Abstract
Photosynthetic gas exchange in the leaves of wheat plants growing in a nutrient solution containing 0 or 2 mM silicon (Si) and inoculated with Pyricularia oryzae was investigated. The blast severity, the gas exchange parameters such as net carbon assimilation rate (A), stomatal conductance to water vapor (g s), internal CO2 concentration (C i) and transpiration rate (E) and the concentration of pigments (chlorophyll a, chlorophyll b and carotenoids) were determined. The blast severity was reduced by 67.66 % on +Si plants compared with the −Si plants. There were significant increases of 29.3, 17.7 and 45 % for A at 48, 72 and 96 h after inoculation (hai); 26.7 and 49 % for g s at 48 and 96 hai; and 25.2 and 31.4 % for E at 48 and 96 hai, respectively, for +Si inoculated plants when compared with the −Si inoculated plants. The C i was significantly lower for +Si inoculated plants than for −Si inoculated plants at 48, 72 and 96 hai. For inoculated plants, the concentrations of chlorophyll a and chlorophyll b were significantly higher for the +Si plants compared with the −Si plants at 72 and 96 hai. The results of this study clearly demonstrated that the supply of Si to the wheat plants was associated with lower blast severity in parallel with improved gas exchange performance, resulting in higher energy for mounting successful defense strategies against P. oryzae infection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Blast, caused by the fungus Pyricularia oryzae Sacc. (teleomorph: Magnaporthe grisea (Hebert) Barr), has become one of the most important disease of wheat (Triticum aestivum L.) in Brazil, considering that it can decrease yield by up to 70 %. On the leaves, the symptoms of this disease include gray–green and water-soaked lesions with dark green borders. Indeed, the fungus causes seedling blight, spike tip death and bright black spots on the rachis. The occurrence of gray–brown lesions on the spikes and spikelets significantly contributes to yield reduction due to low levels of nutrient translocation to the grains. Blast epidemics mainly occur during the rainy season, with temperatures ranging from 21 to 27 °C and high relative humidity (Goulart et al. 2007). The spraying of fungicides has demonstrated low efficiency for disease control (Goulart and Paiva 1993; Goulart et al. 2007), and few cultivars with desirable level of blast resistance are available (Urashima et al. 2004).
Although silicon (Si) is not considered to be an essential nutrient for plants, its beneficial effects on disease control have been very well documented (Datnoff et al. 2007). Plants absorb Si from the soil solution in the form of monosilicic acid, but differences in their ability to accumulate Si in their shoots differ among plant species (Datnoff et al. 2007). Dallagnol et al. (2009) demonstrated that low Si concentration in the tissues of the lsi1 mutant plants, which were defective in active Si uptake ability, affected the basal level of resistance to brown spot, suggesting that a minimum foliar Si concentration is necessary for increasing the resistance of rice to the disease. Sorghum plants supplied with Si and infected with Colletotrichum sublineolum were able to maintain carbon fixation and keep an efficient antioxidant system (Resende et al. 2012).
When plants are attacked by pathogens, several physiological processes, such as respiration, water and nutrient translocation, photosynthesis and transpiration are negatively impaired (Lucas 1998). Some studies have shown that attacks by pathogens reduce photosynthesis due to damage to the photosynthetic machinery (Bassanezi et al. 2002; Petit et al. 2006; Dallagnol et al. 2011). Bassanezi et al. (2002) measured gas exchange in the leaves of bean plants infected with Uromyces appendiculatus, Pseudocercospora griseola and Colletotrichum lindemuthianum and observed that reductions in photosynthesis caused by U. appendiculatus and P. griseola were due to a lower carboxylation capacity. In contrast, the reductions in internal CO2 concentrations were due to increased stomatal resistance, which was the main factor associated with decreases in photosynthesis due to infection by C. lindemuthianum. Symptoms of esca disease on the leaves of vines were associated with stomatal closure and alterations in the photosynthetic apparatus due to decreases in the assimilation of CO2 and a reduction in the concentration of chlorophyll (Petit et al. 2006).
