Abstract
In the present study, we investigated the effects of l-DOPA (l-3,4-dihydroxyphenylalanine), an allelochemical exuded from the velvetbean (Mucuna pruriens L DC. var. utilis), on the growth and cell viability of soybean (Glycine max L. Merrill) roots. We analyzed the effects of l-DOPA on phenylalanine ammonia lyase (PAL), cinnamyl-alcohol dehydrogenase (CAD) and cell wall-bound peroxidase (POD) activities as well as its effects on phenylalanine, tyrosine and lignin contents in the roots. 3-day-old seedlings were cultivated in half-strength Hoagland nutrient solution (pH 6.0), with or without 0.5 mM l-DOPA, in a growth chamber at 25 °C for 6, 12, 18 or 24 h with a day/night regime of 1:1, and a photon flux density of 280 μmol m−2 s−1. In general, the length, fresh weight and dry weight of the roots decreased followed by a significant loss of cell viability. Phenylalanine, tyrosine and lignin contents as well as PAL, CAD and cell wall-bound POD activities increased after l-DOPA treatment. These results reinforce the susceptibility of soybean to l-DOPA, which increases the enzyme activity in the phenylpropanoid pathway and, therefore, provides precursors for the polymerization of lignin. In brief, these findings suggest that the inhibition of soybean root growth induced by exogenously applied l-DOPA may be due to excessive production of lignin in the cell wall.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants release secondary metabolites into the soil environment that can influence the growth and development of neighboring plants, both positively and negatively. This phenomenon is called allelopathy, defined as the ability of plants to protect themselves using natural allelochemicals (Gniazdowska and Bogatek 2005) and also the chemical communication between microbe–microbe, plant–microbe and plant–insect or plant–herbivore (Weir et al. 2004). Investigation into the mode of action of allelochemicals is a challenging endeavor because of the multitude of potential molecular targets that they may influence (Reigosa and Pazos-Malvido 2007). Allelochemicals typically inhibit seed germination and seedling growth. Moreover, they alter several physiological and biochemical processes including water utilization, mineral uptake, foliar expansion, photosynthesis, amino acid metabolism, protein synthesis, glycolysis, mitochondrial respiration and ATP synthesis among others (Weir et al. 2004).
The velvetbean (Mucuna pruriens L DC. var. utilis) is widely used in tropical regions for intercropping with corn, sorghum and millet and for providing benefits, such as suppression of the nematode population, weed smothering, symbiotic nitrogen fixation, nutrient recycling, control of erosion and soil improvement in its physical structure (Anaya 1999). Many secondary compounds are produced by velvetbean, but the main compound is the non-protein amino acid l-DOPA, which constitutes 0.5–1.5 % of the fresh leaves weight and 6–9 % of the dry seed weight (Fujii 1999; Vranova et al. 2011). The yield of fresh weight leaves and stems from plants ranges from 20 to 30 ton ha−1 and, by consequence, velvetbean can add 100–450 kg ha−1 of l-DOPA to the soils (Fujii 1999; Golisz et al. 2011). Several studies have addressed the effects of l-DOPA on plants and have revealed that it has attributes typical of an allelochemical; for example, l-DOPA reduces seed germination and suppresses root growth (Fujii 1999; Nakajima et al. 1999; Nishihara et al. 2004, 2005; Hachinohe et al. 2004). However, l-DOPA’s mechanism of action in plants is poorly understood.
The phenylpropanoid pathway is one of the most important metabolic pathways involved in the synthesis of a wide range of secondary compounds in plants, including phenolic acids, flavonoids, tannins, coumarins (Solecka 1997; Kovácik et al. 2007) and lignin (Boerjan et al. 2003). The first rate-limiting enzyme of this pathway is phenylalanine ammonia lyase (PAL), which in association with cinnamate 4-hydroxylase, 4-coumarate: CoA ligase, and cinnamoyl-CoA reductase enables the synthesis of p-coumaryl, coniferyl and sinapyl aldehydes. Cinnamyl-alcohol dehydrogenase (CAD) catalyzes the conversion of the corresponding cinnamyl aldehydes to cinnamyl alcohols. In the last step of the pathway, cell wall-bound peroxidase (POD) catalyzes the oxidative polymerization of p-hydroxycinnamyl alcohols to lignin.
