Abstract
l-Ascorbic acid (Vitamin C, AsA) is an important component of human nutrition. Plants and several animals can synthesize their own ascorbic acid, whereas humans lack the gene essential for ascorbic acid biosynthesis and must acquire from their diet. In the present study, we developed transgenic potato (Solanum tuberosum L. cv. Taedong Valley) over-expressing l-gulono-γ-lactone oxidase (GLOase gene; NCBI Acc. No. NM022220), isolated from rat cells driven by CaMV35S constitutive promoter that showed enhanced AsA accumulation. Molecular analyses of four independent transgenic lines performed by PCR, Southern and RT-PCR revealed the stable integration of the transgene in the progeny. The transformation frequency was ca. 7.5% and the time required for the generation of transgenic plants was 6–7 weeks. Transgenic tubers showed significantly enhanced AsA content (141%) and GLOase activity as compared to untransformed tubers. These transgenics were also found to withstand various abiotic stresses caused by Methyl Viologen (MV), NaCl or mannitol, respectively. The T1 transgenic plants exposed to salt stress (100 mM NaCl) survived better with increased shoot and root length when compared to untransformed plants. The elevated level of AsA accumulation in transgenics was directly correlated with their ability to withstand abiotic stresses. These results further demonstrated that the overexpression of GLOase gene enhanced basal levels of AsA in potato tubers and also the transgenics showed better survival under various abiotic stresses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
l-Ascorbic acid (Vitamin C, AsA) is required for the maintenance and normal functioning of immune system as well as for the synthesis of collagens. A mutation in the gene encoding the enzyme which catalyzes the terminal step in AsA biosynthesis has rendered humans incapable of synthesizing AsA. Therefore AsA must be acquired regularly from dietary sources, primarily from plants. Biosynthesis of AsA in animals occurs via d-glucuronic acid, l-gulonic acid, and l-gulono-γ-lactone, which is then oxidized to AsA by the l-gulono-γ-lactone oxidase (GLOase) (Loewus 1963; Smirnoff 2001). However, in higher plants AsA synthesis occurs via d-mannose/l-galactose pathway, where d-mannose is converted to GDP-d-mannose and then to GDP-l-galactose. The l-galactose is further oxidized to l-galactono-γ-1actone, a precursor to AsA (Wheeler et al. 1998). Part of the AsA pool is synthesized via galacturonic acid, a principal component of cell wall pectins (Agius et al. 2003). Furthermore, part of the ‘animal pathway’ also known as myo-inositol pathway with l-gulono-γ-lactone as the final precursor of AsA biosynthesis appears to be operating in plants, but the enzymes involved have not yet been identified (Wolucka and Van Montagu 2003; Davey et al. 1999). The proposed AsA biosynthesis pathway in plants and animals is shown in Fig. 1 (Wheeler et al. 1998; Lorence et al. 2004).
Proposed pathways for ascorbic acid biosynthesis in plants and animals (Wheeler et al. 1998; Lorence et al. 2004; Agius et al. 2003). 1–4: Plant pathways; 5: animal pathway. PGI phosphogluco isomerase, PMI phosphomanno isomerase, PMM phosphomanno mutase, GMPase GDP-d-mannose pyrophosphorylase, GME GDP-mannose epimerase, GGalPP GDP-l-galactose pyrophosphatase, GalPP l-galactose-1-phosphate phosphatase, GalDH l-galactose dehydrogenase, GLDH l-galactono-γ-lactone dehydrogenase, GGulPP GDP-l-gulose-pyrophosphatase, GulPP l-gulose-1-phosphate phosphatase, GulDH l-gulose dehydrogenase, GLOase l-gulono-γ-lactone oxidase, MIOX myo-inositol oxygenase, GlcUR d-glucuronic acid reductase, AL aldonolactonase, GalUR d-galacturonic acid reductase, PGM phosphogluco mutase, UGPase UDP-glucose pyrophosphorylase, UGDH UDP-glucose dehydrogenase, GlcPUT glucuronate-1-phosphate uridyltransferase, GlcK glururonokinase
Recently, several ascorbate biosynthetic pathway genes have been introduced into plants through metabolic engineering to elevate the AsA level. These include l-gulono-γ-lactone oxidase (GLOase) cDNA isolated from mouse in tobacco and lettuce (Jain and Nessler 2000), a human dehydroascorbate (DHAR) gene in tobacco (Kwon et al. 2003), d-galacturonic acid reductase (GalUR) cDNA of strawberry in Arabidopsis and potato (Agius et al. 2003; Hemavathi et al. 2009), and wheat DHAR cDNA in tobacco and maize (Chen et al. 2003).
