Abstract
Velvetbean (Mucuna pruriens) plants impede the growth of neighboring plants. One compound, 3-(3′,4′-dihydroxyphenyl)-l-alanine (l-DOPA), is responsible for the allelopathic capacity of velvetbean. This compound is an active allelochemical that decreases root growth of several plant species. In mammals, l-DOPA is a well-known therapeutic agent for the symptomatic relief of Parkinson’s disease. However, its mode of action in plants is still not well understood. To address such issues, gene expression in Arabidopsis thaliana plants, which had been exposed to l-DOPA, was analyzed using DNA microarrays. After 6 h of l-DOPA exposure, the expression of 110 genes was significantly upregulated, and the expression of 69 genes was significantly downregulated. These induced genes can be divided into different functional categories, mainly on the basis of subcellular localization, metabolism, and proteins with a binding function or cofactor requirement. Based on these results, we suggest that l-DOPA acts by two mechanisms: it influences amino acid metabolism and deregulates metal homeostasis, especially that of iron, which is required for the fundamental biological processes of all organisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allelopathy is a phenomenon of chemical interaction among plants. Plants with allelopathic capabilities play important roles in agricultural production. Natural toxins, including allelochemicals that suppress or eliminate competing plant species near the source plant, have been extensively studied because of their agricultural potential as herbicides (Dayan et al. 2000). A component of velvetbean (Mucuna pruriens L. DC.), 3-(3′,4′-dihydroxyphenyl)-l-alanine (l-DOPA), is an allelochemical (Fujii et al. 1991). Because velvetbean has special abilities such as weed smothering, tolerance to pests, suppression of the nematode population, and soil improvement in its physical structure, the usage of plants could drastically reduce the application of artificial chemicals to agricultural crops (Fujii 2003). Fresh velvetbean leaves contain from 0.5 to 1.5% l-DOPA (by weight), and the yield of fresh leaves and stems from velvetbean plants ranges from 20 to 30 t ha−1 (Fujii et al. 1991, Fujii 1999). Therefore, velvetbean could produce 100–450 kg ha−1 of l-DOPA. l-DOPA is quite unstable in soils; especially those with a high pH value have been shown to possess the ability to eliminate l-DOPA through transformation and adsorption reactions, resulting in the detoxification of l-DOPA (Hiradate et al. 2005; Furubayashi et al. 2005, 2007; Dayan and Duke 2009). The phytotoxic activity of l-DOPA in the absence of soil has been observed at concentrations as low as 1 × 10−4–1 × 10−5 M (Fujii 2003; Nishihara et al. 2004; Furubayashi et al. 2005; Hiradate et al. 2005). Concentrations in this range are high enough to reduce the growth of neighboring plants and to reduce the rate of root growth in several plant species (Fujii et al. 1991; Hachinohe et al. 2004; Nishihara et al. 2004). Velvetbean seeds also contain high concentrations of l-DOPA (6–9%) (Damodaran and Ramaswamy 1937; Rehr et al. 1973), which acts as a chemical barrier against insect attacks (Bell and Janzen 1971; Premchand 1981).
In the mammalian brain, l-DOPA is the precursor of the neurotransmitter dopamine and is the most effective therapeutic agent for the symptomatic relief of Parkinson’s disease (Mercuri and Bernardi 2005). In animal skin, hair, feathers, and fur as well as in insect cuticle, l-DOPA is oxidized to dopaquinone and finally converted to melanin (Fujii 2003).
In plants, it is a precursor of melanin and many alkaloids, catecholamines, flavonoids, and phenylpropenoids (Hahlbrock and Scheel 1989; Pattison et al. 2002). According to Hachinohe and Matsumoto (2005, 2007), the phytotoxicity of l-DOPA in barnyard grass and lettuce is attributed to the oxidative damage caused by reactive oxygen species and/or free radical species that are generated from the melanin synthesis pathway. Soares et al. (2007) suggested that l-DOPA-induced inhibition in soybean roots occurs because of a cell wall stiffening process related to cross-linking between cell wall polymers that occurs during lignin production.
