Abstract
l-Galactono-1, 4-lactone dehydrogenase (GalLDH; EC 1.3.2.3) is the last key enzyme in the putative l-ascorbic acid (AsA) biosynthetic pathway of higher plants. To evaluate the effect of the gene on manipulating AsA accumulation, a cDNA encoding GalLDH (RrGalLDH, Acc. No. AY643403), isolated from Rosa roxburghii fruit known to be rich in AsA, was introduced into tobacco plants by Agrobacterium-mediated transformation under CaMV 35S constitutive promoter in the present study. Southern blotting revealed the stable integration of the transgene with single copy in four independent transgenic lines, among which, L3 and L4 showed the significantly enhanced RrGalLDH transcript levels, GalLDH activities and AsA accumulations as compared to untransformed (WT) plants. So, we developed the AsA-overproducing tobacco plant by overexpressing GalLDH. As exposed to salt stress (100 mM NaCl), these AsA-overproducing transgenic lines were found to grow better with increased shoot length and fresh weight than WT. Furthermore, L3, which demonstrated the highest AsA accumulation (2.1-fold higher than WT) and expression level of RrGalLDH, showed a higher resistance to oxidative stress caused by paraquat when compared to WT. These results further justify that the overexpression of GalLDH gene confers an elevated AsA accumulation and tolerance against environmental stresses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
l-Ascorbic acid (AsA) is the major antioxidant in plant cells, protecting the plant from oxidative damage by scavenging free radicals and other active oxygen species that are generated during photosynthesis, oxidative metabolism, and environmental stresses (Noctor and Foyer 1998). AsA is an enzymatic cofactor for many reactions (Davey et al. 2000; Arrigoni and De Tullio 2002) and also functions as a regulator of cell division, cell expansion, and elongation in plants (Citterio et al. 1994; Conklin 2001). Also, it plays an important role in the process and regulation of hormone biosynthesis, signaling and plant defense responses (Pastori et al. 2003; Conklin and Barth 2004). Furthermore, AsA is of vital importance to human beings by protecting the body against oxidative stress and acting in collagen synthesis reactions and its deficiency causes scurvy (Padayatty et al. 2003). In animals these reactions are especially important in wound-healing and in preventing bleeding from capillaries. Since human beings have lost the ability to synthesize AsA, this vitamin must be acquired regularly from dietary uptake, primarily from fruits and vegetables.
To date, several AsA biosynthetic routes have been proposed in plants and the pathway through l-galactose is the best described (Wheeler et al. 1998). In the l-galactose pathway, d-mannose is converted to GDP-d-mannose and then to GDP-l-galactose. The l-galactose is produced from GDP-l-galactose and further oxidized to form l-galactono-1,4-lactone (GalL), which is the final precursor of AsA biosynthesis. GalL is oxidized to AsA by l-galactono-1,4-lactone dehydrogenase (GalLDH; EC 1.3.2.3), which is located on the inner mitochondrial membrane and plays important roles in AsA biosynthetic regulation (Bartoli et al. 2000; Millar et al. 2003). It has been suggested that there are other alternative pathways to AsA through galacturonic acid (Loewus 1999; Agius et al. 2003), l-gulose (Wolucka and Van Montagu 2003, 2007) or myo-inositol (Lorence et al. 2004). Several of the genes involved in the AsA biosynthetic pathway, such as l-gulono-1,4-lactone oxidase (GLO) (Jain and Nessler 2000; Radzio et al. 2003; Hemavathi et al. 2010), l-galactose dehydrogenase (GalDH) (Gatzek et al. 2002), d-galacturonic acid reductase (GalUR) (Agius et al. 2003), myo-inositol oxygenase (MIOX) (Lorence et al. 2004), GalLDH (Tokunaga et al. 2005; Imai et al. 2009), GDP-l-galactose guanyltransferase (GGT) (Bulley et al. 2009), GDP-l-galactose phosphorylase (GGP) (Bulley et al. 2011), and GDP-d-mannose-3,5-epimerase (GME) (Zhang et al. 2011), have been overexpressed to regulate the content of AsA in transgenic plants. In these experiments, the AsA content in the transgenic plants (or cells) varied depending on the studies. For example, overexpression of rat GLO in tobacco, lettuce, Arabidopsis plants and potato tubers led to a 1.5 to 7-fold increase in the AsA content (Jain and Nessler 2000; Radzio et al. 2003; Hemavathi et al. 2010). Overexpression of Arabidopsis MIOX or strawberry GalUR in Arabidopsis showed two to threefold elevations of the AsA level (Agius et al. 2003; Lorence et al. 2004). Interestingly, overexpression of GalLDH in tobacco BY-2 cells showed approximately fourfold increase in the AsA pool size (Tokunaga et al. 2005), while GalLDH-overexpressing tobacco plant showed only 30 % (Bauw et al. 2002) to no increase (Imai et al. 2009) in AsA compared to non-transformed plants. Thus, the effect of GalLDH overexpression on promoting AsA accumulation remains elusive in plants. Yet, the transgenic tobacco plant with high level of AsA using GalLDH has not been achieved. Rosa roxburghii Tratt, a perennial rosebush native to China, belonging to the family Rosaceae has recently sparked great interest nationwide due to the high AsA content (1,100–3,000 mg/100 g fresh weight) of its fruits (He et al. 1984; Fan et al. 1997; Wen et al. 2004). Previous work has demonstrated that expression of GalLDH in R. roxburghii Tratt is organ- or development-specific, and that the expression patterns in different tissues or developing fruits are correlated with AsA accumulation (An et al. 2004, 2007), indicating that GalLDH is important for AsA biosynthesis and its regulation in plants. In the present study, to verify further the effect of GalLDH gene on manipulating AsA content in plants and to obtain transgenic tobacco plants with enhanced AsA, we overexpressed the cDNA of RrGalLDH (Acc. No. AY643403) isolated from Rosa roxburghii fruit under the control of CaMV 35S promoter for promoting AsA accumulation in tobacco. Some of the transgenic lines showed significantly enhanced GalLDH transcript levels, GalLDH activities, and AsA accumulation as compared to WT plants, and the most AsA level of the transgenic line, L3, was 2.1-fold higher than the non-transgenic plant. Subsequently, the tolerance of AsA-overproducing transgenes to abiotic stresses induced by either paraquat or NaCl was further evaluated herein.
Materials and methods
Generation of transgenic tobacco overexpressing Rosa roxburghii GalLDH
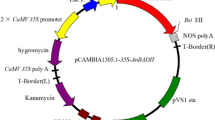
pCAMBIA1301 and pBI121 were used to construct the transformation vector. The CaMV 35S promoter and NOS terminator regions were excised from the binary vector, pBI121 with HindIII/BamHI and SacI/EcoRI sites, respectively. These were then introduced into the multi-cloning sites of the pCAMBIA1301. A full-length cDNA encoding Rosa roxburghii GalLDH (RrGalLDH, Acc. No. AY643403), isolated from Rosa roxburghii fruit by RT-PCR amplification as described previously (An et al. 2007), was introduced into the KpnI site between the CaMV 35S promoter and NOS terminator in the sense orientation (Fig. 1). The obtained pCAMBIA-RrGalLDH vector, confirmed by DNA sequencing, was introduced into Agrobacterium tumefaciens strain LBA4404 and transformed into tobacco (Nicotiana tabacum cv. xanthin) as described by Jones et al. (1995). The leaf discs were selected in MS medium containing 2.25 mg/L 6-benzylaminopurine (6-BA), 0.3 mg/L a-naphthalene acetic acid (NAA), 20 mg/L hygromycin and 500 mg/L cefotaxime (sodium salt) for adventitious bud regeneration. A total of 23 regenerated shoots obtained from 220 explants inoculated with Agrobacterium were transferred to MS medium supplemented with 0.2 mg/L Indole-3-butytric acid for rooting, at 25 ± 2 °C under a photosynthetic photon flux density of 120 μmol m−2 s−1 in 14/10 h day night cycle. Among them, six shoots survived and developed roots on MS medium containing hygromycin.
