suggested that NO treatment declined oxidative injuries to cell membrane, which is obvious by the lower MDA content in soybean plants. Manai et al. (2014) identified that application of NO alleviated salt-induced oxidative damage in tomato plants.
Ashraf (2009) recommended that plants with more antioxidant capacity are better capable to ROS detoxification and therefore have better stress tolerance. According to our data, supplementation with NO heightened the activities of CAT and SOD and SOD expression under drought stress, which aided to decline ROS content and established a reducing environment for metabolic activities. These results propose that NO plays an important function in regulating soybean tolerance to drought stress. A cysteine residue from CAT that can be post-translationally modified by S-nitrosation (Rodríguez-Ruiz et al. 2019). Nitric oxide can efficiently maintain plants from damage by improving the activities of antioxidant enzymes (Akram et al. 2018; Boogar et al. 2014; Singh et al. 2021). Since NO could react with superoxide radical to generate peroxynitrite which is a very oxidant molecule, thus NO can serve as a direct scavenger of ROS and antioxidant system inducer to increase the expression of antioxidant enzyme-encoding genes (Groß et al. 2013). Exogenous application of NO may enhance the synthesis of endogenous NO which can active like signaling molecule or ROS scavenger under stress condition by modulating/increasing the antioxidant enzymes activities (Fan and Liu 2012; Hao et al. 2008). The increment in SOD activity by NO application could be due to an increased expression of SOD gene in soybean plants. Nitric oxide regulates the expression of genes (Grun et al. 2006). Nitric oxide augmented the demethylation ratio of methylated locations and can induce gene expression under drought condition (Fan et al. 2012). In support of our data, some studies described the up-regulation of antioxidant enzymes-encoding genes under stress with NO application (Ahmad et al. 2016; Wu et al. 2017). Ahmad et al. (2016) exhibited that NO may trigger the expression of antioxidant enzymes-related biosynthetic genes and cause induction of antioxidant enzymes.
In the present experiment, accumulation of proline was recorded in soybean plants under drought stress. Proline is one of main players in conversing plant abiotic stress tolerance by protecting osmotic regulation and energy source under stress (Ashraf and Foolad 2007; Kaur and Asthir 2015). Antioxidant capacity of proline is limited to reduce.OH toxicity. Thus, proline performances as selective scavenger of.OH, situated as a second line of defense (Signorelli et al. 2016). A similar result was obtained in Salicornia persica and S. europaea plants under stress (Aghaleh et al. 2011). Application of NO to drought-stressed soybean plants triggered a notable increase in proline content, possibly to provide a better maintain to plants exposed to stress. This NO-induced rise in biomass production in soybean plants may have been due to proline accumulation. NO-mediated acceleration of proline content has been recorded in other plants (Dong et al. 2014; Kausar et al. 2013). A more induction in proline after NO application in stressed plants shows a possible role of NO in proline biosynthesis. According to our data, exogenous application of NO has been known to induce the P5CS1 gene encoding pyrroline-5-carboxylate synthetase, a key enzyme involved in the proline synthesis. The rise in proline accumulation by NO application could be due to an increased expression of P5CS1 gene. The improved amount of proline in drought-treated plants might be due to the increase biosynthesis of endogenous NO. Ahmad et al. (2016) showed that NO treatment up-regulated expression of P5CS1 gene in Cicer arietinum L. under stress.