Little is known about the function of allelochemicals and the defensive responses of plants to them. Arabidopsis thaliana L. has been a good model for studying plant responses to allelochemicals and other environmental toxins (Baerson et al. 2005). Moreover, microarray techniques have become a standard tool for genome-wide monitoring of gene expression. Hence, the present work uses microarrays to analyze the gene expression profiles of A. thaliana L. plants exposed to l-DOPA. To the best of our knowledge, this is the first study to evaluate gene responses to this compound using microarrays. This study may lead to a better understanding of the mode of action of l-DOPA in plants.
Materials and methods
Plant material, growth conditions, and experimental treatment
Seeds of A. thaliana L. (Col-4, source NASC N933), bought from Lehle Seeds Company (Round Rock, TX, USA) were sterilized for 1 min in 70% ethanol, followed by the application of 1% sodium hypochlorite with one drop of Tween 20 (Sigma–Aldrich Group, St. Louis, MO, USA) for 6 min, after which they were rinsed ten times with distilled water. Sterilized seeds were placed on 1% solidified agar (Nacalai Tesque, Inc., Kyoto, Japan) with 0.5 × Murashige and Skoog Plant Salt Mixture (Nihon Pharmaceutical Co., Ltd., Tokyo, Japan) and 1% sucrose (Wako Pure Chemical Industries, Osaka, Japan) in an Agripot (Kirin, Tokyo, Japan). The seeds in the Agripots were cold treated (4°C) for 3 days in darkness and then transferred to a growth chamber. Plants were maintained in the growth chamber on a schedule of 16 h of light (22°C) and 8 h of dark (20°C) (Boyes et al. 2001; Baerson et al. 2005). After 20 days in the growth chamber, the plants were removed from the agar and transferred to a solution culture in distilled water containing 100 mg kg−1 (of water) of l-DOPA (Nacalai Tesque). This concentration reflects specific activities as determined in previous studies (Fujii et al. 1991). The effective concentration of the compound to induce half-maximum inhibition for lettuce in water was 50 mg kg−1. However, we used a higher concentration because we had a shorter time of exposure. As a control, plants were transferred in parallel to distilled water. After 6 h of treatment, plants were collected and immediately frozen in liquid nitrogen, and then stored at −80°C prior to analysis. Each treatment was replicated three times in each of the three independent experiments.
Microarray data analysis
Total RNA from both the treated and the control 20-day-old Arabidopsis plants was isolated using the RNeasy Plant Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The quantity and quality of total isolated RNA were assessed by spectrophotometry and gel electrophoresis, respectively. RNA was amplified and labeled as described in the GeneChip Expression Analysis Technical Manual, Rev. 5 (Affymetrix, Santa Clara, CA, USA). Total RNA (1 μg) was converted into double-stranded cDNA using the One-Cycle cDNA Synthesis Kit (Affymetrix). In vitro transcription reactions were performed using a GeneChip IVT labeling kit (Affymetrix), which includes T7 RNA polymerase and biotin-labeled ribonucleotides. The cDNA (15 μg) was hybridized to an Affymetrix GeneChip Arabidopsis ATH1 Genome Array. The array was incubated for 16 h and then automatically washed and stained using the GeneChip Hybridization, Wash and Stain Kit (Affymetrix). The genome array was scanned using a GeneChip Scanner 3000 7G. Expression analysis was performed using GeneSpring Viewer 5.2 software (Agilent Technologies, Santa Clara, CA, USA). We defined genes as responsive when transcripts were detected in at least two of the three independent experiments (biological replicates) and when the signals were significantly different (P ≤ 0.05) as compared to the control plants.
Quantitative real-time RT-PCR analysis
Total RNA from both the treated and the control 20-day-old Arabidopsis plants was isolated using Trizol (Invitrogen, Carlsbad, CA, USA). The synthesis of cDNA was carried out using the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen). The kits were used according to the manufacturers’ instructions. A primer pair was used to amplify the constitutively expressed control gene encoding elongation factor 1α (EF1α; At5g60390; Becher et al. 2004). The primers for quantitative real-time RT-PCR were designed using Primer Express software (v 2.0, Applied Biosystems, Foster City, CA, USA). The sequences of all primers are presented in Table 1.