The structures of pCAMBIA-RrGalLDH. CaMV 35S promoter and NOS terminator which were excised with HindIII/BamHI and SacI/EcoRI sites from the binary vector pBI121 were introduced into the multi-cloning sites of the vector pCAMBIA1301, respectively. A full-length cDNA clone encoding Rosa roxburghii GalLDH (RrGalLDH, Acc. No. AY643403) was introduced into KpnI site between the CaMV 35S promoter and NOS terminator in the sense orientation. NOS T nopaline synthase terminator, PolyA CaMV 35S PolyA as another terminator, HPT hygromycin phosphotransferase gene, GUS β-glucuronidase, RB and LB right border and left border, respectively
PCR and southern blot analysis
Genomic DNA was extracted from young leaves of the six rooted lines (designated L1–L6) and untransformed (WT) plants by the CTAB method (Porebski et al. 1997) for PCR and southern blot analysis. The PCR amplification was carried out using the CaMV 35S promoter-specific primer, 5′-GCTCCTACAAATGCCATCA-3′ (forward primer) and RrGalLDH-specific primer, 5′-CCACATTGTATAGCAAGATCC-3′ (reverse primer). The amplification parameters were as follows: 94 °C for 5 min, followed by 35 cycles of 94 °C for 30 s, 57 °C for 40 s, 72 °C for 40 s and a final extension time of 10 min at 72 °C. PCR products were viewed on a 1 % agarose gel. For southern blot analysis, genomic DNA (20 μg) of each sample was digested with BamHI, and blotted on to a nylon membrane (Hybond N+; Amersham Pharmacia Biotech) after electrophoresis on an agarose gel. Probes were labeled from cDNAs for HPT gene with the digoxigenin (DIG)-labeling system (Roche Co., Germany). Southern blot analysis was carried out using the Dig Detection Kit II according to the manufacturer’s instructions (Roche Co., Germany).
RNA isolation and RT-PCR
Total RNA was extracted from the leaves of the four southern positive transgenic lines (L1–L4) and WT plants by CTAB-LiCl method as described previously (Chang et al. 1993). The RNA was used to synthesize cDNA by RT-PCR amplification using the RNA PCR Kit Ver.2.1 (TaKaRa, Dalian, China) with RrGalLDH-specific primers and internal standard 18S rRNA primers: 5′-CACAGAAAAATGCAGAGAGCA-3′ (Forward primer) and 5′-CCACATTGTATAGCAAGATCC-3′ (Reverse primer) for RrGalLDH, and 5′-CAAATTTCTGCCCTATCAAC -3′ (Forward primer) and 5′-CAAAATCCAACTACGAGCTT-3′ (Reverse primer) for 18S rRNA. PCR amplification was then performed at 94 °C for 4 min, followed by 35 cycles of 94 °C for 30 s, 57 °C for 40 s, and 72 °C for 40 s. Final extension time of 10 min at 72 °C was used. PCR products were separated by electrophoresis on a 1 % agarose gel.
AsA and DHA quantification
After confirmation of the RrGalLDH transgene by Southern blot and RT-PCR, the four putative transgenic lines were maintained and propagated, respectively. Ten 8-week-old transgenic lines and WT plants were used for AsA analysis, GalLDH activity assay and abiotic stress evaluation. For AsA and DHA quantification, 1 g of fresh leaves from the same position of plants was ground in 5 % (w/v) metaphosphoric acid at 4 °C to prevent AsA oxidation and DHA degradation. The homogenate was then centrifuged at 16,000g for 20 min and the supernatant was collected to measure AsA and DHA contents according to the method of Takahama and Oniki (1992). For AsA quantification, a 50 μL aliquot was assayed at 265 nm in 2.0 ml 100 mM potassium phosphate buffer (pH 6.8) following addition of 1.25 U mL−1 ascorbate oxidase (Sigma-Aldrich Co.). DHA was assayed by subtraction, after reducing the DHA to AsA using 2 mM dithiothreitol (DTT) in another 50 μL aliquot of the extract, which was then measured at 265 nm in 2.0 ml 100 mM potassium phosphate buffer (pH 6.8). Complete reduction of DHA took no longer than 8 min. Measurements were then corrected to account for the absorbance of DTT at 265 nm. A series of AsA and DHA solutions of known concentration (Sigma-Aldrich Co.) were measured using the same methods to produce standard curves. Three independent analyses were carried out with each sample.
Assays for GalLDH activities
The crude enzyme extract of leaves from transgenic lines and WT plants was prepared according to the method of Tabata et al. (2001). GalLDH activity was assayed spectrophotometrically by measuring the increase in absorbance at 550 nm accompanied by the l-GalL-dependent reduction of cytochrome c at 25 °C, according to the method of Ôba et al. (1995). All chemicals were purchased from Sigma-Aldrich Co (St Louis, USA). One unit of enzyme was defined as the reductions of 1 mol of cytochrome c per min and mean values from three independent measurements are shown.