Considering the lack of information on physiological changes on wheat leaves of plants supplied with Si during the infection process for P. oryzae, this study investigated the potential for this element to minimize the negative effects of P. oryzae infection on leaf gas exchange parameters and photosynthetic pigments concentration.
Materials and methods
Nutrient solution preparation
A nutrient solution was prepared based on Clark (1975), with some modifications. It included the following macronutrients: 1.04 mM Ca(NO3)2·4H2O, 1 mM NH4NO3, 0.8 mM KNO3, 0.069 mM KH2PO4, 0.931 mM KCl and 0.6 mM MgSO4·7H2O. It also included the following micronutrients: 19 μM H3BO3, 2 μM ZnSO4·7H2O, 0.5 μM CuSO4·5H2O, 7 μM MnCl2·4H2O, 0.6 μM Na2MoO4·4H2O, 90 μM FeSO4·7H2O and 90 μM EDTA bisodium. Silicon (2 mM) was supplied to plants in the form of silicic acid, which was prepared by passing potassium silicate through a cation exchange resin (Amberlite IR-120B; H+ form; Sigma-Aldrich). The pH of the nutrient solution was 5.6 and was not affected by the addition of silicic acid.
Plant growth
Wheat seeds from cv. BR-18 were germinated on distilled water-soaked germitest paper (Fisher Scientific Co., Pittsburgh, PA, USA) in a germination chamber at 25 °C for 6 days. Germinated seedlings were transferred to plastic pots with one-half strength nutrient solution without the presence of Si for 2 days. After this period, the plants were transferred to new plastic pots with 5 dm3 of nutrient solution prepared with or without Si. The nutrient solution, with aeration, was changed every 4 days. The electrical conductivity and the pH of the nutrient solution were checked daily. The pH was kept at ≈5.6 by using NaOH or HCl (1 M) as needed.
Inoculation of plants with P. oryzae
A pathogenic isolate of P. oryzae (UFV/DFP-01), obtained from ears of wheat plants (cv. BR-18), was used to inoculate the leaves of the wheat plants. Pieces of filter paper with fungal mycelia were transferred to Petri dishes containing oatmeal agar medium. After 3 days, plugs of oatmeal agar medium containing fungal mycelia were transferred to new Petri dishes containing the same medium. These Petri dishes were kept in a growth chamber at 25 °C with a 12-h photoperiod for 10 days. After this period, conidia were carefully removed from the Petri dishes with a rubber policeman to obtain a suspension of conidia. Plants were inoculated with a conidial suspension of P. oryzae (105 conidia cm−3) at 60 days after emergence (growth stage 65) (Zadoks et al. 1974). Twenty-five cm3 of suspension was applied as a fine mist to the adaxial leaf blades of each plant until runoff using a VL Airbrush atomizer (Paasche Airbrush Co., Chicago, IL, USA). Gelatin (1 %, wt vol−1) was added to the suspension to aid in conidial adhesion to the leaf blades. Immediately after inoculation, the plants were transferred to a growth chamber with a temperature of 25 ± 2 °C and a relative humidity of 90 ± 5 % and were subjected to an initial 24 h dark period. After this period, the plants were transferred to a plastic mist growth chamber (MGC) inside a greenhouse for the duration of the experiments. The MGC was made of wood (2 m wide, 1.5 m high and 5 m long) and was covered with 100-µm-thick transparent plastic. The temperature inside the MGC ranged from 25 ± 2 °C (day) to 20 ± 2 °C (night). The relative humidity was maintained at 92 ± 3 % using a misting system in which the nozzles (model NEB-100; KGF Company, São Paulo, Brazil) sprayed mist every 30 min above the plant canopy. The relative humidity and temperature were measured with a thermo-hygrograph (TH-508, Impac, Brazil). The maximum natural photon flux density at plant canopy height was approximately 900 μmol m−2 s−1.