Potato is the fourth most important source of calories for mankind after cereals wheat, rice and maize. Although several studies in plants with enhanced AsA have been reported, the accumulation of high level of AsA in potato tubers using GLOase gene has not yet been achieved. In this communication, we overexpressed the GLOase gene from rat under the control of CaMV35S promoter for higher AsA accumulation in potato. We also evaluated the transgenic plants for their enhanced tolerance to various abiotic stresses.
Materials and methods
Plant materials
Potato cv. ‘Taedong Valley’ was propagated on MS (Murashige and Skoog’s 1962) basal medium supplemented with 30 g sucrose l−1. The cultures were maintained in growth chamber at 22 ± 2°C and 16 h light period with an intensity of 100 μmol m−2 s−1. Four week old in vitro rooted shoots were planted in pots (10 cm in size) containing biopeat (Seminis Asia, Korea) and transferred to greenhouse for hardening. Young expanding leaves were used as explants for transformation study. Leaves were surface sterilized with 10% sodium hypochlorite for 15 min followed by three washes with sterile distilled water in laminar flow.
Agrobacterium strain, vector construction and potato transformation
Transformation was carried out using Agrobacterium tumefaciens strain EHA 105 harbouring the modified pCAM2300 binary vector, kindly provided by Dr. H. J. Hak (Nong Woo Bio., Ltd, Korea). The T-DNA region of the plasmid vector contains GLOase and nptII genes driven under the control of CaMV35S promoter. Transgenic plants were generated via Agrobacterium tumefaciens mediated transformation, as described (Hemavathi et al. 2009). The regenerated shoots were transferred to MS basal medium supplemented with kanamycin (50 mg l−1) and carbenicillin (300 mg l−1) for rooting. Rooted plantlets were transferred to pots (25 cm in size) containing mixture of biopeat and soil and maintained in greenhouse for further growth and tuber formation. These plants were referred as T0 transgenic lines. The putative T0 transformants were screened by PCR for the presence of GLOase gene using gene specific primers. The efficiency of transformation was calculated as the number of shoots rooted on selection medium to the total number of explants kept on co-cultivation medium.
Generation of T1 transgenic potato plants
The putative transgenic and untransformed control plants were maintained under greenhouse conditions in pots (25 cm in size) with a day temperature of 22°C and night 15°C for tuber formation. The T0 tubers collected from 12 week old transgenic and untransformed control plants were kept at room temperature for sprouting. The sprouted T0 tubers were planted in the pots (25 cm) for obtaining T1 plants as well as T1 tubers. The T1 plants and tubers were taken for the molecular, biochemical and physiological analyses. Single node cuttings from T1 transgenic line were used for the in vitro salt stress analyses.
Molecular characterization of transgenic potato plants
PCR and Southern blot analyses
Genomic DNA was isolated from 0.5 g young leaves by the CTAB method (Doyle and Doyle 1987). The PCR amplification was carried out using GLOase specific primers (F: 5′-TCC ATG GGT ACA AAG GGG TC-3′ and R: 5′-TAG AAG ACT TTC TCC AGG TAC G-3′). Southern blot analysis was performed according to the standard protocol (Sambrook et al. 1989). Approximately 20 μg of DNA was digested with XbaI and hybridized using a Dig-labelled GLOase probe prepared by PCR Dig Labelling Mix (Roche Co., Germany) with the above mentioned primers. Southern blot was detected using the Dig Detection Kit according to the manufacturer’s instructions (Roche Co., Germany).
Semi-quantitative and quantitative RT-PCR
Total RNA was extracted from the leaves of transgenic as well as untransformed control plants using TRI reagent (Sigma, USA). First-strand cDNA was synthesized using SuperScript Reverse Transcriptase (Invitrogen, USA). The GLOase gene was amplified using the above mentioned primers and PCR conditions. Actin mRNA served as loading control.