Physiological evaluation of transgenic plants for stress tolerance
Tobacco node cuttings or leaf discs of transgenic lines and WT were used to assess the tolerance to NaCl and paraquat (SinoChemtech, Shanghai, China) stresses. For NaCl treatment, six single node cuttings from WT and each transgenic plant were transferred into the MS medium containing 0.2 mg/L IBA with 100 mM NaCl and cultured under a 16 h photoperiod at 25 °C. Salt stress tolerance was estimated by measuring the shoot length and biomass after 10 weeks of growth. For paraquat treatment, leaf discs (1 cm2) were excised from healthy and fully expanded leaves of 8-week-old transgenic (L3) and the WT plants according to the protocol of Fan et al. (1997). The discs were dipped in the 2 μM paraquat solution for 30 min, and then transferred into the MS medium. The leaf discs were incubated at 22 ± 2 °C under continuous white light of 120 μmol m−2 s−1 intensity. The effect of various treatments on leaf discs was observed by monitoring the phenotypic changes and by measuring the chlorophyll content.
Chlorophyll measurement
Chlorophyll content was measured according to Lichtenthaler (1987) with some modifications. The leaf discs were briefly rinsed in deionized water and then homogenized in 1 mL of 80 % acetone (v/v) and the homogenate was filtered. The filtrate was retained and the absorbance was recorded at 663 and 645 nm. Total chlorophyll content was expressed as mg g−1 FW.
Results and discussion
Generation of transgenic tobacco plants overexpressing RrGalLDH
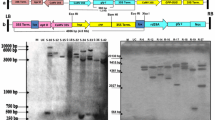
The cDNA encoding GalLDH from Rosa roxburghii fruit, which is known to be extremely rich in AsA (Fan et al. 1997; Wen et al. 2004; An et al. 2007), was introduced into tobacco plants (Nicotiana tabacum cv. xanthin) by Agrobacterium-mediated transformation under CaMV 35S promoter (Fig. 1). From 220 explants inoculated with Agrobacterium, a total of 23 regenerated shoots obtained, among which, six shoots survived and developed roots on MS medium containing hygromycin. The six independent hygromycin-resistant tobacco plantlets were recovered and designated as L1–L6. Analysis by PCR using genomic DNA isolated from both the transgenic and WT plants confirmed the presence of the transgene in five transgenic lines, while no amplification was observed in WT plants and L5 (Fig. 2a). Southern blot analyses verified that four RrGalLDH-transgenic lines (L1–L4) had independently incorporated single copy of the introduced transgene (Fig. 2b), while no hybridization signal was detected in the L5, L6 or WT plants. Furthermore, RT-PCR was performed to evaluate the expression levels of RrGalLDH in the four Southern positive transgenic plants. A specific 1.9 kb amplification corresponding to RrGalLDH was detected with all the four transgenic lines, and no expected band was observed in WT plant (Fig. 2c). The result also showed that L3 and L4 had shown significantly enhanced RrGalLDH transcript levels as compared with other transgenic lines, which was probably ascribed to their positional effect (Meyer 1995; Spiker and Thompson 1996).