Recent studies have shown that the reduction in plant root growth by some allelochemicals is characterized by increases in the activity of enzymes, such as PAL and POD, and by enhanced lignin production (dos Santos et al. 2004, 2008; Politycka and Mielcarz 2007; Zanardo et al. 2009; Bubna et al. 2011). In a previous report (Soares et al. 2007), we observed that after 24 h of exposure to l-DOPA, soybean seedlings had impaired root growth and increased lignin production. Based on these reports, the purpose of this study was to investigate further the mode of action of l-DOPA in soybean (Glycine max L. Merrill) roots, evaluating its effects on several parameters related to the lignification process. The root growth, cell viability and content of aromatic amino acids (phenylalanine and tyrosine) and lignin as well as the activity of some enzymes of the phenylpropanoid pathway, including PAL, CAD and cell wall-bound POD were monitored.
Materials and methods
General procedures
Soybean seeds were surface-sterilized for 2 min with 2 % sodium hypochlorite, rinsed extensively with deionized water and dark-germinated (at 25 °C) on two sheets of moistened filter paper. Twenty-five 3-day-old seedlings of uniform size were placed on an adjustable acrylic plate and dipped into a glass container (10 × 16 cm) filled with 200 ml of half-strength Hoagland’s solution (pH 6.0), with or without 0.5 mM l-DOPA. This concentration was selected based on the previous report that evaluated the effects of different concentrations of l-DOPA on soybean root growth (Soares et al. 2007). All nutrient solutions were buffered with 17 mM potassium phosphate buffer. The containers were kept in a growth chamber at 25 °C for 6, 12, 18 or 24 h with a day/night regime of 1:1, and a photon flux density of 280 μmol m−2 s−1. The roots were measured before incubation and again at the end of the experiment, and the difference in root length was calculated for each sample. The fresh root weight was determined immediately after incubation, and the dry weight was recorded after oven drying at 80 °C until the sample reached a constant weight. l-DOPA was purchased from Sigma-Aldrich (St. Louis, MO, USA), and all other reagents were of the purest available grade.
Cell viability
After incubation, the loss of cell viability in the seedlings was determined using the Evans blue staining spectrophotometric assay (Zanardo et al. 2009). All freshly harvested roots were incubated for 15 min with 30 ml of 0.25 % Evans blue solution. Next, the roots were washed in distilled water for 30 min to remove excess and unbound dye, and the excised root tips (3 cm) were soaked in 3 ml of N,N-dimethylformamide for 50 min at room temperature. The absorbance of released Evans blue solution was measured at 600 nm, using deionized water as a blank. The loss of cell viability is expressed as the absorbance at 600 nm of treated roots in relation to untreated roots (control).
Phenylalanine and tyrosine quantification
Samples (5.0 mg of dry root) were transferred to Pyrex® ampoules (10 × 150 mm) that had been pyrolyzed at 400 °C for 8 h. Next, 0.5 ml of aqueous 6 N HCl, doubly distilled at 104 °C and containing 0.1 % phenol, was added to each sample. Vials were sealed under vacuum and placed in an oven at 110 °C for 22 h. After acid hydrolysis, the solution was dried using rotary evaporation and resuspended in 0.17 M sodium citrate buffer (pH 2.2) containing 15 % polyethylene glycol (PEG 400) and 0.4 % thiodiglycol. Samples (0.9 ml) were loaded into a cation exchange column (resin: PC 6A amino acid analysis resin pierce) and eluted by pH and ionic strength (short column pH 5.28; long column pH 3.25, and additional pH 4.25). After chromatographic separation, the amino acids eluted from the column were reacted with ninhydrin in a boiling water bath (100 °C for 15 min), and the products of the reaction were detected colorimetrically at 570 nm (Moore et al. 1958). The phenylalanine and tyrosine contents are expressed as mg g−1 dry weight.
Enzymatic assays
PAL was extracted from root fresh, its activity was determined using the HPLC method described by Ferrarese et al. (2000), and the results are expressed as nmol t-cinnamate h−1 g−1 fresh weight. Cinnamyl-alcohol dehydrogenase (CAD) was extracted from root fresh, its activity was determined using the HPLC method as described in detail previously (dos Santos et al. 2006), and the results are expressed as nmol sinapaldehyde consumed h−1 g−1 fresh weight. Cell wall-bound POD was extracted from root fresh, its activity was determined spectrophotometrically (dos Santos et al. 2008), and the results are expressed as nmol tetraguaiacol h−1 g−1 fresh weight.