Assessment of blast severity
The blast severity was assessed on the fourth, fifth and sixth leaves, from the base to the apex, of each plant, 96 h after inoculation (hai) using a standard area diagram sets (SADs) proposed by Rios et al. (2013). The SADs proposed by these authors has ten levels of blast diseased wheat leaves with a range of severity from 0.1 to 72 %.
Determination of the leaf gas exchange parameters
Gas exchange parameters were measured on the fifth leaf, from the base to the apex, of non-inoculated and inoculated plants at 48, 72 and 96 hai. The net carbon assimilation rate (A), stomatal conductance to water vapor (g s), internal CO2 concentration (C i) and transpiration rate (E) were estimated from 09:00 to 12:00 h under artificial and saturating photon irradiance (1,000 μmol m−2 s−1) and an external CO2 concentration of 400 μmol mol−1 using a portable open-system infrared gas analyzer (LI-6400, LI-COR Inc., Lincoln, NE, USA).
Determination of pigments concentration
The fourth and fifth leaves, from the base to the apex, of non-inoculated and inoculated plants were collected at 24, 48, 72 and 96 hai. Samples were kept in liquid nitrogen during sampling and were then stored at −80 °C until further analysis. A total of 200 mg of leaves was ground into a fine powder with liquid nitrogen using a mortar and pestle with the addition of 1 mg of calcium carbonate. Next, the fine powder was homogenized with 2 mL of 80 % acetone (v/v) for 1 min in a room with reduced light intensity. The suspension was filtered through a Whatman Number 1 filter paper and the residue was washed four times with 80 % acetone. The volume was increased to 25 ml with the same solvent in a volumetric flask. The absorbance of the samples was recorded at 470, 646.8 and 663.2 nm and the concentrations of photosynthetic pigments (chlorophyll a, chlorophyll b and carotenoids) were estimated according to Lichtenthaler (1987) and expressed in mg per gram of fresh matter.
Determination of foliar Si concentration
After the termination of the experiments, fourth and fifth leaves were collected from plants from each replication of each treatment, washed in deionized water, dried for 72 h at 65 °C and ground with a Thomas Wiley mill (Thomas Scientific, Swedesboro, NJ, USA) until they could pass through a 40-mesh screen. The foliar Si concentration was determined by colorimetric analysis of 0.1 g of dried and alkali-digested tissue (Resende et al. 2012) and was expressed in g kg−1.
Experimental design and data analysis
A 2 × 2 factorial experiment was performed consisting of two Si concentrations (0 and 2 mM, hereafter referred to as the −Si and +Si treatments, respectively) and plant inoculation was arranged in a completely randomized design with four replications. Each experimental unit corresponded to a plastic pot containing five wheat plants. The experiment was repeated once. Data from blast severity, foliar Si concentration, leaf gas exchange parameters and pigments concentration were combined for statistical analysis after determining the homogeneity of variance and the mean squares (Gomes and Garcia 2002). For leaf gas exchange parameters, the analysis of variance was considered to be a 2 × 2 × 3 factorial experiment consisting of two Si concentrations, plant inoculation and three evaluation times. The analysis of variance for pigments concentration was considered to be a 2 × 2 × 4 factorial experiment consisting of two Si concentrations, plant inoculation and four evaluation times. Data for all variables were subjected to analysis of variance and the mean from the treatments were compared using a t test (P ≤ 0.05) using SAS software (SAS Institute Inc., Cary, NC, USA). The Pearson correlation was used to determine the relationships among the gas exchange parameters, foliar Si concentration and blast severity.
Results
Foliar Si concentration and blast severity
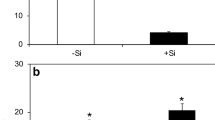
The foliar Si concentration was significantly higher for the +Si plants compared with the −Si plants (4.30 and 45.8 g kg−1, respectively). The blast severity was significantly lower on the leaves of the +Si plants than on the leaves of the −Si plants (9.22 and 28.53 %, respectively).