According to our data, drought stress induced total phenol in soybean plants. Enhancement of phenolic compounds is very vital to lessen the deleterious effects of drought stress in plants (Naikoo et al. 2019). Phenolic compounds operated such as antioxidants and maintained plants against negative impacts of water deficit (Nichols et al. 2015). Linic et al. (2019) reported that different phenolic acids involved in stress tolerance in some species of Brassicaceae family. In this study, NO application to drought-stressed plants heightened the different phenolic acids, which reflected that foliar-applied NO powerfully suppressed oxidative injury triggered by drought stress in soybean plants via promoting the antioxidant defense. The rise of phenolic acids may be connected to the synthesis of amino acids which control the osmotic regulation in plant cell (Ayaz et al. 1999). Phenolic compounds involved in suppressing free radicals, catalyzing oxygenation reactions by establishment of metallic complexes and preventing the activity of oxidizing enzymes (Amarowicz and Weidner 2009; Petridis et al. 2012). Zafari et al. (2017) showed effect of NO on phenolic acids in Prosopis farcta under stress. Nitric oxide treatment increased SA in soybean plants under stress. Salicylic acid is a signaling molecule that plays a significant role in plant tolerance against abiotic stresses by the modification of some physiological processes (Alavi et al., 2014). The possible interaction between NO and SA enhanced stress tolerance (Fatima et al. 2021). The content of caffeic acid increased in stressed soybean plants by NO treatment. Caffeic acid played an important role in the adjustment of cell elongation, turgor potential, water fluctuation and growth (Lattanzio et al. 2006). Klein et al. (2015) described that caffeic acid by rise in NO content prompted salinity tolerance in soybean plants. Marked increase in the coumaric acid and caffeic acid content aid to decline oxidative stress, since coumaric acid and caffeic acid have high free radical suppressing ability because of their hydroxyl nature. Nitric oxide application caused a significant increase in ferulic acid content. Phenolic compounds act like photoprotectors and inhibit the chlorophyll excitation under drought stress (Hura et al. 2008). Phenolic compounds especially ferulic acid convert high energy and destructive radiations into the blue radiation that is fewer destructive for the cell components of the plant such as photosynthetic apparatus (Bilger et al. 2001). Our findings confirm the effective role of NO on production of phenolic compounds and stimulation of antioxidant defense system.
Flavonoids are a main component of secondary metabolites and have defense roles in plants against abiotic stresses. Flavonoids act such as an alleviator of drought and oxidative stresses (Dixon and Paiva 1995). The protecting function of these compounds is due to their special structure, e.g., double carbon bonds, hydroxyl group and alterations for example methylation, glycosylation and prenylation, successfully counter the oxidative injury (Rice-Evans et al. 1997). Mohamed and Latif (2017) presented that drought stress increased flavonoid compounds in soybean plants. There was a significant rise in total flavonoid and some of flavonoid compounds including catechin, luteolin and myricetin at all plants treated with NO in combination with drought stress as compared to stressed plants. These findings showed that flavonoid content may be regulated by NO. Our results identified these flavonoids compounds were connected with the drought tolerance strength of soybean. The antioxidant ability of catechin is employed by direct ways such as ROS detoxification, metal ions chelation; and indirect ways comparing preventing pro-oxidant enzymes and inducing antioxidant enzymes (Youn et al. 2006). Luteolin prevents ROS-induced hurts to protein, DNA and lipids. Luteolin inhibits O2•− production by decreasing xanthine oxidase activity (Brown and Rice-Evans 1998; Nagao et al. 1999). Myricetin is one of the most effective suppressers of ROS in flavonoids group. The antioxidant capacity of myricetin was higher that of α-tocopherol (Bennett et al. 2004; Shahidi and Wanasundara 1995). Hao et al. (2009) proved an enhanced accumulation of flavonoids due to NO application, through regulation of PAL activity. Nitric oxide application improved flavonoid content in Solanum Lycopersicum under salt stress Ahmad et al. (2018) that is agreement with our results.
PAL causes the production of cinnamic acid from phenylalanine, which is rate-limiting step on the phenylpropanoid pathway leading to biosynthesis of phenolic acids and flavonoids. Enhancement of PAL activity may be associated to the plant defense mechanism through synthesis of secondary metabolites (Mandal et al. 2009). This study confirmed that PAL activity was induced by NO in soybean plants. According to our results, a direct relationship was witnessed between the induction of PAL activity and increase of its gene expression in NO-treated soybean plants. These observations proposed that PAL gene expression relates with PAL activity. Current results also suggested PAL activity to play an important function in drought resistance. Expression of PAL gene presented significant levels of PAL transcripts in the plants exposed to NO. These results proposed that NO facilitated the drought-induced phenolic acid accumulation by improving PAL activity. Nitric oxide may act as a signal molecule for improvement of PAL activity (Kováčik et al. 2009). In this concern, under environmental stresses NO increases biosynthesis of secondary metabolites by stimulation of PAL enzyme (Hao et al. 2009; Kovácik et al. 2009). It has been obviously revealed that NO actives transcription of PAL gene in plants (Durner 1998). Enhancement of PAL activity means more efficiency in exchanging phenylalanine into phenolic compounds; and, so, content of phenolic compounds heighten subsequent the application of NO.