Lignin quantification
The lignin content of dry tissues was determined spectrophotometrically at 280 nm by the thioglycolic reaction (Ferrarese et al. 2002), and the results are expressed as mg lignin-thioglycolic acid (LTGA) g−1 dry weight.
Statistical analysis
The experimental design was completely randomized, and each point on the plot represents one glass container of 25 seedlings. The data are expressed as the means of three independent experiments ± SE and were analyzed using the Prism® package (Version 3.0, GraphPad, USA). Mean group differences were evaluated using Student’s t tests, and P ≤ 0.05 was adopted as the minimum criterion for statistical significance.
Results
Effects of l-DOPA on root growth, cell viability and amino acids contents
The effects of short-term exposure (6–24 h) to l-DOPA on root growth in soybean seedlings were evaluated (Table 1). Root length was significantly reduced by 18.6–62.4 % with increasing time of exposure, as compared to controls. These effects were also evident in fresh and dry root weight. As compared to controls, the root fresh weight decreased by 9.3, 21.3 and 19.4 % after 12, 18 and 24 h of exposure to l-DOPA, respectively, whereas the dry root weight decreased by 27.2 and 22.2 % after 18 and 24 h of exposure, respectively. The viability of root cells was also significantly affected by 6–24 h exposures to l-DOPA. The uptake of Evans blue, which indicates loss of cell viability, was significantly increased by 32, 255, 272 and 124 % after 6, 12, 18 and 24 h treatment, respectively, compared to controls (Fig. 1). In addition, phenylalanine and tyrosine contents in the roots increased by 50 and 114 %, respectively, after a 24-h treatment (Fig. 2).
Effects of l-DOPA on PAL, CAD and POD activities and lignin content
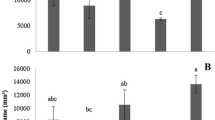
To determine whether l-DOPA treatment affects the phenylpropanoid pathway, PAL, CAD and POD activities in roots were evaluated. As shown in Fig. 3a, roots exposed to l-DOPA had significantly increased PAL activity with respect to controls instead (48 and 130 % after 18 and 24 h exposures, respectively).
Time-course of a phenylalanine ammonia lyase (PAL), b cinnamyl-alcohol dehydrogenase (CAD) and c cell wall-bound peroxidase (POD) activities in soybean roots treated with 0.5 mM l-DOPA. *Mean ± SE (n = 3) of l-DOPA treatment differing statistically (P ≤ 0.05) from values of untreated roots at the same experimental time. ns not significant
The activity of CAD was significantly increased by 50, 96 and 156 % after 12, 18 and 24 h of treatment, respectively, in comparison to controls (Fig. 3b). In addition to these findings, cell wall-bound POD activity was significantly increased by 19, 46 and 42 %, after 12, 18 and 24 h of l-DOPA treatment, respectively (Fig. 3c). As a consequence of l-DOPA treatment, lignin content increased from 26–152 % after 12–24 h of exposure (Fig. 4).
Discussion
The findings of the present study showed that the soybean root growth (length and weight) decreased during a short-term exposure to l-DOPA (Table 1). The data also revealed a significant loss of cell viability, which was confirmed by enhanced Evans blue uptake. In addition, phenylalanine, tyrosine and lignin contents as well as PAL, CAD and cell wall-bound POD activities were increased (Figs. 1, 2, 3, 4).