Quantitative real-time RT-PCR was performed on the ABI Prism 7700 Sequence Detection System (Applied Biosystems) using SYBR Green. cDNA was diluted 1:50 with nuclease-free water. Reactions were performed in 20 μl of liquid, containing 9 μl of qPCR Master Mix (Eurogentec, Liege, Belgium), 0.6 μl of SYBR Green (Eurogentec), and 0.5 μmol of forward and reverse primers (Operon Biotechnologies, Tokyo, Japan). The following standard thermal profile was used: 2 min at 50°C, 10 min at 95°C, followed by 40 cycles of 15 s at 95°C, and 60 s at 60°C. Each sample was analyzed in three technical replicates, and the resulting data were analyzed using ABI Prism 7000 SDS Software. For the calculation of the threshold cycle (C T) values, the auto-C T function was used. For further calculations, the mean value of each triplicate was used. To normalize the target gene expression, the difference between the C T of the target gene and the C T of EF1α (constitutive control) for the respective template was calculated (∆ C T value). To calculate fold changes in gene expression, the ∆C T value was calculated as follows: ∆C T = C T (target gene) − C T (constitutive control gene). Relative transcript levels were calculated as 1,000 × \( 2^{{ - \Updelta C_{\text{T}} }} \).
Results
The results demonstrated that l-DOPA influenced gene expression in A. thaliana plants. Microarray analysis showed that the expression of 110 genes was significantly upregulated, and that the expression of 69 genes was significantly downregulated (P ≤ 0.05). Among the upregulated genes, 32 were induced ≥ threefold as compared with their expression in the control plants (Table 2). The highest levels of expression were for an expressed protein of unknown function (At1g47400) and for a protein bHLH (At5g04150) with fold changes of 27.67- and 16.90-fold changes, respectively. Besides these proteins (transcription factor), l-DOPA affected the expression of another bHLH protein (At3g47640) and the expression of the WRKY family transcription factor (At4g23810) with 6.06- and 7.84-fold changes, respectively (Table 2). Furthermore, more than ten upregulated genes were responsible for metal homeostasis, especially iron homeostasis. Apart from the genes listed above, other notable genes included OPT (At4g16370), FRO (At1g23020), and NRAMP4 metal ion transporter (At5g67330) with 7.60-, 4.06-, and 3.20-fold changes, respectively (Table 2). In addition, two other notable genes were two alcohol dehydrogenase genes (At1g77120, At1g64710) with fold changes of 6.67 and 2.26, respectively. These two genes are involved in the response to osmotic stress (TAIR database http://www.arabidopsis.org/index.jsp). Furthermore, other upregulated genes included those involved in the defensive response to biotic stress such as ankyrin repeat protein (At4g14400), disease resistance protein (At4g16890), and pathogenesis-related protein 1 (At2g14610; Table 2). It is possible that the phytotoxicity of l-DOPA influences the expression of genes involved in responses to both abiotic and biotic stresses.
The upregulated genes were distributed into 17 functional categories (Fig. 1a) based on FunCat assignments available through the MIPS A. thaliana database (http://mips.gsf.de/proj/funcatDB/search_main_frame.html). The largest categories were “subcellular localization”, “protein with binding function or cofactor requirement”, and “metabolism”, representing 30.8, 25.2, and 25.2% of all functions assigned, respectively. Also noteworthy functional categories were “cellular transport, transport facilities, and transport routes” (17.7%), “cell rescue, defence, and virulence” (14.0%), and “transcription” (3.7%; Fig. 1a).
The 69 downregulated genes were distributed into 16 functional categories based on FunCat database (Fig. 1b). The main categories were “subcellular localization” and “metabolism”, representing 46.3 and 30.4% of all functions assigned, respectively. Among these downregulated genes were those induced by photo-oxidative stress (TAIR database http://www.arabidopsis.org/index.jsp), such as FER1 (At5g01600) and expressed protein (At3g20340), with 0.28- and 0.40-fold changes, respectively (Table 3). Moreover, ferredoxin–nitrate reductase (At2g15620), which is involved in the second step of nitrate assimilation (TAIR), was also downregulated with a 0.47-fold change. The protein product of this gene functions in chloroplasts as an electron carrier in the photosynthetic electron transport chain. Notably in the presence of l-DOPA, more than ten genes that function in the transport of ions such as zinc (At4g36050), copper (At3g43670 and At4g14940), calcium (At1g76650), and ferric iron (At2g40300 and At3g56090; Table 3) were downregulated, suggesting that l-DOPA influences ion transport and homeostasis in plants. Notably six peroxidase genes that are involved in the response to oxidative stress were also downregulated following l-DOPA exposure. These include At1g05250, At4g30170, At4g08390, At4g26010, At5g15180, and At2g37130 with 0.18-, 0.27-, 0.31-, 0.37-, 0.44-, and 0.50-fold changes, respectively (Table 3). Moreover, two important factors for plant growth were also downregulated: one was phytosulfokines 5 (At5g65870), which encodes a unique plant peptide growth factor, with a 0.47-fold change and the second was rapid alkalinization factor (At5g67070) with a 0.50-fold change; the protein encoded by this gene has an essential role in the physiology of Arabidopsis, where it regulates growth and development (Table 3).