Quantitative RT-PCR was carried out using ABI Prism 7700 sequence detector (Applied Biosystems). Total RNA extracted from leaves of transgenic and untransformed control plants was used for first strand cDNA synthesis using SuperScript Reverse Transcriptase (Invitrogen, USA). The amplification of GLOase gene (200 bp) was carried out using cDNA specific primers (F: 5′-TGG ATC CGG AAC ACA TAA CA-3′ and R: 5′-GAA ACT GAG GCA CAC ACT GC-3′). The PCR was performed using SYBR green PCR kit (Qiagen GmbH, Hilden, Germany). Actin was used as an internal control.
Determination of ascorbic acid (AsA) content by HPLC
Ascorbic acid was extracted from potato tubers according to the method of Han et al. (2004). Approximately 0.5 g frozen tubers were ground using a commercial blender in 5% metaphosphoric acid and the homogenate was filtered through Whatman No. 1 filter paper and centrifuged at 18,000g for 10 min at 1°C. Supernatants, 20 μl, were analysed by HPLC using an Atlantis dC18 column (4.6 mm × 250, 5 μm) with a mobile phase of 0.2% TFA (A) and 100% methanol (B) at 1 ml/min. The eluants were monitored at 254 nm. Three independent analyses were carried out with each sample.
GLOase enzyme assay
Protein was isolated from potato tubers according to the method described by Ulloa et al. (1997). The extracted protein was quantified by the Bradford method with bovine serum albumin as the standard. The GLOase enzyme activity assayed according to the method of Dabrowski (1990) with slight modifications. The enzyme reaction was initiated by the addition of 50 μl of enzyme extract to reaction mixture containing 50 mM phosphate buffer (pH 7.4), 50 mM reduced glutathione (GSH) and 100 mM l-gulono-1,4-lactone. The GLOase activity was determined by the increase in the absorbance at 265 nm for a period of 20 min and quantified using its molar extinction coefficient (18.13 mM−1 cm−1).
Precursor feeding assay
The precursor feeding assay was followed according to the protocol of Jain and Nessler (2000) with fully expanded leaves from transformed and untransformed potato plants. The leaves were placed in 100 ml flasks containing deionized water and freshly prepared 30 mM l-gulonic acid (l-gul). Leaves were maintained at 22°C under a 16 h light/8 h dark period. The leaf samples were collected after 72 h for AsA analysis by HPLC.
Physiological evaluation of transgenic plants for stress tolerance: leaf disc assay for sensitivity against Methyl Viologen, mannitol and salinity stress
Leaf disc assay were carried out to assess the tolerance of transgenic and untransformed potato plants to oxidative, salt and drought stresses caused by Methyl Viologen (MV), NaCl and mannitol, respectively according to the protocol of Fan et al. (1997). Leaf discs (1 cm2) were excised from healthy and fully expanded leaves of 6–8 week old transgenic and untransformed potato plants. Leaf discs were floated in 5 ml of MV (0, 5, 10 μM) and NaCl (0, 400, 600 mM) or mannitol (0, 200, 300 mM) for 4 days. The leaf discs were incubated at 22 ± 2°C under continuous white light of 100 μmol m−2 s−1 intensity. The effect of various treatments on leaf discs was observed by monitoring the phenotypic changes and by measuring the chlorophyll content.
Estimation of the chlorophyll content
The chlorophyll content in the leaf discs floated on MV, NaCl and mannitol was estimated according to the procedure of Arnon (1949). The leaf discs were homogenized in 1 ml of 80% acetone and the homogenate was centrifuged at 3,500 g/n for 5 min. The supernatant was retained and the absorbance was recorded at 663 and 646 nm. The chlorophyll content was expressed in μg g−1 FW.
In vitro salinity stress tolerance
Single node cuttings from the T1 transgenic and untransformed (UT) plants were cultured on MS basal medium supplemented with NaCl (0, 100 mM). The shoots were cultured in a growth chamber under a 16 h photoperiod with light intensity of 100 μM m−2 s−1 at 20 ± 2°C. Salt stress tolerance was estimated by measuring the shoot and root length after 30 days of growth.
Statistical analysis
Three replicates of each sample were used for statistical analysis and the means were analyzed using Statistical Analysis Software (SAS, Inc., USA) package program 9.1. Statistically differences were determined using a one way analysis of variance (ANOVA) and means were considered significantly different at P < 0.01.