Leaf gas exchange parameters
At least one of the factors Si concentrations, plant inoculation and evaluation times and some of their interactions was significant for the parameters A, g s, C i and E (Table 1). For the non-inoculated plants at 96 hai, A was significantly higher for the +Si plants than for the −Si plants (Fig. 1). For the inoculated plants supplied with Si, A significantly increased by 29.3, 17.7 and 45 % at 48, 72 and 96 hai, respectively, compared with the −Si plants (Fig. 1). For the non-inoculated plants, the g s was significantly higher for the +Si plants in comparison to the −Si plants at 48 hai (Fig. 1). For the inoculated plants supplied with Si, the g s significantly increased by 26.7 and 49 % at 48 and 96 hai, respectively, compared with the −Si plants. For C i on the non-inoculated plants, there was no significant difference between the −Si and +Si treatments (Fig. 1). For the inoculated plants supplied with Si, the C i was significantly lower than for the −Si plants at 48, 72 and 96 hai (Fig. 1). The E was significantly higher for non-inoculated +Si plants than for the −Si plants at 48 hai. For the inoculated plants, the E was significantly higher at 48 and 96 hai for the +Si plants than for the −Si plants (Fig. 1).
Leaf gas exchange parameters net carbon assimilation rate (A) (a, b), stomatal conductance to water vapor (g s) (c, d), internal CO2 concentration (C i) (e, f) and transpiration rate (E) (g, h) determined on the leaves of wheat plants grown in hydroponic culture containing 0 mM (−Si) or 2 mM (+Si) of silicon and non-inoculated (NI) or inoculated (I) with Pyricularia oryzae. The mean for the −Si and +Si treatments followed by an asterisk at each evaluation time are significantly different using Student’s t test (P ≥ 0.05). Bars represent the standard errors of the mean. n = 8
Pigments concentration
At least one of the factors Si concentrations, plant inoculation and evaluation times and some of their interactions was significant for the concentrations of chlorophyll a, chlorophyll b and carotenoids (Table 2). For non-inoculated plants, the concentration of chlorophyll a was significantly higher at 24, 48 and 96 hai for the +Si plants when compared with the −Si plants (Fig. 2). There was no significant difference between the −Si and +Si treatments regardless of plant inoculation for the concentrations of chlorophyll b and the carotenoids (Fig. 2). For the inoculated plants, the concentrations of chlorophyll a and chlorophyll b were significantly higher at 72 and 96 hai for the +Si plants in comparison to the −Si plants (Fig. 2). The concentration of carotenoids was significantly higher at 48 and 96 hai for the −Si plants compared with the +Si plants (Fig. 2).
Concentrations of chlorophyll a (a, b), chlorophyll b (c, d) and carotenoids (e, f) in the leaves of wheat plants grown in hydroponic culture containing 0 mM (−Si) or 2 mM (+Si) of silicon and non-inoculated (NI) or inoculated (I) with Pyricularia oryzae. The mean for the −Si and +Si treatments followed by an asterisk at each evaluation time are significantly different using Student’s t test (P ≥ 0.05). Bars represent the standard errors of the mean. FM fresh matter. n = 8
Pearson correlation
The correlation between blast severity and foliar Si concentration was negatively significant (Table 3). Parameter A was negatively correlated with the C i and blast severity, but positively correlated with the g s, E and foliar Si concentration (Table 3). The g s parameter correlated significantly and positively with the E but significantly and negatively with the C i (Table 3). The correlation of the C i with the E was significantly negative (Table 3). Parameter E was positively correlated with the foliar Si concentration and negatively correlated with blast severity.