To verify the microarray results, quantitative real-time RT-PCR assays were carried out for three upregulated and three downregulated genes (Fig. 2). The fold changes for a given gene were very similar for both the methods. In the case of the upregulated genes, both microarray and real-time RT-PCR results showed that the relative transcript level of expressed protein (At1g47400) was the highest among other tested genes as compared with the control sample, while the transcript with the greatest reduction in expression was peroxidase (At1g05250) for both methods (Fig. 2).
Quantitative real-time RT-PCR analysis of expression of six selected genes encoding expressed protein (At1g47400), WRKY family transcription factor (At4g23810), bHLH family protein (At5g04150), peroxidase (At1g05250), expressed protein (At3g20340), and FER1 (At5g01600) in Arabidopsis thaliana. The transcript levels in plants were assessed by the quantitative real-time RT-PCR following the 6-h exposure of l-DOPA the 20-day-old plants. The ∆C T values and the relative transcript levels were means of three technical replicates. The ∆C T values were calculated as follows: ∆C T (target gene) = C T (target gene) − C T (constitutive control gene: EF1α), where C T is the cycle number at which the PCR product exceeded a set threshold. Relative transcript levels (RTL) were calculated as follows: RTL = 1,000 × \( 2^{{ - \Updelta C_{\text{T}} }} \)
Discussion
In the past few decades lots of allelochemicals have been identified but the mode of actions of many of them are not yet clear. In the present study, we focused on the analysis of transcriptome response in Arabidopsis plants that were exposed to the allelochemical l-DOPA. In this experiment, phytotoxic compounds were applied directly to water to avoid the barrier that produces soil or agar. Thus, plants directly absorbed the allelochemicals through the roots. The plants were exposed to l-DOPA for only a short time (6 h) to study the primary response. Based on previous studies (Nakajima et al. 1999; Hachinohe and Matsumoto 2005, 2007; Soares et al. 2007), we expected that the response of plants will affect genes involved in oxidative stress, the pathway of melanin synthesis, lipid peroxides, phenolic compounds, and lignification, and that they will also influence on amino acid metabolism.
It has been suggested that all catechol-like compounds, including l-DOPA, may have a similar mode of action (Fujii 1999). Microarray analyses revealed that the gene expression profiles of Arabidopsis plants that were exposed to the catechol compounds, such as rutin, gallic acid, and fagomine differ from those described in the present report (Golisz et al. 2008).
According to current literature, the mode of l-DOPA action in plants differs depending on its source and the treatment conditions. Dose/response relationships and time of exposure are crucial to allelopathy studies (Belz et al. 2007). The phytotoxic effects of l-DOPA that occur in the roots of lettuce and barnyard grass after 5 days of exposure at a concentration of 10−4 M, are due to oxidative damage caused by reactive oxygen species and/or free radical species generated by the melanin synthesis pathway (Hachinohe and Matsumoto 2005, 2007). In the roots of cucumber after 8 days of treatment with 250–500 μM l-DOPA, the amount of tyrosine and phenylalanine increased remarkably (Nakajima et al. 1999). Similar results were found in soybean roots, where PAL (EC 4.3.1.5) and POD (EC 1.11.1.7) activities, phenolic compounds, and lignin content increased, whereas length and fresh and dry weight of the roots decreased after 24 h of treatment with 0.1–1.0 mM l-DOPA (Soares et al. 2007). These results are consistent with our finding that l-DOPA influenced the amino acid metabolism of phenylalanine and tyrosine. Compared with previous studies (Nakajima et al. 1999; Hachinohe and Matsumoto 2007; Soares et al. 2007), our treatment was relatively short (only 6 h); nevertheless, despite this short period of exposure to l-DOPA, an increased expression of genes that play an important role in amino acid metabolism, such as tyrosine aminotransferase (At5g36160 and At5g53970; 1.27- and 1.17-fold change; EC 2.6.1.5) and all five types of aspartate aminotransferase, particularly ASP2 (At5g19550; 1.52-fold change; EC 2.6.1.1) was observed. Moreover, the tryptophan synthase genes At3g54640 and At4g27070 (EC 4.2.1.20) were notably affected with 1.62- and 1.46-fold changes, respectively. However, the expression level of the gene that encodes PAL was almost unchanged as compared with that of control plants (data not shown).