Plants can acclimate to drought stress through the change of fatty acid profile in cellular membranes (Yordanov et al. 2000). Nitric oxide treatment changed fatty acid profiling in Capsicum annuum L. plants (Gonzalez-Gordo et al. 2019). In our experiment, drought stress increased PUFA in soybean plants. Enhancement of unsaturated fatty acids content can be due to drought stress modifying the fatty acids content by influencing fatty acid desaturases enzymes (Bellaloui et al. 2013). A rise in length and unsaturation fatty acids increases thickness and membrane fluidity, respectively, which influence membrane penetrability to ions (Elkahoui et al. 2004). Plants could be maintained against the oxidative damage by rearrangement membranes with less PUFA (Malkit et al. 2002). The treatment with NO caused significant decrease in index unsaturation as well as unsaturated fatty acids. These positive findings of decreased unsaturated fatty acids content may be illuminated as a consequence of cellular defense against oxidative injury by the prevention of lipid peroxidation and detoxification of free radicals. This is considered one of the good results from the NO application. Thus, these data showed that NO declined unsaturated fatty acid and reduced the oxidative damage and leakage of ions.
Conclusion
Several drought stress-eliciting responses, including lipid peroxidation, ROS production, antioxidative enzymes activities, gene expression, osmolyte production, secondary metabolites and fatty acid composition were intermediated by NO. Modulations of antioxidant defense mechanisms by NO associated with conferring drought tolerance in soybean plants. In fact, increased antioxidant enzymes can detoxify and neutralize drought-induced oxidative stress in soybean plants. Nitric oxide supported soybean plants to tolerate drought stress by increasing osmolyte compounds. Nitric oxide treatment enhanced phenolic acid and flavonoid compounds. These results showed that the higher accumulation of these metabolites could possibly associate with the tolerance mechanism. This was also demonstrated that exogenously applied NO triggered the SA content which may play a role in enhancing antioxidant enzymes activities and decreasing MDA to develop drought tolerance in soybean plants. So, NO and SA are both jointly responsible signaling molecules which function in combination in conferring tolerance in soybean plants to drought stress. These findings offer information that may help to realize the biochemical and physiological pathways underlying drought stress tolerance in soybean plants induced by NO treatment. Additionally, exogenous application of NO in improving drought tolerance in plants is an economically possible method.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Aghaleh M, Niknam V, Ebrahimzadeh H, Razavi Kh (2011) Effect of salt stress on physiological and antioxidative responses in two species of Salicornia (S. persica and S. europaea). Acta Physiol Plant 33:1261–1270
Ahmad P, Abdel Latef AA, Hashem A, Abd_Allah EF, Gucel S, Tran L-SP (2016) Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front Plant Sci 7:347–358
Ahmad P, Ahanger MA, Alyemeni MN, Wijaya L, Alam P, Ashraf M (2018) Mitigation of sodium chloride toxicity in Solanum lycopersicum L. by supplementation of jasmonic acid and nitric oxide. J Plant Interact 13:64–72
Alavi SM, Arvin MJ, Manoochehri Kalantari K (2014) Salicylic acid and nitric oxide alleviate osmotic stress in wheat (Triticum aestivum L.) seedlings. J Plant Interact 9:683–688
Akram NA, Iqbal M, Muhammad A, Ashraf M, Al-Qurainy F, Shafiq S (2018) Aminolevulinic acid and nitric oxide regulate oxidative defense and secondary metabolisms in canola (Brassica napus L.) under drought stress. Protoplasma 255:163–174