Reduction in root growth by l-DOPA has been reported in other plant species. Topal and Kocaçalişkan (2006) tested the herbicidal effects of l-DOPA against four weed species, including Sinapis arvensis, Cirsium arvense, Papaver rhoeas and Lamium amplexicaule. At 1.5 and 3 g L−1 concentrations, l-DOPA suppressed root growth of all weeds. They also conducted similar experiments with other plant species such as Triticum vulgare and Hordeum vulgare, which were not affected by l-DOPA. Using a treatment of 0.25 g L−1 l-DOPA, Nishihara et al. (2004) showed that the allelochemical strongly inhibited the root growth of Cucurbita moschata, Lactuca sativa, Cucumis sativus, Brassica oleraceae, Brassica campestris, Brassica chinensis, Nemophila menziesii and Nasturtium officinale. Hachinohe and Matsumoto (2007) reported that the amount of growth suppression caused by 0.02 g L−1 l-DOPA differed remarkably between Echinochloa crus galli and Lactuca sativa. Root of Echinochloa crus galli was completely suppressed by l-DOPA whereas the root length of Lactuca sativa was reduced by 20 % in comparison to control plants. Because l-DOPA reduced soybean root growth in addition to fresh and dry root weight in the present study (Table 1), its categorization as a strong allelochemical has been reinforced.
Some reports suggest that l-DOPA can be metabolized by plants. In fact, Nakajima et al. (1999) observed a significant accumulation of l-DOPA and free amino acids in the roots of Cucumis sativus seedlings compared with untreated plants. Hachinohe et al. (2004) reported remarkable increases in phenylalanine and tyrosine that were supposedly due to the metabolism of l-DOPA. In that study, amino acid content was higher in Echinochloa crus galli than in Lactuca sativa, and this metabolic process is one mechanism by which l-DOPA is detoxified by the plant. In addition, high level of phenylalanine and tyrosine can be also caused by the diversion of glycolysis and photosynthesis intermediates of the shikimate pathway, suggesting a link between allelopathic stress and lignification (Devi and Prasad 1996; dos Santos et al. 2008). The shikimate pathway, which leads to the synthesis of aromatic amino acids, such as phenylalanine and tyrosine, and the phenylpropanoid pathway, which leads to the synthesis of lignin, are clearly linked (Boerjan et al. 2003; Wildermurth 2006). This fact indicates that any effect (biotic or abiotic stress, for example) on enzymes can change the flux of metabolites into these pathways, and consequently, plant metabolism and development.
There is evidence that the effects of l-DOPA on soybean reported here could be related to the premature lignification of roots, because PAL, CAD and POD activities increased with increased lignin production (Figs. 3, 4). For example, the accumulation of the substrate phenylalanine (Fig. 2) can increase PAL activity (Fig. 3a), which is considered a stress response in plant species. In the phenylpropanoid pathway, PAL catalyzes the deamination of l-phenylalanine to produce t-cinnamate, which is converted into p-coumarate followed by caffeate, ferulate, 5-hydroxyferulate, sinapate and lignin (Boerjan et al. 2003). In agreement, Soares et al. (2007) reported large increases in phenolic compounds in the roots of soybean seedlings that had been stressed by l-DOPA. As verified in the current research, l-DOPA significantly increased PAL activity in soybean roots, which is an indicative of its toxicity to the plant. These results are also consistent with previous research, because other allelochemicals have been shown to increase PAL activity in soybean roots (Politycka 1998; dos Santos et al. 2004). We also measured the activity of tyrosine ammonia lyase (TAL), which catalyzes the deamination of tyrosine to produce phenylpropanoid p-coumaric acid. However, TAL activity was unchanged compared to controls (data not shown).
To test the hypothesis that l-DOPA induces the lignification process, CAD activity was measured in treated soybean roots and shown to be increased (Fig. 3b). Phenylpropanoid intermediates can be catalyzed by CAD, which is a key enzyme involved in the conversion of p-hydroxycinnamyl aldehydes to the corresponding alcohols during monolignol biosynthesis in the cell wall (Zhang et al. 2010). As observed here, increased lignin content (Fig. 4) in conjunction with increases in CAD activity (Fig. 3b) strengthens the hypothesis that l-DOPA enhances lignification of the cell wall of soybean root because this enzyme is considered a marker of this metabolic process (Boerjan et al. 2003). These findings suggest that the impact of CAD on lignin production in l-DOPA-stressed soybean roots may be critical.
The increased activity of cell wall-bound POD (Fig. 3c) that is associated with l-DOPA-induced reduction in root growth and weight (Table 1) is consistent with the function of this enzyme in plants. Cell wall-bound POD is often considered to be the enzyme that is most directly involved in the lignification of xylem cells. In the last step of the phenylpropanoid pathway, POD catalyzes the oxidative polymerization of monolignols from ferulate, p-coumarate and caffeate. This process requires oxidative coupling, which depends on the presence of H2O2. The H2O2 molecule triggers secondary defenses in the plant that induce cell wall lignification, and consequently the loss of cellular viability and growth (Passardi et al. 2005). It is feasible that effects on this pathway could explain the observed increase in cell wall-bound POD in treated soybean roots (Fig. 3c), which coincides with increased lignin production (Fig. 4).