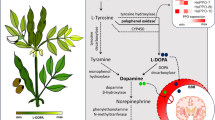
Our results substantiated the findings of previous studies (Nakajima et al. 1999; Hachinohe and Matsumoto 2007; Soares et al. 2007) that l-DOPA affects amino acid metabolism; however, this current research shows that the response of Arabidopsis plants to l-DOPA is similar to the response that follows iron deficiency. After l-DOPA treatment, the regulation of metal homeostasis, especially iron homeostasis, was disrupted in Arabidopsis plants. Iron is an essential nutrient for all organisms. Ferric reduction oxidase (FRO) is the enzyme responsible for the plasma membrane Fe(III) chelate reductase activity that is induced by iron deficiency in Arabidopsis (Thimm et al. 2001; Grotz and Guerinot 2006). In our results, FRO3 (At1g23020) in treated plants was upregulated 4.06-fold compared with that in control plants (Table 2), which is in accordance with published reports of iron deficiency effects on the expression of this gene (Wintz et al. 2003; Puig et al. 2007; Buckhout et al. 2009). AtFRO3 may possibly function both in iron metabolism in roots and in iron reduction on the root surface (Wu et al. 2005). FRO is able to reduce copper (Mukherjee et al. 2006). Therefore, under conditions of low copper, the expression of FRO3 would be induced, as occurred in our experiment. Iron deficiency also induces rapid changes in zinc homeostasis (Buckhout et al. 2009) including increased ZIF1, which is responsible for transport of Zn ligands into vacuoles. These results suggest that l-DOPA influences not only on iron homeostasis but also copper and zinc homeostasis. The upregulation of FRO3 under l-DOPA stress correlated with many essential genes that are regulated by iron deficiency: transcription factors (At5g04150-bHLH101, At3g47640-bHLH047, At3g18290-EMB2454); transporters (At4g16370-OPT3, At5g67330-NRAMP4, At5g13740-ZIF1), and expressed protein (At1g47400, At3g56360, At1g48300) (Fig. 3). These results from our study correspond well with a previous analysis by Buckhout et al. (2009) of gene transcripts that were upregulated by early iron deficiency. In our results, the highest upregulated genes were expressed protein (At1g47400) and transcription factor bHLH101 (At5g04150) belonging to a subgroup of bHLH Ib genes (Table 2). Both of them were strongly up regulated by iron deficiency in the roots and leaves of Arabidopsis (Wang et al. 2007; Buckhout et al. 2009).
Co-expression of selected upregulated genes by network of ATTED-II (http://atted.jp/) in response to l-DOPA treatment in Arabidopsis thaliana plant
In response to iron deficiencies, plants accumulate metal transporters of the NRAMP family, such as AtNRAMP1, AtNRAMP3, and AtNRAMP4 (Grotz and Guerinot 2006). Metal transporters of the NRAMP family have the ability to transport both iron and manganese (Lanquar et al. 2005, 2010). In our results, AtNRAMP4 (At5g67300) was upregulated 3.20-fold compared with the control plants (Table 2). This gene accumulates in both the roots and shoots of Arabidopsis and plays a role in the intracellular transport of iron, specifically in mobilizing iron from the vacuole (Grotz and Guerinot 2006). Furthermore, OPT family members are also regulated by metals, and AtOPT3 is highly induced during iron deficiency (Wintz et al. 2003; Wu et al. 2005; Stacey et al. 2008; Buckhout et al. 2009). In our data, AtOPT3 (At4g16370) was 7.84-fold higher in Arabidopsis plants treated with l-DOPA compared with that in the control plants (Table 2).