Discussion
In support of previous findings that Si can improve the resistance of several monocots against foliar pathogens (Datnoff et al. 2007), including the wheat-P. oryzae pathosystem (Xavier Filha et al. 2011), the present study describes the first physiological features associated with the increase in resistance to P. oryzae infection of wheat plants supplied with Si. Several studies have demonstrated the efficiency of Si in reducing the negative effects of pathogen infections on plant photosynthesis (Fortunato et al. 2012; Resende et al. 2012). However, to the best of our knowledge, the effects of Si on enhancing the resistance of wheat to P. oryzae infection, specifically at the level of photosynthesis, have never been investigated.
The high Si concentration in the wheat leaves contributed to decreased blast severity. Supplying Si to plants has become an important strategy for reducing the intensity of diseases in many economically important crops, such as bananas, barley, beans, cucumbers, oats, rice and wheat (Dallagnol et al. 2009; Xavier Filha et al. 2011; Polanco et al. 2012; Fortunato et al. 2012). The mechanisms, however, are not completely understood. Originally, it was proposed that Si increased plant disease resistance by the deposition of silica in the leaves, which was believed to act as a physical barrier that hampers pathogen penetration into epidermis (Jones and Handreck 1967). Indeed, Kim et al. (2002) suggested that Si-induced cell wall fortification of rice leaves was closely associated with enhanced resistance to blast. Although important recent evidences suggest that the passive role of Si is not solely determinant for the Si-mediated resistance to pathogens. Therefore, some studies have demonstrated that Si potentiates inducible defense responses. Wheat plants supplied with Si produced phytoalexins in response Blumeria graminis f.sp. tritici infection (Rémus-Borel et al. 2005). According to Xavier Filha et al. (2011), the wheat resistance to blast was reduced by Si primarily due to an increase in the concentration of lignin-thioglycolic acid derivatives and higher activities of the defense enzymes chitinase and peroxidase. The production of two diterpenoids phytoalexins in response to P. grisea infection has been potentiated in rice plants supplied with Si (Rodrigues et al. 2004).
As reported by several authors, photosynthesis in several crops is impaired by foliar diseases (Scholes and Rolfe 1996; Chou et al. 2000; Berger et al. 2007; Dallagnol et al. 2011; Resende et al. 2012). In the present study, infection by P. oryzae on wheat leaves caused devastating effects on photosynthetic gas exchange in the plants not supplied with Si. As the wheat blast progressed, the A values dramatically decreased, but they decreased more slowly in the +Si plants. The reduction of A due to an increase in the severity of diseases has been demonstrated in other studies (Godoy et al. 2001; Meyer et al. 2001). One cause by which pathogens reduce photosynthesis is related to the stomatal closure impeding CO2 inflow, in addition to directly affecting the chloroplasts (Erickson et al. 2003). Stomatal closure due to low water availability in diseased tissues compromises the electron transport chain and reduces the entry of CO2 into the Calvin cycle in chloroplasts, increasing the photo-oxidation of photosystem antennae (Bacelar et al. 2006). Even at 48 hai, when the incubation period for blast had not yet occurred, there was some reduction of A in the −Si plants, which might be explained by lower photosynthetic efficiency in the asymptomatic tissues due to stomatal resistance. Meyer et al. (2001) showed that the infection of bean leaves with C. lindemuthianum caused stomatal closure in areas of apparently healthy tissues, thus reducing photosynthesis, even when the anthracnose severity remained at low levels. The reduction in the g s is a major factor affecting photosynthesis in diseased plants because of the reduction in CO2 influx (Erickson et al. 2003). The g s values were dramatically lower in the −Si plants upon infection by P. oryzae. However, the higher C i values suggest that the reduced CO2 influx was not the main factor associated with the reduction in the A, in contrast to some limitations at the chloroplast level. Decreases in the A might be due to the low activities of photosynthetic enzymes such as the Rubisco (Guo et al. 2005), carbonic anhydrase, which converts CO2 to HCO3 or enzymes involved in the degradation of photoassimilates (Baker et al. 1997). In soybean plants subjected to an abiotic stress, it was showed Si increased the photosynthesis due to higher activities of photosynthetic enzymes (Shen et al. 2010) and this may explain the higher A values observed in the present study for the inoculated +Si plants relative to their −Si counterparts. A recent study showed that Si increased the mesophyll conductance in rice plants (Detmann et al. 2012), which also may have occurred in the present study explaining the higher values of A for the +Si plants.