In conclusion, the present findings suggest that one mode of action of the allelochemical l-DOPA may be related to its effects on the phenylpropanoid pathway, which triggers excessive production of lignin and a reduction in soybean root growth.
Author contribution
A. R. Soares carried out all experiments. R. C. Siqueira-Soares helped chemical measurements and realization of experiments. V. H. Salvador helped in lignin quantification. M. L. L. Ferrarese planned all experiments and helped in data interpretation. O. Ferrarese-Filho was the mastermind and wrote the manuscript.
Abbreviations
- CAD:
-
Cinnamyl-alcohol dehydrogenase
- l-DOPA:
-
l-3,4-dihydroxyphenylalanine
- EDTA:
-
Ethylene diamide tetraacetic acid
- H2O2 :
-
Hydrogen peroxide
- LTGA:
-
Lignin-thioglycolic acid
- PAL:
-
Phenylalanine ammonia lyase
- POD:
-
Peroxidase
- PVPP:
-
Polyvinylpolypirrolidone
- ROS:
-
Reactive oxygen species
- O ·−2 :
-
Superoxide anion
- SOD:
-
Superoxide dismutase
References
Anaya AL (1999) Allelopathy as a tool in the management of biotic resources in agroecosystems. Crit Rev Plant Sci 18:697–739
Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annu Rev Plant Biol 54:519–546
Bubna GA, Lima RB, Zanardo DIL, dos Santos WD, Ferrarese MLL, Ferrarese-Filho O (2011) Exogenous caffeic acid inhibits the growth and enhances the lignification of the roots of soybean (Glycine max). J Plant Physiol 168:1627–1633
Devi SR, Prasad MNV (1996) Ferulic acid mediated changes in oxidative enzymes of maize seedlings: implications in growth. Biol Plant 38:387–395
dos Santos WD, Ferrarese MLL, Finger A, Teixeira ACN, Ferrarese-Filho O (2004) Lignification and related enzymes in Glycine max root growth-inhibition by ferulic acid. J Chem Ecol 30:1199–1208
dos Santos WD, Ferrarese MLL, Ferrarese-Filho O (2006) High performance liquid chromatography method for the determination of cinnamyl alcohol dehydrogenase in soybean. Plant Physiol Biochem 44:511–515
dos Santos WD, Ferrarese MLL, Nakamura CV, Mourão KSM, Mangolin CA, Ferrarese-Filho O (2008) Soybean (Glycine max) root lignification induced by ferulic acid. The possible mode of action. J Chem Ecol 34:1230–1241
Ferrarese MLL, Rodrigues JD, Ferrarese-Filho O (2000) Phenylalanine ammonia lyase activity in soybean roots extract measured by reversed-phase high performance liquid chromatography. Plant Biol 2:152–153
Ferrarese MLL, Zottis A, Ferrarese-Filho O (2002) Protein-free lignin quantification in soybean (Glycine max) roots. Biologia 57:541–543
Fujii Y (1999) Allelopathy of hairy vetch and mucuna: their application for sustainable agriculture. In: Chou CH (ed) Biochemistry and allelopathy: from organisms to ecosystems in the Pacific. Acad Sinica, Taipei, pp 289–300
Gniazdowska A, Bogatek A (2005) Allelopathic interactions between plants. Multi site action of allelochemicals. Acta Physiol Plant 27:395–407
Golisz A, Sugano M, Hiradate S, Fujii Y (2011) Microarray analysis of Arabidopsis plants in response to allelochemical l-DOPA. Planta 233:231–240
Hachinohe M, Matsumoto H (2007) Mechanism of selective phytotoxicity of l-3,4-dihydroxyphenylalanine (l-DOPA) in barnyard glass and lettuce. J Chem Ecol 33:1919–1926
Hachinohe M, Sunohara Y, Matsumoto H (2004) Absorption, translocation and metabolism of l-DOPA in barnyard grass and lettuce: their involvement in species-selective phytotoxic action. Plant Growth Regul 43:237–243