Amarowicz R, Weidner S (2009) Biological activity of grapevine phenolic compounds. Grapevine Molecular Physiology & Biotechnology. Springer, New York, pp 389–405
Ashraf M, Foolad M (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Ashraf M (2009) Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol Adv 27:84–93
Ayaz FA, Kadioglu AR, Turgut R (1999) Water stress effects on the content of low molecular weight carbohydrates and phenolic acids in Ctenanthe setosa (Rosc.) Eichler. Can J Plant Sci 80:373–378
Babaei S, Niknam V, Behmanesh M (2021) Nitric oxide induced carotenoid contents in Crocus sativus under salinity. Nat Prod Res 35:888–892
Barchet GLHHG, Dauwe R, Guy RD, Schroeder WR, Soolanayakanahally RY, Campbell MM, Mansfield SD (2014) Investigating the drought-stress response of hybrid poplar genotypes by metabolite profiling. Tree Physiol 34:1203–1219
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. J Plant Soil 39:205–207
Bellaloui N, Mengistu A, Kassem A (2013) Effects of genetics and environment on fatty acid stability in soybean seed. Food Nutr Sci 4:165–175
Bennett CJ, Caldwell ST, McPhail DB, Morrice PC, Duthie GG, Hartley RC (2004) Potential therapeutic antioxidants that combine the radical scavenging ability of myricetin and the lipophilic chain of vitamin E to effectively inhibit microsomal lipid peroxidation. Bioorg Med Chem 12:2079–2098
Berner M, Krug D, Bihlmaier C, Vente A, Muller R, Bechthold A (2006) Genes and enzymes involved in caffeic acid biosynthesis in Actinomycete Saccharothrix espanaensis. J Bacteriol 188:2666–2673
Bilger W, Johnsen T, Schreiber U (2001) UV-excited chlorophyll fluorescence as a tool for the assessment of UV protection by the epidermis of plants. J Exp Bot 52:2007–2014
Boogar AR, Salehi H, Jowkar A (2014) Exogenous nitric oxide alleviates oxidative damage in turfgrasses under drought stress. S Afr J Bot 92:78–82
Brown JE, Rice-Evans CA (1998) Luteolin-rich artichoke extract protects low density lipoprotein from oxidation in vitro. Free Radic Res 29:247–255
Buezo J, Sanz-Saez Á, Moran JF, Soba D, Aranjuelo I, Esteban R (2019) Drought tolerance response of high-yielding soybean varieties to mild drought: physiological and photochemical adjustments. Physiol Plant 166:88–104
Caldwell CR, Britz SJ, Mirecki RM (2005) Effect of temperature, elevated carbon dioxide, and drought during seed development on the isoflavone content of dwarf soybean [Glycine max (L.) Merrill] grown in controlled environments. J Agric Food Chem 53:1125–1129
Cechin I, Cardoso GS, de Fátima FT, Corniani N (2015) Nitric oxide reduces oxidative damage induced by water stress in sunflower plants. Bragantia Campinas 74:200–206
Chang C, Yang M, Wen H, Chern J (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10:178–182
Conde E, Cadahia E, Garcia-Vallejo M (1995) HPLC analysis of flavonoids and phenolic acids and aldehydes in Eucalyptus spp. Chromatographia 41:657–660
Delfine S, Tognettir R, Loreto F, Alvino A (2002) Physiological and growth responses to water stress in field grown bell pepper (Capsicum annuum L.). J Hortic Sci Biotechnol 77:697–704
Del Río LA, Corpas FJ, Barroso JB (2004) Nitric oxide and nitric oxide synthase activity in plants. Phytochem 65:783–792
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085–1097
Dong YJ, Jinc SS, Liu S, Xu LL, Kong J (2014) Effects of exogenous nitric oxide on growth of cotton seedlings under NaCl stress. J Soil Sci Plant Nutr 14:1–13
Dong S, Jiang Y, Dong Y, Wang L, Wang W, Ma Z, Yan C, Ma C, Liu L (2019) A study on soybean responses to drought stress and rehydration. Saudi J Biolog Sci 26:2006–2017
Durner J, Wendehenne D, Klessig DF (1998) Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc Natl Acad Sci USA 95:10328–10333