Molecular analysis of transgenic lines of tobacco plants. a PCR analysis for the presence of the RrGalLDH gene in hygromycin-resistant tobacco transgenic lines. Lanes P positive control pCAMBIA-RrGalLDH, WT untransformed control plants, L1–L6 hygromycin-resistant tobacco plants. b Southern blot analysis following digestion of genomic DNA with BamHI and hybridization with an HPT-specific gene probe. Lanes P positive control pCAMBIA-RrGalLDH; WT untransformed control plants; L1–L6 transgenic plants. c Reverse transcriptase-PCR analysis of total RNA from transgenic tobacco with RrGalLDH-specific primers. Lanes WT untransformed control plants, L1–L4 transgenic plants
GalLDH activity and AsA content in RrGalLDH-overexpressing tobacco lines
The enzyme assays performed in the four transgenic lines showed that the L3 and L4 had significantly higher GalLDH activities as compared to the L1, L2, or WT plants (Fig. 3a), which were well correlated with the RrGalLDH transcript levels (Fig. 2c). The maximum GalLDH activity observed in L3 demonstrated 2.2-fold increase in comparison with that in the control. It is difficult to explain the reason for the failure to increase the GalLDH activities in L1 or L2 in which higher RrGalLDH transcript levels as compared with WT were observed, but the position effect of the transgene might result in insufficient translation in these lines (Meyer 1995). The levels of AsA in leaves of WT and transgenic plants were measured to determine the metabolic consequences of RrGalLDH overexpression. The result proved that two of the transgenic lines, L3 and L4, had significantly elevated AsA levels as compared to WT, and L3 was found to contain 2.1-fold higher (2.06 mg/g FW) AsA in the leaves compared to WT (Fig. 3b). Therefore, we developed the AsA-overproducing tobacco plant by overexpressing GalLDH. Similar results were reported by Tokunaga et al. (2005) having successfully created tobacco BY-2 cells, in which GalLDH-overexpressing BY-2 cells showed a 1.3 to 1.8-fold increase in AsA content and a five to sevenfold increase in GalLDH activity. Bauw et al. (2002) showed that overexpression of GalLDH gene in the tobacco leads to a two to threefold increase in GalLDH activity while there was a 30 % increase in AsA content. Recently, Shi et al. (2011) reported that overexpression of GalLDH from apple in Lanzhou lily (Lilium davidii) via particle bombardment-mediated transformation caused two to sevenfold increase in AsA content compared to that of controls. These results indicated that overexpression of GalLDH in plants could effectively increase AsA production. Essentially, GalLDH is not only the last key enzyme in the main pathway for AsA biosynthesis but it is also involved in the d-galacturonic acid pathway as well (Agius et al. 2003). A high correlation had been reported between GalLDH activity, transcription level, and AsA content (Tabata et al. 2001; Pateraki et al. 2004; An et al. 2007; Li et al. 2009), which provide evidences that GalLDH functions as a final enzyme in a major pathway of AsA biosynthesis in higher plants. In another study, however, despite the elevated GalLDH activity, overexpression of sweet potato GalLDH in tobacco strain SR1 did not exhibit an increased foliar AsA content (Imai et al. 2009). This difference in response could be due to different source materials or genes, position effect of transgene (Meyer 1995; Spiker and Thompson 1996), or post-transcriptional regulation (Alhagdow et al. 2007; Loscos et al. 2008).
GalLDH activity and ascorbate contents in the leaves of RrGalLDH-transformed (L1–L4) and non-transformed tobacco plants (WT). Each line represents mean ± SD of three independent measurements (n = 6). Asterisk indicates values that are significantly different from that of WT plants according to the Duncan’s multiple range test at p < 0.05 level
Abiotic stress tolerance of transgenic plants overexpressing RrGalLDH gene
The transgenic lines and WT were cultured in a medium containing 100 mM NaCl for salt stress. Although no obvious morphological damage appeared under NaCl stress, the increments of shoot length and biomass of those lines tested were diversely affected (Fig. 4). The transgenic shoots grown at 100 mM NaCl demonstrated significantly higher fresh weight as compared with WT, but the response of shoot growth to salt stress varied within these lines. The fresh weight and shoot length of L3 and L4 maintained significantly higher than that of WT after 10 weeks of treatment. These results suggested that overexpression of the RrGalLDH gene conferred salt tolerance to the transgenic lines via elevation of AsA levels. Therefore, overexpression of GalLDH gene could be an effective strategy for breeding salt tolerant crops.