Another iron-storage protein is ferritin, which is repressed during iron starvation (Puig et al. 2007). The FER repressor is ubiquitinated and degraded after iron treatment in a process in which nitric oxide plays a key role (Puig et al. 2007). Excess iron is trapped with oxygen in the ferritin mineral to minimize radical chemistry and reactive oxygen species. The FER family is central to the natural regulation of iron in cells (Hintze and Theil 2006; Ravet et al. 2009). The absence of ferritin leads to a strong deregulation of the expression of several metal transporter genes in the stem, the over-accumulation of iron in reproductive organs, and a decrease in fertility (Ravet et al. 2009). Arabidopsis possesses four ferritin family members that are differentially expressed in response to various environmental signals and during the course of plant growth and development (Petit et al. 2001a, b). AtFer1 and AtFer3 accumulate in response to high iron and oxidative stress (Petit et al. 2001a; Ravet et al. 2009). In our results, AtFer1 (At5g01600), AtFer3 (At3g56090), and AtFer4 (At2g40300) were downregulated (Table 3), which is in agreement with published reports of iron deficiency (Thimm et al. 2001; Wintz et al. 2003). In the absence of ferritin, plants have higher levels of reactive oxygen species and increased activity of enzymes involved in their detoxification (Ravet et al. 2009).
The results of this study show that the allelopathic effect of l-DOPA may be acting via its effects on amino acid metabolism and/or on the regulation of metal homeostasis, not only that of iron but also that of zinc and copper. l-DOPA seems to be a promising allelochemical for the control of weeds because of its influence on many fundamental biological processes (i.e., photosynthesis) in plants.
Abbreviations
- ASP2:
-
Aspartate aminotransferase
- bHLH:
-
Basic helix-loop-helix
- EMB2454:
-
Embryo defective, zinc ion binding
- FER:
-
Ferritin
- FRO:
-
Ferric reduction oxidase
- l-DOPA:
-
3-(3′,4′-Dihydroxyphenyl)-l-alanine
- NADP+ :
-
Nicotinamide adenine dinucleotide phosphate
- NRAMP:
-
Natural resistance-associated macrophage protein
- OPT:
-
Oligopeptide transporter
- PAL:
-
Phenylalanine ammonia-lyase
- POD:
-
Peroxidase
- ZIF:
-
Zinc induced facilitator
References
Baerson SR, Sanchez-Moreiras A, Pedrol-Bonjoch N, Schulz M, Kagan IA, Agarwal AK, Reigosa MJ, Duke SO (2005) Detoxification and transcriptome response in Arabidopsis seedlings exposed to the allelochemical benzoxazolin-2(3H)-one. J Biol Chem 280:21867–21881
Becher M, Talke IN, Krall L, Kramer U (2004) Cross-species microarray transcript profiling reveals high constitutive expression of metal homeostasis genes in shoots of the zinc hyperaccumulator Arabidopsis halleri. Plant J 37:251–268
Bell EA, Janzen DH (1971) Medical and ecological considerations of l-DOPA and 5-HTP in seeds. Nature 229:136–137
Belz RG, Velini ED, Duke SO (2007) Dose/response relationships in allelopathy research. In: Fujii Y, Hiradate S (eds) Allelopathy new concepts and methodology. Science Publishers, USA, pp 3–29
Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis KR, Gorlach J (2001) Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell 13:1499–1510
Buckhout TJ, Yang TJW, Schmidt W (2009) Early iron-deficiency-induced transcriptional changes in Arabidopsis roots as revealed by microarray analyses. BMC Genomics 10:147–163
Damodaran M, Ramaswamy R (1937) Isolation of l-3, 4-dihydroxyphenylalanine from the seeds of Mucuna pruriens. Biochem J 31:2149–2152
Dayan FE, Duke SO (2009) Biological activity of allelochemicals. In: Osbourn AE, Lanzotti V (eds) Plant derived natural products. Synthesis, functions, and application. Springer, NY, pp 361–384