Results
Generation of transgenic potato plants overexpressing GLOase gene
Transgenic potato plants were raised via Agrobacterium-mediated transformation. Regeneration of the kanamycin resistant shoots were observed from the callus formed from the leaf explants after 4 weeks on MS medium supplemented with zeatin (2 mg l−1), NAA (0.01 mg l−1) and GA3 (0.1 mg l−1). Elongated shoots (ca. 2 cm length) were transferred to rooting medium (MS basal) containing 50 mg kanamycin l−1. Well-rooted plantlets were transferred to a greenhouse for hardening and further growth. The transgenic plants were phenotypically uniform and undistinguishable from the untransformed control plants. The time required to generate the transformed plants from initiation of culture was 6–7 weeks.
A total of 13 shoots were obtained from 120 explants (taken from four different experiments) inoculated with Agrobacterium. Among them, nine shoots developed roots on MS medium containing kanamycin. Subsequently these nine shoots were transferred to pots where five of them survived. In the PCR analysis all the five plants were found to be PCR positive for GLOase gene (data not shown). The transformation efficiency of potato with GLOase gene was found to be 7.5%. The tubers (T0) from five PCR positive lines were selected and planted for generation of T1 transgenic plants. Among them four lines were able to sprout and produce T1 plants and subsequently formed tubers.
Molecular analysis of T1 transgenics
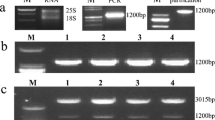
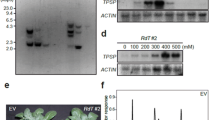
All the four T1 transgenic lines were PCR positive with the GLOase gene amplified with specific primers showing the amplification of 1.3 kb band on agarose gel (Fig. 2a), while no amplification was observed in the untransformed control plant. Southern blot analysis was performed to check the stable integration and copy number of the transgene. The estimated copy number of the GLOase gene inserted in different transgenic plants ranged from one to two. Three lines (T1 1.6, T1 2.1 and T1 5.2) contained single copy of the gene, while the line T1 9.1 had two integrated copies (Fig. 2b). However, the hybridization signal was not detected in untransformed control plants.
Molecular analysis of transgenics. a PCR analysis of T1 putative transgenic lines using GLOase gene specific primers. Lanes T1 1.6–T1 9.1 are T1 transgenic lines. b Southern blot analysis of different independent T1 potato transgenic lines showing copy number of transgene. c RT-PCR analysis of GLOase using gene specific primers in T1 transgenic lines. Lane UT: Untransformed plants; Lanes T1 1.6–T1 9.1 transgenic lines. Total RNA (2 μg) was used in the synthesis of the first strand cDNA, which was subsequently used in PCR amplification using specific primers. The actin gene fragment (600 bp) was amplified in RT-PCR as an internal control. d The GLOase transcript levels of T1 transgenic (T1 1.6, T1 2.1, T1 5.2 and T1 9.1) lines. The comparative threshold (Ct) values were normalized to actin control and compared to obtain relative expression levels. Each line represents mean ± SD of three independent measurements
Semi-quantitative RT-PCR amplifications were performed to evaluate the expression levels of GLOase in the Southern positive T1 transgenic plants. An expected 1.3 kb amplification corresponding to GLOase was detected with all the four transgenic lines, where as no amplification was detected with the untransformed control plants (Fig. 2c). The mRNA expression level of the GLOase determined by quantitative RT-PCR showed elevated level of the GLOase transcripts in all the four transgenic lines with no significant variation among the different transgenic lines (Fig. 2d).
AsA content and GLOase activity in T1 transgenic tubers
All the four T1 transgenic tubers (T1 1.6, T1 2.1, T1 5.2 and T1 9.1) contained 2.7, 2.6, 2.7 and 3 μmol FW AsA g−1 representing ca. 141% increase over untransformed control tubers (Fig. 3a). These results indicated that transgene expressed under the control of CaMV35S promoter is stable and capable of increasing the AsA contents in potato plants. Similarly all these T1 transgenic tubers showed ca. 350% enhanced GLOase activity when compared to untransformed controls (Fig. 3b).
AsA contents and GLOase activity in T1 transgenics and untransformed (UT) control potato tubers. a The AsA contents of transgenics (T1 1.6, T1 2.1, T1 5.2 and T1 9.1) and untransformed (UT) control lines. Data represents the means ± SD of three independent measurements. b The GLOase activity of T1 transgenic (T1 1.6, T1 2.1, T1 5.2 and T1 9.1) and untransformed (UT) control lines. Data represents the means ± SD of three independent measurements
Precursor feeding assay
The AsA levels of T1 transgenic leaves treated with l-gul was significantly higher as compared to the transgenic leaves treated with distilled water (Table 1).