Effect of salt stress on transgenic and WT tobacco plants. Graph representing the fresh weight (g plant−1) and shoot length (cm) of transgenic and WT plants after 10 weeks growing in MS medium supplemented with 100 mM NaCl. Values are the mean ± SD of three independent experiments (n = 6). Asterisk indicates values that are significantly different from those of WT plants according to the Duncan’s multiple range test at p < 0.05 level
L3 plants, which had the highest level of AsA were further used to estimate the tolerance to the oxidative stresses caused by paraquat. In the leaf disc assay, the phenotypic differences were observed from the leaf discs derived from WT plant and the transgenic line after 48 h of paraquat (2 μM) treatment (Fig. 5a). Leaf discs from the WT plants showed complete senescence, while the transgenic discs remained green. The chlorophyll content of the leaf discs of L3 plants was significantly higher than that of WT after the treatment (Fig. 5b), indicating higher tolerance of the transgenic tobacco plants to oxidative stress. This is consistent with the results obtained by Tokunaga et al. (2005) in which GalLDH-overexpressing tobacco BY-2 cells had much higher viability than non-transformed cells when treated with paraquat. Furthermore, increased tolerance of transgenic plants with elevated AsA levels to salt or oxidative stresses were reported with the overexpression GLO (Hemavathi et al. 2010), GalUR (Hemavathi et al. 2009), MDAR (Eltayeb et al. 2007), DHAR (Kwon et al. 2001; Chen et al. 2003; Ushimaru et al. 2006), or APX (Badawi et al. 2004). These evidences, together with our experiment above, led us to conclude that AsA plays key roles in plants as an antioxidant in defense against different abiotic stresses, and an increase in AsA content could confer tolerance to these stresses. Whether the manipulation of the upstream genes in the AsA biosynthetic pathway from this rosebush would result in a greater increase in AsA would be an interesting finding.
Effect of oxidative stress induced by paraquat on leaf discs of transgenic and WT tobacco plants. a Phenotypic differences in the leaf discs from transgenic (L3) versus the WT lines after paraquat treatment. b Chlorophyll content (mg g−1FW) of leaf discs of transgenic line L3 versus the WT lines after 0, 48 and 60 h treatment of paraquat, under continuous light at 22 ± 2 °C. Values are the mean ± SD of three independent measurements (n = 3). Asterisk indicates values that are significantly different from that of WT plants according to the Duncan’s multiple range test at p < 0.05 level
Author contribution
Hua-Ming An designed and instructed the research work. Wei Liu and Man Yang performed the experiments. Hua-Ming An and Wei Liu were involved in data interpretation and paper preparing. All authors have read and approved the final manuscript.
References
Agius F, Gonzlezolez LR, Caballer JL (2003) Engineering increased vitamin C levels in plants by over- expression of a d-galacturonic acid reductase. Nat Biotechnol 21:177–181
Alhagdow M, Mounet F, Gilbert L, Nunes-Nesi A, Garcia V, Just D, Petit J, Beauvoit B, Fernie AR, Rothan C (2007) Silencing of the mitochondrial ascorbate synthesizing enzyme l-galactono-1,4-lactone dehydrogenase affects plant and fruit development in tomato. Plant Physiol 145:1408–1422
An HM, Chen LG, Fan WG (2004) cDNA fragment cloning of l-galactono-1, 4-lactone dehydrogenase and its expression in different organs of R. roxburghii Tratt. Agri Sci in China 11:807–811
An HM, Fan WG, Chen LG, Asghar S, Liu QL (2007) Molecular characterisation and expression of l-galactono-1,4-lactone dehydrogenase and l-ascorbic acid accumulation during fruit development in Rosa roxburghii. J Hortic Sci Biotech 82:627–635
Arrigoni O, de Tullio MC (2002) Ascorbic acid: much more than just an antioxidant. Biochem Biophys Acta 1569:1–9
Badawi GH, Kawano N, Yamauchi Y, Shimada E, Sasaki R, Kubo A, Tanaka K (2004) Over-expression of ascorbate peroxidase in tobacco chloroplasts enhances the tolerance to salt stress and water deficit. Physiol Plant 121:131–238
Bartoli CG, Pastori GM, Foyer CH (2000) Ascorbate biosynthesis in mitochondria is linked to the electron transport chain between complexes III and IV. Plant Physiol 123:335–343