Elkahoui S, Smaoui A, Mokhtar Zarrouk M, Ghrir R, Limam F (2004) Salt-induced lipid changes in Catharanthus roseus cultured cell suspensions. Phytochem 65:1911–1917
Fan QJ, Liu JH (2012) Nitric oxide is involved in dehydration/drought tolerance in Poncirus trifoliata seedlings through regulation of antioxidant systems and stomatal response. Plant Cell Rep 31:145–154
Fan H, Li T, Guan L, Li Z, Guo N, Cai Y, Lin Y (2012) Effects of exogenous nitric oxide on antioxidation and DNA methylation of Dendrobium huoshanense grown under drought stress. Plant Cell Tissue Organ Cult 109:307–314
Fatima A, Husain T, Suhel M, Prasad SM, Singh VP (2021) Implication of nitric oxide under salinity stress: the possible interaction with other signaling molecules. J Plant Growth Regul 4:1–5
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: II. Purification and quantitative relationship with water soluble protein in seedlings. J Plant Physiol 59:315–318
Gonzalez-Gordo S, Bautista R, Claros MG, Canas A, Palma M, Corpas F (2019) Nitric oxide-dependent regulation of sweet pepper fruit ripening. J Exper Bot 70:4557–4570
Groß F, Durner J, Gaupels F (2013) Nitric oxide, antioxidants and prooxidants in plant defence responses. Front Plant Sci 4:419
Grun S, Lindermayr C, Sell S, Durner J (2006) Nitric oxide and gene regulation in plants. J Exper Bot 57:507–516
Gudej J, Tomczyk M (2004) Determination of flavonoids, tannins and ellagic acid in leaves from Rubus L. species. Arch Pharm Res 27:1114–1119
Hao GP, Xing Y, Zhang JH (2008) Role of nitric oxide dependence on nitric oxide synthase-like activity in the water stress signaling of maize seedling. J Integr Plant Biol 50:435–442
Hao G, Du X, Zhao F, Shi R, Wang J (2009) Role of nitric oxide in UV-Binduced activation of PAL and stimulation of flavonoid biosynthesis in Ginkgo biloba callus. Plant Cell Tissue Org Cult 97:175–185
Hassanpour H, Khavari-Nejad RA, Niknam A, Najafi F, Razavi Kh (2012) Effects of penconazole and water deficit stress on physiological and antioxidative responses in pennyroyal (Mentha pulegium L.). Acta Physiol Plant 34:1537–1549
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hura T, Hura K, Grzesiak S (2008) Contents of total phenolics and ferulic acid, and PAL activity during water potential changes in leaves of maize single-cross hybrids of different drought tolerance. J Agron Crop Sci 194:104–112
Kaur G, Asthir B (2015) Proline: a key player in plant abiotic stress tolerance. Biol Plant 59:609–619
Kausar F, Shahbaz M (2013) Interactive effect of foliar application of nitric oxide (NO) and salinity on wheat (Triticum aestivum L.). Pak J Bot 45:67–73
Kaya C, AshraF M, Sonmez O, Tuna AL, Aydemir S (2015) Exogenously applied nitric oxide confers tolerance to salinity-induced oxidative stress in two maize (Zea mays L.) cultivars differing in salinity tolerance. Turk J Agric for 39:909–919
Keinänen M, Oladham NJ, Baldwin LT (2001) Rapid HPLC screening of jasmonate induced increases in tobacco alkaloids, phenolics and diterpene glycosides in Nicotiana attenuate. J Agric Food Chem 49:3553–3558
Khan N, Bano A, Babar MA (2019) Metabolic and physiological changes induced by plant growth regulators and plant growth promoting rhizobacteria and their impact on drought tolerance in Cicer arietinum L. PLoS ONE 14(3):e0213040
Klein A, Keyster M, Ludidi N (2015) Response of soybean nodules to exogenously applied caffeic acid during NaCl-induced salinity. S Afr J Bot 96:13–18
Kohli SK, Khanna K, Bhardwaj R, Abd_Allah EF, Ahmad P, Corpas F (2019) Assessment of subcellular ROS and NO metabolism in higher plants: multifunctional signaling molecules. Antioxidants 8:641. https://doi.org/10.3390/antiox8120641
Kováčik J, Klejdus B, Bačkor M (2009) Nitric oxide signals ROS scavenger-mediated enhancement of PAL activity in nitrogen-deficient Matricaria chamomilla roots: side effects of scavengers. Free Radic Biol Med 46:1686–1693