Reductions in the E for the −Si plants infected with P. oryzae can be linked to reductions in the g s values and, therefore, can be associated with stomatal closure. Some studies have also demonstrated concomitant reductions in both E and g s for the pathosystems wheat-Puccinia triticina (McGrath and Pennypacker 1990), common bean–Uromyces appendiculatus, common bean–Phaeoisariopsis griseola and common bean–Colletotrichum lindemuthianum (Duniway and Durbin 1971; Bassanezi et al. 2002), eucalyptus–Puccinia psidii (Alves et al. 2011) and rice–Bipolaris oryzae (Dallagnol et al. 2011). Moreover, the reductions in the E might be the result of the symptoms of wilting and drying observed in the leaves due to the massive colonization of the leaf tissue by P. oryzae. According to Resende et al. (2012), in sorghum plants not supplied with Si and infected by C. sublineolum, there were decreases of 60 % for the A, 61 % for the g s and 57 % for the E in comparison to plants supplied with Si at 8 days after inoculation. Those authors showed that the reduction in the leaf gas exchange parameters caused by the C. sublineolum infection was attenuated in the +Si plants which was associated to the increased activity of some antioxidant enzymes such as superoxide dismutase, catalase, ascorbate peroxidase and glutathione reductase, limiting, therefore, the cellular damage in those plants compared to the −Si ones.
Infection with P. oryzae negatively affected the concentration of pigments, especially in the −Si plants. This finding can be linked to the actions of lytic enzymes or to non-selective toxins released by the pathogen during tissue colonization. Reductions in pigments concentration are typical features of the oxidative stresses caused by pathogen infections and might be a consequence of accelerated chlorophyll degradation or reductions in its synthesis due to changes in the composition of the thylakoid membrane (Smirnoff 1995). The higher concentration of carotenoids in the −Si plants infected by P. oryzae might be interpreted as a strategy to protect the photosynthetic apparatus against damage by photoinhibitory singlet oxygen produced by the excited triplet state of chlorophyll (Bacelar et al. 2006).
In conclusion, the results of this study clearly demonstrated that Si supplied to the wheat plants was associated with lower blast severity in parallel with improved gas exchange performance, giving the plants more energy for mounting successful defense strategies against P. oryzae infection.
Author contribution
Jonas Alberto Rios: Graduate student that conducted the experiments and analyzed the data. Fabrício de Avila Rodrigues: Advisor of the Ph.D. students Jonas A. Rios and Daniel Debona. Conceived the idea and helped to write the manuscript. Daniel Debona: Graduate student that conducted the experiments and analyzed the data. Leandro Castro Silva: Undergraduate student that helped on the experiments and on data analysis.