Bauw GJC, Davey MW, Ostergaard J, Van Montagu MCE (2002) Production of ascorbic acid in plants. US Patent 6,469,149
Bulley S, Rassam M, Hose D, Otto W, Schunemann N, Wright M, Macrae E, Gleave A, Laing W (2009) Gene expression studies in kiwifruit and gene over-expression in Arabidopsis indicates that GDP-L-galactose guanyl transferase is a major control point of vitamin C biosynthesis. J Exp Bot 60:765–778
Bulley S, Wright M, Rommens C, Yan Hua, HuaYan M, Wang KL, Andre C, Brewster D, Karunairetnam S, Allan AC, Laing WA (2011) Enhancing ascorbate in fruits and tubers through over-expression of the L-galactose pathway gene GDP-L-galactose phosphorylase. Plant Biotechnol J 10:390–397
Chang SJ, Jeff P, John C (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 11:113–116
Chen Z, Young TE, Ling J, Chang SC, Gallie DR (2003) Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc Natl Acad Sci USA 100:3525–3530
Citterio S, Sgorbati S, Scippa S, Sparvoli E (1994) Ascorbic acid effect on the onset of cell proliferation in pea root. Physiol Plant 92:601–607
Conklin PL (2001) Recent advance in the role and biosynthesis of ascorbic acid in plants. Plant Cell Environ 24:383–394
Conklin PL, Barth C (2004) Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant Cell Environ 27:959–970
Davey MW, Montagu VM, Inzh D, Sanmartin M, Kanellis A (2000) Plant l-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. Sci Food Agric 80:825–860
Eltayeb A, Kawano N, Badawi G, Kaminaka H, Sanekata T, Shibahara T, Inanaga S, Tanaka K (2007) Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta 225:1255–1264
Fan WG, Xia GL, Luo YC (1997) Utilization of Rosa roxburghii resources and its development strategy in Guizhou province. Southwest China. J Agric Sci 10:109–115
Gatzek S, Wheeler GL, Smirnoff N (2002) Antisense suppression of l-galactose dehydrogenase in Arabidopsis thaliana provides evidence for its role in ascorbate synthesis and reveals light modulated l-galactose synthesis. Plant Physiol 30:541–553
He ZF, Niu AZ, Xiang XH, Wang SM (1984) A study on the nutrition and variation in the vitamin C content in the fruits of Rosa roxburghii Tratt. Acta Hortic Sinica 11:271–273
Hemavathi, Upadhyaya CP, Ko EY, Nookaraju A, Kim HS, Heung J, Oh MO, Reddy AC, Chun SC, Kim DH, Park SW (2009) Over-expression of strawberry d-galacturonic acid reductase in potato leads to accumulation of vitamin C with enhanced abiotic stress tolerance. Plant Sci 177:659–667
Hemavathi, Upadhyaya CP, Akula N, Young KE, Chun SC, Kim DH, Park SW (2010) Enhanced ascorbic acid accumulation in transgenic potato confers tolerance to various abiotic stress. Biotechnol Lett 32:321–330
Imai T, Niwa M, Ban Y (2009) Importance of the l-galactonolactone pool for enhancing the ascorbate content revealed by l-galactonolactone dehydrogenase-overexpressing tobacco plants. Plant Cell Tiss Organ Cult 96:105–112
Jain AK, Nessler CL (2000) Metabolic engineering of an alternative pathway for ascorbic acid biosynthesis in plants. Mol Breed 6:73–78
Jones H, Gallois P, Marinho P (1995) Leaf disk transformation using Agrobacterium tumefaciens expression of heterologous genes in tobacco. In: Walker JM (ed) Plant gene transfer and expression protocols, vol 49. Methods in molecular biology, pp 39–48
Kwon SY, Ahn YO, Lee HS, Kwak SS (2001) Biochemical characterization of transgenic tobacco plants expressing a human dehydroascorbate reductase gene. J Biochem Mol Biol 34:316–321
Li M, Liang D, Pu F, Ma F, Hou C, Lu T (2009) Ascorbate levels and the activity of key enzymes in ascorbate biosynthesis and recycling in the leaves of 22 Chinese persimmon cultivars. Sci Hortic 120:250–256
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Loewus FA (1999) Biosynthesis and metabolism of ascorbic acid in plants and of analogs of ascorbic acid in fungi. Phytochemistry 52:193–210
Lorence A, Chevone BI, Mendes P, Nessler CL (2004) Myo-inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiol 134:1200–1205
Loscos J, Matamoros MA, Becana M (2008) Ascorbate and homoglutathione metabolism in common bean nodules under stress conditions and during natural senescence. Plant Physiol 146:1282–1292
Meyer P (1995) Understanding and controlling transgene expression. Trends Biotechnol 13:332–337