Lamattina L, García-Mata C, Graziano M, Pagnussat G (2003) Nitric oxide: the versatility of an extensive signal molecule. Ann Rev Plant Biol 54:109–136
Lattanzio V, Lattanzio VM, Cardinali A (2006) Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochem Adv Res 661:23–67
Lau SE, Hamdan MF, Pua TL, Saidi NB, Tan BC (2021) Plant nitric oxide signaling under drought stress. Plants 10(2):360
Linic I, Šamec D, Grúz J, Vujcic Bok V, Strnad M, Salopek-Sondi B (2019) Involvement of phenolic acids in short-term adaptation to salinity stress is species-specific among Brassicaceae. Plants 8:155–173
Malkit A, Sadka A, Fisher M, Goldshlag P, Gokhman I, Zamir A (2002) Salt induction of fatty acid elongase and membranes lipid modifications in the extreme halotolerant Alga Dunaliella salina. Plant Physiol 129:1320–1329
Maltas E, Dageri N, Vurral C, Yildiz S (2011) Biochemical and molecular analysis of soybean seed from Turkey. J Med Plants Res 5:1575–1581
Manai J, Gouia H, Corpas FJ (2014) Redox and nitric oxide homeostasis are affected in tomato (Solanum lycopersicum) roots under salinity-induced oxidative stress. J Plant Physiol 171:1028–1035
Mandal S, Mallick N, Mitra A (2009) Salicylic acid-induced resistance to Fusarium oxysporumf sp. lycopersici in tomato. Plant Physiol Biochem 47:642–649
Mohamed HI, Latif HH (2017) Improvement of drought tolerance of soybean plants by using methyl jasmonate. Physiol Mol Biol Plants 23:545–556
Mostofa MG, Fujita M, Tran LSP (2015) Nitric oxide mediates hydrogen peroxide-and salicylic acid-induced salt tolerance rice (Oryza sativa L.) seedlings. Plant Growth Regul 77:265–277
Nagao A, Seki M, Kobayashi H (1999) Inhibition of xanthine oxidase by flavonoids. Biosci Biotechnol Biochem 63:1787–1790
Naikoo MI, Dar MI, Raghib F, Jaleel H, Ahmad B, Raina A, Khan FA, Naushin F (2019) Role and regulation of plants phenolics in abiotic stress tolerance: an overview. In: Plant signaling molecules. Elsevier, Amsterdam, pp 157–168
Nichols SN, Hofmann RW, Williams WM (2015) Physiological drought resistance and accumulation of leaf phenolics in white clover interspecific hybrids. Environ Exp Bot 119:40–47
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Biol 49:249–279
Noctor G, Mhamdi A, Foyer CH (2014) The roles of reactive oxygen metabolism in drought: not so cut and dried. Plant Physiol 164:1636–1648
Osakabe Y, Osakabe K, Shinozaki K, Tran LSP (2014) Response of plants to water stress. Front Plant Sci 5:86
Owen RW, Haubner R, Mier W, Giacosa A, Hull WE, Spiegelhalder B, Bartsch H (2003) Isolation, structure elucidation and antioxidant potential of the major phenolic and flavonoid compounds in brined olive drupes. Food Chem Toxicol 41:703–717
Planchet E, Kaiser WM (2006) Nitric oxide production in plants. Plant Signal Behav 1:46–51
Petridis A, Therios I, Samouris G, Tananaki C (2012) Salinity induced changes in phenolic compounds in leaves and roots of four olive cultivars (Olea europaea L.) and their relationship to antioxidant activity. Environ Exp Bot 79:37–43
Razavizadeh R, Ehsanpour AA (2009) Effects of salt stress on proline content, expression of delta-1-pyrroline-5-carboxylate synthetase, and activities of catalase and ascorbate peroxidase in transgenic tobacco plants. Biol Lett 46:63–75
Rezayian M, Niknam V, Ebrahimzadeh H (2018a) Positive effects of Penconazole on growth of Brassica napus under drought stress. Arch Agron Soil Sci 64:1791–18006
Rezayian M, Niknam V, Ebrahimzadeh H (2018b) Penconazole and calcium improves drought stress tolerance and oil quality in canola. Soil Sci Plant Nutr 64:606–615
Rice-Evans CA, Miller NJ, Paganga G (1997) Antioxidant properties of phenolic compounds. Trends Plant Sci 2:152–159