Abiotic stress tolerance of transgenic plants overexpressing GLOase gene
Only three transgenic lines (T1 1.6, T1 2.1 and T1 5.2) containing single copy of the transgene were taken for all the physiological analysis. In the leaf discs assay, the phenotypic differences were observed from the leaf discs derived from untransformed control and transgenic lines after 4 days of MV (10 μM) treatments (Fig. 4a). Leaf discs from the untransformed control line showed complete senescence, while the transgenic line remained green. Leaf discs treated with distilled water devoid of MV appeared green in both untransformed and transgenic lines. The chlorophyll retention in the leaf discs of T1 transgenic lines exposed 10 μM MV stress was significantly high as compared to the leaf discs of untransformed control lines (Fig. 4b). Similarly the leaf discs of T1 transgenic lines floated on NaCl (400 and 600 mM) and mannitol (200 and 300 mM) showed delayed senescence. The leaf discs from untransformed control plants showed complete loss of color at 600 mM NaCl and 300 mM mannitol after 4 days of treatment (Fig. 4c, e), while the transgenic leaf discs remained green. The differences in the chlorophyll content was significant in the leaf discs from transgenic lines than the untransformed controls (Fig. 4d, f), thus indicating greater tolerance of the former to NaCl and mannitol stress as compared to the later.
Methyl Viologen, NaCl and mannitol stress induced senescence of leaf discs from the transgenic lines of potato over-expressing the GLOase gene. a Phenotypic differences in the leaf discs from transgenic versus the untransformed control lines after 4 days of MV treatment. b Chlorophyll content (μg g−1 FW) of leaf discs of transgenic lines T1 1.6, T1 2.1 and T1 5.2 versus the untransformed control (UT) line floated on 0, 5 and 10 μM MV solution, respectively, for 4 days under continuous light at 24 ± 2°C. c Phenotypic differences in the leaf discs from transgenic versus the untransformed control lines after 4 days of NaCl treatment (0, 400 and 600 mM). d Chlorophyll content (μg g−1 FW) of leaf discs of transgenic lines T1 1.6, T1 2.1 and T1 5.2 versus the untransformed control (UT) line floated on 400 and 600 mM NaCl solution, respectively, for 4 days under continuous light at 24 ± 2°C. e Phenotypic differences in the leaf discs from transgenic versus the untransformed control lines after 4 days of mannitol treatment (0, 400 and 600 mM). f Chlorophyll content (μg g−1 FW) of leaf discs of transgenic lines T1 1.6, T1 2.1 and T1 5.2 versus the untransformed control (UT) line floated on 200 and 300 mM mannitol solution, respectively for 4 days under continuous light at 24 ± 2°C
Response of transgenic plants to salt stress
When shoots of T1 transgenic plants were grown in vitro on MS basal medium supplemented with 100 mM NaCl this caused significant reduction in the plant growth in both transgenic and untransformed lines. However, the transgenic shoots grown at 100 mM NaCl had significantly higher shoot and root length compared to untransformed control shoots (Fig. 5a, b).
Effect of salt stress on transgenic (T1 1.6, T1 2.1 and T1 5.2) and untransformed (UT) potato plants. a Graph representing the shoot length (cm) of transgenic and untransformed control plants after 30 days growing in MS medium supplemented with 100 mM NaCl. b Graph representing the root length (cm) of the transgenic and untransformed control plants after 30 days growing in MS medium supplemented with 100 mM NaCl. Values are the means ± SD of three independent measurements
Discussion
In the present study, we report the overexpression of GLOase gene isolated from rat cells, driven by CaMV 35S promoter for enhanced accumulation of AsA in potato. Overexpression of the GLOase gene in potato leads to enhanced accumulation of AsA (141%) in the transgenic potato tubers. Earlier, Jain and Nessler (2000) also reported the expression of GLOase (isolated from rat) cDNA in lettuce and tobacco with increased AsA content correlated with high levels of mRNA transcript expression. The increase in tuber AsA content in potato by overexpression of GLOase gene indicated that this gene can also operate well through AsA biosynthetic pathway in plants. Recently, a substantial increase in α-tocopherol content was reported in potato tubers by overexpression of At-HPT (Crowell et al. 2008) wherein, the transgene was driven under the control of CaMV35S promoter. Similarly, we used CaMV35S promoter to drive GLOase gene for its strong and constitutive expression in potato tubers. Potato plants overexpressing GLOase gene contained elevated levels of AsA and these levels were positively correlated with GLOase activity. Incubation of potato leaves with the precursor (l-gulono-γ-lactone, l-gul) enhanced the AsA levels in transgenic plants compared to untransformed control. This indicated that the transgenic plants were capable of utilizing l-gul for l-AsA synthesis, either directly or through its conversion to other AsA intermediates. It was earlier reported that exogenous supply of l-galactono-γ-lactone (l-gal) and (l-gul) had increased the ascorbate synthesis in potato tubers and pea seedlings (Oba et al. 1994; Pallanca and Smirnoff 1999).