Millar AH, Mittova V, Kiddle G, Heazlewood JL, Bartoli CG, Theodoulou FL, Foyer CH (2003) Control of ascorbate synthesis by respiration and its implications for stress responses. Plant Physiol 133:443–447
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Ann Rev Plant Physiol Mol Biol 49:249–279
Ôba K, Ishikawa S, Nishikawa M, Mizuno H, Yamamoto T (1995) Purification and properties of l-galactono-1,4-lactone dehydrogenase, a key enzyme for ascorbic acid biosynthesis, from sweet potato root. J Biol Chem 117:120–124
Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK, Levine M (2003) Vitamin C as an antioxidant: a valuation of its role in disease prevention. J Am Coll Nutri 22:18–35
Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, Noctor G, Foyer CH (2003) Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell 15:939–951
Pateraki I, Sanmartin M, Kalamak MS, Gerasopoulos D (2004) Moleclar characterization and expression studies during melon fruit development and ripening of l-galactono-1, 4-lactone dehydrogenase. J Exp Bot 55:1623–1633
Porebski SL, Bailey G, Baum BR (1997) Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol Biol Rep 15:8–15
Radzio JA, Lorence A, Chevone BI, Nessler CL (2003) Gulono-1,4-lactone oxidase expression rescues vitamin C-deficient Arabidopsis (vtc) mutants. Plant Mol Biol 53:837–844
Shi SG, Ma FM, Li YH, Feng FJ, Shang ZZ (2011) Overexpression of l-galactono-1, 4-lactone dehydrogenase (GLDH) in Lanzhou lily (Lilium davidii var. unicolor) via particle bombardment- mediated transformation. In Vitro Cell Dev Biol Plant 1:1–6
Spiker S, Thompson WF (1996) Nuclear matric attachment regions and transgene expression in plants. Plant Physiol 110:15–21
Tabata K, Ôba K, Suzuki K, Esaka M (2001) Generation and properties of ascorbic acid-deficient transgenic tobacco cells expressing antisense RNA for l-galactono-1,4-lactone dehydrogenase. Plant J 27:139–148
Takahama U, Oniki T (1992) Regulations of peroxidase-dependent oxidation of phenolics in the apoplast of spinach leaves by ascorbate. Plant Cell Physiol 33:379–387
Tokunaga T, Miyaharal K, Tabata K, Esaka M (2005) Generation and properties of ascorbic acid-overproducing transgenic tobacco cells expressing sense RNA for l-galactono-1,4-lactone dehydrogenase. Planta 220:854–863
Ushimaru T, Nakagawa T, Fujioka Y, Daicho K, Naito M, Yamuchi Y, Nonaka H, Amako K, Yamawaki K, Murata N (2006) Transgenic Arabidopsis plants expressing the rice dehydroascorbate reductase gene are resistant to salt stress. J Plant Physiol 163:1179–1184
Wen XP, Pang XM, Deng XX (2004) Characterization of genetic relationships of Rosa roxburghii Tratt and its relatives using morphological traits, RAPD and AFLP markers. J Hortic Sci Biotechnol 79:189–196
Wheeler GL, Jones MA, Smirnoff N (1998) The biosynthetic pathway of vitamin C in higher plants. Nature 393:65–369
Wolucka BA, Van Montagu M (2003) GDP-mannose 3′,5′-epimerase forms GDP-L-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. J Biol Chem 278:47483–47490
Wolucka BA, Van Montagu M (2007) The VTC2 cycle and the de novo biosynthesis pathways for vitamin C in plants: an opinion. Phytochemistry 68:2602–2613
Zhang C, Liu J, Zhang Y, Cai X, Gong P, Zhang J, Wang T, Li H, Ye Z (2011) Overexpression of SlGMEs leads to ascorbate accumulation with enhanced oxidative stress, cold and salt tolerance in tomato. Plant Cell Rep 30:389–398
Acknowledgments
The authors would like to thank Prof. Xiaopeng Wen for help in revising our English composition and Guizhou Key Laboratory of Agricultural Bioengineering for providing the tobacco material. This work was supported by the National Natural Science Foundation of China (31060257), the Excellent Youth Scientific and Technological Talent Cultivation Program (200704), and the special fund project for the outstanding talents in science and education of Guizhou Province, P. R. China (201012).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.-H. Liu.
Rights and permissions
About this article
Cite this article
Liu, W., An, HM. & Yang, M. Overexpression of Rosa roxburghii l-galactono-1,4-lactone dehydrogenase in tobacco plant enhances ascorbate accumulation and abiotic stress tolerance. Acta Physiol Plant 35, 1617–1624 (2013). https://doi.org/10.1007/s11738-012-1204-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-012-1204-7