Kovácik J, Klejdus B, Backor M, Repcak M (2007) Phenylalanine ammonia lyase activity and phenolic compounds accumulation in nitrogen-deficient Matricaria chamomilla leaf rosettes. Plant Sci 172:393–399
Moore S, Spackman DH, Stein WH (1958) Chromatography of amino acid on sulfonated polystyrene resins. Anal Chem 30:1185–1190
Nakajima N, Hiradate S, Fujii Y (1999) Characteristics of growth inhibitory effect of l-3,4-dihydroxyphenylalanine (l-DOPA) on cucumber seedlings. J Weed Sci Tech 44:132–138
Nishihara E, Parvez MM, Araya H, Fujii Y (2004) Germination growth response of different plant species to the allelochemical l-3,4-dihydroxyphenylalanine (l-DOPA). Plant Growth Regul 42:181–189
Nishihara E, Parvez MM, Araya H, Kawashima S, Fujii Y (2005) l-3-(3,4-dihydroxyphenyl)alanine (l-DOPA), an allelochemical exuded from velvetbean (Mucuna pruriens) roots. Plant Growth Regul 45:113–120
Passardi F, Cosio C, Penel C, Dunand C (2005) Peroxidases have more functions than a Swiss army knife. Plant Cell Rep 24:255–265
Politycka B (1998) Phenolics and the activities of phenylalanine ammonia lyase, phenol-beta-glucosyltransferase and beta-glucosidase in cucumber roots as affected by phenolic allelochemicals. Acta Physiol Plant 20:405–410
Politycka B, Mielcarz B (2007) Involvement of ethylene in growth inhibition of cucumber roots by ferulic and p-coumaric acids. Allelopath J 19:451–460
Reigosa MJ, Pazos-Malvido E (2007) Phytotoxic effects of 21 plant secondary metabolites on Arabidopsis thaliana germination and root growth. J Chem Ecol 33:1456–1466
Soares AR, Ferrarese MLL, Siqueira RC, Böhm FMLZ, Ferrarese-Filho O (2007) l-DOPA increases lignification associated with Glycine max root growth-inhibition. J Chem Ecol 33:265–275
Solecka D (1997) Role of phenylpropanoids compounds in plant responses to different stress factors. Acta Physiol Plant 19:257–268
Topal S, Kocaçalişkan I (2006) Allelopathic effects of DOPA against four weed species. DPÜ Fen Bil Enst Dergisi 11:27–32
Vranova V, Rejsek K, Skene KR, Formanek P (2011) Non-protein amino acids: plant, soil and ecosystem interactions. Plant Soil 342:31–48
Weir TL, Park SW, Vivanco JM (2004) Biochemical and physiological mechanisms mediated by allelochemicals. Curr Opin Plant Biol 7:472–479
Wildermurth MC (2006) Variations on a theme: synthesis and modification of plant benzoic acids. Curr Opin Plant Biol 9:288–296
Zanardo DIL, Lima RB, Ferrarese MLL, Bubna GA, Ferrarese-Filho O (2009) Soybean root growth inhibition and lignification induced by p-coumaric acid. Environ Exp Bot 66:25–30
Zhang L, Wang G, Chang J, Liu J, Cai J, Rao X, Zhang L, Zhong J, Xie J, Zhu S (2010) Effects of 1-MCP and ethylene on expression of three CAD genes and lignification in stems of harvested Tsai Tai (Brassica chinensis). Food Chem 123:32–40
Acknowledgments
Research was financially supported by the National Council for Scientific and Technological Development (CNPq), Brazil. O. Ferrarese-Filho and M. L. L. Ferrarese are research fellows of CNPq. A. R. Soares is the recipient of a CNPq fellowship. The authors kindly thank A. M. D. Ramos for their technical assistance.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Weidner.
Rights and permissions
About this article
Cite this article
Soares, A.R., de Cássia Siqueira-Soares, R., Salvador, V.H. et al. The effects of l-DOPA on root growth, lignification and enzyme activity in soybean seedlings. Acta Physiol Plant 34, 1811–1817 (2012). https://doi.org/10.1007/s11738-012-0979-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-012-0979-x