AsA is involved in free radical scavenging enzymatically or non-enzymatically in plants (Smirnoff 1996). However, increasing evidence suggests that ascorbate peroxidase provides resistance to various environmental stresses in plants (Mano et al. 2001; Kwon et al. 2002). In our study, the GLOase transgenic plants with elevated AsA levels displayed increased tolerance to various abiotic stresses imposed by Methyl Viologen (10 μM), NaCl (600 mM) and mannitol (300 mM) in the leaf disc assay. However, the higher retention of chlorophyll in the transgenic leaf disc was directly proportional to the AsA content and hence the increase in tolerance. The higher level of AsA in transgenic lines was directly correlated with their ability to withstand abiotic stress. Similar result was obtained in the transgenic potato plants expressing d-galacturonic acid reductase (Hemavathi et al. 2009). The ability of AsA to interact with various reactive oxygen species signifies its modulation in plant tolerance to various stresses (Conklin and Barth 2004). Increased tolerance of transgenic tobacco with elevated levels of AsA to salt and oxidative stresses was reported with the overexpression of DHAR (Kwon et al. 2003) and GalDH (Tokunaga et al. 2005). The association between salt environment and endogeneous levels of water-soluble antioxidant enzymes has also been reported (Lechno et al. 1997; Shalata and Tal 1998). In this report, the T1 transgenic lines also showed enhanced tolerance to NaCl (100 mM) with significantly higher shoot and root lengths as compared to untransformed control plants. This is consistent with the result obtained by (Kwon et al. 2003) where the human DHAR gene, overexpressed in tobacco lead to increased AsA levels and the transgenics were able to grow in high salt concentrations.
In conclusion, overexpression of GLOase gene under the constitutive expression of CaMV35S promoter resulted in enhanced AsA content in transgenic potato plants. The increased level of AsA was positively correlated with the increased mRNA transcript expression and also GLOase enzyme activity. Transgenic potato plants showed enhanced tolerance to various abiotic stresses like oxidative, salt and drought stresses caused by MV, NaCl and mannitol, respectively under in vitro conditions. Our study thus demonstrated that overexpression of GLOase gene to increase AsA biosynthesis also imparted increased tolerance in potato plants to various abiotic stresses.