References
Alves AA, Guimarães LMS, Chaves ARM, DaMatta FM, Alfenas AC (2011) Leaf gas exchange and chlorophyll a fluorescence of Eucalyptus urophylla in response to Puccinia psidii infection. Acta Physiol Plant 33:1831–1839
Bacelar EA, Santos DL, Moutinho-Pereira JM, Gonçalves BC, Ferreira HF, Correia CM (2006) Immediate responses and adaptative strategies of three olive cultivars under contrasting water availability regimes: changes on structure and chemical composition of foliage and oxidative damage. Plant Sci 170:596–605
Baker NR, Nogués S, Allen DJ (1997) Photosynthesis and photoinhibition. In: Lumsden PJ (ed) Plants and UV-B: Responses to environmental change. Cambridge University Press, Cambridge, pp 95–111
Bassanezi RB, Amorim L, Bergamin Filho A, Berger RD (2002) Gas exchange and emission of chlorophyll fluorescence during the monocycle of rust, angular leaf spot and anthracnose on bean leaves as a function of their trophic characteristics. J Phytopathol 150:37–47
Berger S, Sinha AK, Roitsch T (2007) Plant physiology meets phytopathology: plant primary metabolism and plant-pathogen inter-actions. J Exp Bot 58:4019–4026
Chou H, Bundock N, Rolfe SA, Scholes JD (2000) Infection of Arabidopsis thaliana leaves with Albugo candida (white blister rust) causes a reprogramming of host metabolism. Mol Plant Pathol 1:99–113
Clark RB (1975) Characterization of phosphatase of intact maize roots. J Agric Food Chem 23:458–460
Dallagnol LJ, Rodrigues FA, Mielli MVB, Ma JF, Datnoff LE (2009) Defective active silicon uptake affects some components of rice resistance to brown spot. Phytopathology 99:116–121
Dallagnol LJ, Rodrigues FA, Martins SCV, Cavatte PC, DaMatta FM (2011) Alterations on rice leaf physiology during infection by Bipolaris oryzae. Australas Plant Pathol 40:360–365
Datnoff LE, Rodrigues FA, Seebold KW (2007) Silicon and Plant Disease. In: Datnoff LE, Elmer WH, Huber DM (eds) Mineral Nutrition and Plant Disease. St Paul, The American Phytopathological Society, pp 233–243
Detmann KC, Araújo WL, Martins SCV, Sanglard LMVP, Reis JV, Detmann E, Rodrigues FA, Nunes-Nesi A, Fernie AR, DaMatta FM (2012) Silicon nutrition increases grain yield, which, in turn, exerts a feed-forward stimulation of photosynthetic rates via enhanced mesophyll conductance and alters primary metabolism in rice. New Phytol 196:752–762
Duniway JM, Durbin RD (1971) Some effects of Uromyces phaseoli on the transpiration rate and stomatal response of bean leaves. Phytopathology 61:114–119
Erickson JE, Stanosz GR, Kruger EL (2003) Photosynthetic consequences of marssonina leaf spot differ between two poplar hybrids. New Phytol 161:577–583
Fortunato AA, Rodrigues FA, Nascimento KJT (2012) Physiological and biochemical aspects of the resistance of banana plants to Fusarium wilt potentiated by silicon. Phytopathology 102:957–966
Godoy CV, Amorim L, Bergamin Filho A (2001) Alterações na fotossíntese e na transpiração de folhas de milho infetadas por Phaeosphaeria maydis. Fitopatol Bras 26:209–215
Gomes FP, Garcia CH (2002) Análise de grupos de experimentos. In: Gomes FP, Garcia CH (eds) Estatística aplicada a experimentos agronômicos e florestais. Piracicaba, FEALQ, pp 169–191
Goulart ACP, Paiva FA (1993) Avaliação de fungicidas no controle da brusone (Pyricularia grisea) do trigo (Triticum aestivum). Fitopatol Bras 18:167–173
Goulart ACP, Sousa PG, Urashima AS (2007) Danos em trigo causados pela infecção de Pyricularia grisea. Summa Phytopathol 33:358–363
Guo DP, Guo YP, Zhao JP, Liu H, Peng Y, Wang QM, Chen JS, Rao GZ (2005) Photosynthetic rate and chlorophyll fluorescence in leaves of stem mustard (Brassica juncea var. tsatsai) after turnip mosaic virus infection. Plant Sci 168:57–63
Jones LHP, Handreck KA (1967) Silica in soils, plants and animals. Adv Agron 19:107–149