Rimbach G, De Pascual-Teresa S, Ewins BA, Matsugo S, Uchida Y, Minihane AM, Turner R, Vafei Adou K, Weinberg PD (2003) Antioxidant and free radical scavenging activity of isoflavone metabolites. Xenobiotica 33:913–925
Rodriguez-Ruiz J, Belarbi EL-H, Garcia Sanchez GL, Alonso DL (1998) Rapid simultaneous lipid extraction and transesterification for fatty acid analyses. Biotechnol Technol 12:689–691
Rodríguez-Ruiz M, González-Gordo S, Cañas A, Campos MJ, Paradela A, Corpas J (2019) Sweet pepper (Capsicum annuum L.) fruits contain an atypical peroxisomal catalase that is modulated by reactive oxygen and nitrogen species. Antioxidants 8:374. https://doi.org/10.3390/antiox8090374
Shahidi F, Wanasundara U (1995) Effect of natural antioxidants on the stability of Canola oil. Develop Food Sci 37:469–479
Shinozaki K, Yamaguchi-Shinozaki K (2007) Gene networks involved in drought stress response and tolerance. J Exper Bot 58:221–227
Signorelli S, Imparatta C, Rodríguez-Ruiz M, Borsani O, Corpas FJ, Monza J (2016) In vivo and in vitro approaches demonstrate proline is not directly involved in the protection against superoxide, nitric oxide, nitrogen dioxide and peroxynitrite. Funct Plant Biol 43:870–879
Signorelli S, Corpas FJ, Rodrıguez-Ruiz M, Valderrama R, Barroso JB, Borsani O, Monza J (2019) Drought stress triggers the accumulation of NO and SNOs in cortical cells of Lotus japonicus L. roots and the nitration of proteins with relevant metabolic function. Environ Exper Bot 161:228–241
Singh S, Husain T, Kushwaha BK, Suhel M, Fatima A, Mishra V, Singh SK, Bhatt JA, Rai M, Prasad SM, Dubey NK (2021) Regulation of ascorbate-glutathione cycle by exogenous nitric oxide and hydrogen peroxide in soybean roots under arsenate stress. J Hazard Mater 5(409):123686
Thao NP, Tran LS (2012) Potentials toward genetic engineering of drought-tolerant soybean. Crit Rev Biotechnol 32:349–362
Tian X, Lei Y (2006) Nitric oxide treatment alleviates drought stress in wheat seedlings. Biol Plant 50:775–778
Yordanov I, Velikova V, Tsonev T (2000) Plant responses to drought, acclimation and stress tolerance. Photosynthetica 38:171–186
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66
Wendehenne D, Durner J, Klessig DF (2004) Nitric oxide: a new player in plant signaling and defense responses. Curr Opin Plant Biol 7:449–455
Wu S, Hu C, Tan Q, Xu S, Sun X (2017) Nitric oxide mediates molybdenum-induced antioxidant defense in wheat under drought stress. Front Plant Sci 8:1085
Yang X, Lu M, Wang Y, Wang Y, Liu Z, Chen S (2021) Response mechanism of plants to drought stress. Hortic 7(3):50
Youn HS, Lee JY, Saitoh SI, Miyake K, Kang KW, Choi YJ, Hwang DH (2006) Suppression of MyD88- and TRIF-dependent signaling pathways of Toll-like receptor by (-)-epigallocatechin-3-gallate, a polyphenol component of green tea. Biochem Pharmacol 72:850–859
Zafari S, Sharifi M, Ahmadian Chashmi N (2017) Nitric oxide production shifts metabolic pathways toward lignification to alleviate Pb stress in Prosopis farcta. Environ Exper Bot 141:41–49
Zhang L, Li X, Li X, Wei Z, Han M, Li ZL (2016) Exogenous nitric oxide protects against drought-induced oxidative stress in Malus rootstocks. Turk J Bot 40:17–27
Acknowledgements
This study was funded by College of Science, University of Tehran.
Author information
Authors and Affiliations
Contributions
MR has contributed in the major bench experiments. VN and HE equally designed the experiments. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Handling Editor: Saddam Hussain
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rezayian, M., Ebrahimzadeh, H. & Niknam, V. Metabolic and Physiological Changes Induced by Nitric Oxide and Its Impact on Drought Tolerance in Soybean. J Plant Growth Regul 42, 1905–1918 (2023). https://doi.org/10.1007/s00344-022-10668-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-022-10668-4