References
Agius F, Lamothe RG, Caballero JL, Blanco JM, Botella MA, Valpuesta V (2003) Engineering increased vitamin C levels in plants by over-expression of a d-galacturonic acid reductase. Nat Biotechnol 21:177–181
Arnon DI (1949) Copper enzyme in isolated chloroplasts: polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Chen Z, Todd E, Ling YJ, Chang SC, Gallie DR (2003) Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc Natl Acad Sci USA 100:3525–3530
Conklin PL, Barth C (2004) Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant Cell Environ 27:959–970
Crowell EF, Mitchell JM, David SD (2008) Accumulation of vitamin E in potato (Solanum tuberosum) tubers. Transgenic Res 17:205–217
Dabrowski K (1990) Gulonolactone oxidase is missing in teleost fish-the direct spectrophotometric assay. Biol Chem Hoppe-Seyler 37:207–214
Davey MW, Gilot C, Persiau G, Østergaard J, Han Y, Bauw GC, Van Montagu MC (1999) Ascorbate biosynthesis in Arabidopsis cell suspension culture. Plant Physiol 121:535–543
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh tissue. Phytochem Bull 19:11–15
Fan L, Xheng S, Xuemin W (1997) Antisense suppression of phospholipase d-γ retards abscisic acid- and ethylene-promoted senescence of postharvest Arabidopsis leaves. Plant Cell 9:2183–2196
Han JS, Kozukue N, Young KS, Lee KR, Friedman M (2004) Distribution of ascorbic acid in potato tubers and in home-processed and commercial foods. J Agric Food Chem 52:6516–6521
Hemavathi, Upadhyay CP, Ko EY, Nookaraju A, Kim HS, Heung JJ, Oh MO, Reddy AC, Chun SC, Kim DH, Park SW (2009) Over-expression of strawberry d-galacturonic acid reductase in potato leads to accumulation of vitamin C with enhanced abiotic stress tolerance. Plant Sci 177(5) (in press)
Jain AK, Nessler CL (2000) Metabolic engineering of an alternative pathway for ascorbic acid biosynthesis in plants. Mol Breed 6:73–78
Kwon SY, Jeong YJ, Lee HS, Kim JS, Cho KY, Allen RD (2002) Enhanced tolerances of transgenic tobacco plants expressing both superoxide dismutase and ascorbate peroxidase in chloroplasts against methyl viologen mediated oxidative stress. Plant Cell Environ 25:873–882
Kwon SY, Choi SM, Ahn YO, Lee HS, Lee HB, Park YM, Kwak SS (2003) Enhanced stress-tolerance of transgenic plants expressing a human dehydroascorbate reductase gene. J Plant Physiol 160:347–353
Lechno S, Zamski E, Tel-Or E (1997) Salt stress-induced responses in cucumber plants. J Plant Physiol 150:206–211
Loewus FA (1963) Tracer studies on ascorbic acid formation in plants. Phytochemistry 2:109–128
Lorence A, Chevone BI, Mendes P, Nessler CL (2004) Myo-inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiol 134:1200–1205
Mano J, Ohno C, Domae Y, Asada K (2001) Chloroplastic ascorbate peroxidase is the primary target of methylviologen-induced photooxidative stress in spinach leaves: its relevance to monodehydroascorbate radical detected with in vivo ESR. Biochem Biophys Acta 1504:275–287
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Oba K, Fukui M, Imai Y, Iriyama S, Nogami K (1994) l-Galactono-γ-lactone dehydrogenase: partial characterization, induction of activity and role in the synthesis of ascorbic acid in wounded white potato tuber tissue. Plant Cell Physiol 35:473–478
Pallanca JE, Smirnoff N (1999) Ascorbic acid metabolism in pea seedlings. A comparison of d-glucose, l-sorbosone, and l-galactono-1,4-lactone as ascorbate precursors. Plant Physiol 120:453–461
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbour Laboratory Press, Cold Spring Harbor, pp 9.31–9.32
Shalata A, Tal M (1998) The effect of salt stress on lipid peroxidation and antioxidants in the leaf of the cultivated tomato and its wild salt-tolerant relative Lycoperscion pennellii. Physiol Plant 104:169–174
Smirnoff N (1996) The function and metabolism of ascorbic acid in plants. Ann Bot 78:661–669
Smirnoff N (2001) l-Ascorbic acid biosynthesis. Vitam Horm 61:241–266
Tokunaga T, Miyahara K, Tabata K, Esaka M (2005) Generation and properties of ascorbic acid-overproducing transgenic tobacco cells expressing sense RNA for l-galactono-1,4-lactone dehydrogenase. Planta 220:854–863
Ulloa RM, Mac-Intosh GC, Melchiorre M, Mentaberry AN, Dallari P, Moriconi DN, Tellez-Inon MT (1997) Protein kinase activity in different stages of potato (Solanum tuberosum L.) microtuberization. Plant Cell Rep 16:426–429
Wheeler GL, Jones MA, Smirnoff N (1998) The biosynthesis pathway of vitamin C in higher plants. Nature 393:365–369
Wolucka BA, Van Montagu M (2003) GDP-mannose 3′,5′-epimerase forms GDP-l-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. J Biol Chem 278:47483–47490
Acknowledgments
This research was supported by the Konkuk University research fund. The research fellowship to Hemavathi as “Research Fellow” from Konkuk University is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hemavathi, Upadhyaya, C.P., Akula, N. et al. Enhanced ascorbic acid accumulation in transgenic potato confers tolerance to various abiotic stresses. Biotechnol Lett 32, 321–330 (2010). https://doi.org/10.1007/s10529-009-0140-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-009-0140-0