Kim SG, Kim KW, Park EW, Choi D (2002) Silicon-induced cell wall fortification of rice leaves: a possible cellular mechanism of enhanced host resistance to blast. Phytopathology 92:1095–1103
Lichtenthaler HK (1987) Chlorophylls and carotenoids, the pigments of photosynthetic biomembranes. In: Douce R, Packer L (eds) Methods in enzymology. Academic Press Inc., New York, pp 350–382
Lucas JA (1998) Plant pathology and plant pathogens. Blackwell Science, Oxford
McGrath MT, Pennypacker SP (1990) Alteration of physiological processes in wheat flag leaves caused by stem rust and leaf rust. Phytopathology 80:677–686
Meyer S, Saccardy-Adji K, Rizza F, Genty B (2001) Inhibition of photosynthesis by Colletotrichum lindemuthianum in bean leaves determined by chlorophyll fluorescence imaging. Plant Cell Environ 24:947–955
Petit AN, Vaillant N, Boulay M, Clément C, Fontaine F (2006) Alterations of photosynthesis in grapevines affected by esca. Phytopathology 96:1060–1066
Polanco LR, Rodrigues FA, Nascimento KJT, Shulman P, Silva LC, Neves FW, Vale FXR (2012) Biochemical aspects of bean resistance to anthracnose mediated by silicon. Ann Appl Biol 161:140–150
Rémus-Borel W, Menzies JG, Bélanger RR (2005) Silicon induces antifungal compounds in powdery mildew-infected wheat. Physiol Mol Plant Path 66:108–115
Resende RS, Rodrigues FA, Cavatte PC, Martins SCV, Moreira WR, DaMatta FM (2012) Leaf gas exchange and oxidative stress in sorghum plants supplied with silicon and infected with Colletotrichum sublineolum. Phytopathology 102:892–898
Rios JA, Debona D, Duarte HSS, Rodrigues FA (2013) Development and validation of a standard area diagram set to assess blast severity on wheat leaves. European J Plant Pathol 136:603–611
Rodrigues FA, McNally DJ, Datnoff LE, Jones JB, Rollins JB, Labbé C, Benhamou N, Menzies JG, Bélanger RR (2004) Silicon enhances the accumulation of diterpenoid phytoalexins in rice: a potential mechanism for blast resistance. Phytopathology 94:177–183
Scholes J, Rolfe SA (1996) Photosynthesis in localized regions of oat leaves infected with crown rust (Puccinia coronata): quantitative imaging of chlorophyll fluorescence. Planta 199:573–582
Shen X, Zhou Y, Duan L, Li Z, Eneji AE, Li J (2010) Silicon effects on photosynthesis and antioxidant parameters of soybean seedlings under drought and ultraviolet-B radiation. J Plant Physiol 167:1248–1252
Smirnoff N (1995) Antioxidant systems and plant responses to the environment. In: Sminorff N (ed) Environment and plant metabolism: flexibility and acclimation. Bios Scientific Publishers, Oxford, pp 217–243
Urashima AS, Lavorenti NA, Goulart ACP, Mehta YR (2004) Resistance spectra of wheat cultivars and virulence diversity of Magnaporthe grisea isolates in Brazil. Fitopatol Bras 29:511–518
Xavier Filha MS, Rodrigues FA, Domiciano GP, Oliveira HV, Silveira PR, Moreira WR (2011) Wheat resistance to leaf blast mediated by silicon. Australas Plant Pathol 40:28–38
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Res 14:415–421
Acknowledgments
F. A. Rodrigues thanks the National Council for Technological and Scientific Development (CNPq) for his fellowship. Mrs. Jonas A. Rios and D. Debona were supported by CNPq. This study was supported by grants from CAPES, CNPq and FAPEMIG to Prof. F. A. Rodrigues.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. Filek.
Rights and permissions
About this article
Cite this article
Rios, J.A., Rodrigues, F.Á., Debona, D. et al. Photosynthetic gas exchange in leaves of wheat plants supplied with silicon and infected with Pyricularia oryzae . Acta Physiol Plant 36, 371–379 (2014). https://doi.org/10.1007/s11738-013-1418-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-013-1418-3