Dayan FE, Romagni JG, Duke SO (2000) Investigating the mode of action of natural phytotoxins. J Chem Ecol 26:2079–2094
Fujii Y (1999) Allelopathy of velvetbean: determination and identification of l-DOPA as a candidate of allelopathic substances. In: Cutler HG, Cutler SJ (eds) Biologically active natural products: agrochemicals. CRC Press, Boca Raton, pp 33–47
Fujii Y (2003) Allelopathy in the natural and agricultural ecosystems and isolation of potent allelochemicals from Velvet bean (Mucuna pruriens) and Hairy vetch (Vicia villosa). Biol Sci Space 17:6–13
Fujii Y, Shibuya T, Yasuda T (1991) l-3, 4-dihydroxyphenylalanine as an allelochemical candidate from Mucuna pruriens (L.) DC. Var utilis. Agric Biol Chem 55:617–618
Furubayashi A, Hiradate S, Fujii Y (2005) Adsorption and transformation reactions of l-DOPA in soils. Soil Sci Plant Nutr 51:819–825
Furubayashi A, Hiradate S, Fujii Y (2007) Role of catechol structure in the adsorption and transformation reactions of l-DOPA in soils. J Chem Ecol 33:239–258
Golisz A, Sugano M, Fujii Y (2008) Microarray expression profiling of Arabidopsis thaliana L. in response to allelochemicals identified in buckwheat. J Exp Bot 59:3099–3109
Grotz N, Guerinot ML (2006) Molecular aspects of Cu, Fe and Zn homeostasis in plants. Biochim Biophys Acta 1763:595–608
Hachinohe M, Matsumoto H (2005) Involvement of reactive oxygen species generated from melanin synthesis pathway in phytotoxicity of l-DOPA. J Chem Ecol 31:237–246
Hachinohe M, Matsumoto H (2007) Mechanism of selective phytotoxicity of l-3, 4-dihydroxyphenylalanine (l-DOPA) in barnyardgrass and lettuce. J Chem Ecol 33:1919–1926
Hachinohe M, Sunohara Y, Matsumoto H (2004) Absorption, translocation and metabolism of l-DOPA in barnyardgrass and lettuce: their involvement in species-selective phytotoxic action. Plant Growth Regul 43:237–243
Hahlbrock K, Scheel D (1989) Physiology and molecular biology of phenylpropanoid metabolism. Annu Rev Plant Physiol Plant Mol Biol 40:347–369
Hintze KJ, Theil EC (2006) Cellular regulation and molecular interactions of the ferritins. Cell Mol Life Sci 63:591–600
Hiradate S, Furubayashi A, Fujii Y (2005) Changes in chemical structure and biological activity of l-DOPA as influenced by an Andosol and its components. Soil Sci Plant Nutr 50:665–675
Lanquar V, Lielievre F, Bolte S, Hames C, Alcon C, Neumann D, Vansuyt G, Curie C, Schroder A, Kramer U, Barbier-Brygoo H, Thomine S (2005) Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron. EMBO J 24:4041–4051
Lanquar V, Ramos MS, Lelievre F, Barbier-Brygoo H, Krieger-Liszkay A, Kramer U, Thomine S (2010) Export of vacuolar manganese by AtNRAMP3 and AtNRAMP4 is required for optimal photosynthesis and growth under manganese deficiency. Plant Physiol 152:1986–1999
Mercuri V, Bernardi G (2005) The magic of l-DOPA: why is it the gold standard Parkinson’s disease therapy? Trends Pharm Sci 26:341–344
Mukherjee I, Campbell NH, Ash JS, Connolly EL (2006) Expression profiling of the Arabidopsis ferric chelate reductase (FRO) gene family reveals differential regulation by iron and copper. Planta 223:1178–1190
Nakajima N, Hiradate S, Fujii Y (1999) Characteristics of growth inhibitory effect of l-3, 4-dihydroxyphenylalanine (l-DOPA) on cucumber seedlings (in Japanese with English summary). J Weed Sci Tech 44:132–138
Nishihara E, Parvez MM, Araya H, Fujii Y (2004) Germination growth response of different plant species to the allelochemical l-3, 4-dihyroxyphenylalanine (l-DOPA). Plant Growth Regul 42:181–189
Pattison DI, Dean RT, Davies MJ (2002) Oxidation of DNA, proteins and lipids by DOPA, protein-bound DOPA, and related catecho (amine)s. Toxicology 177:23–37
Petit JM, Briat JF, Lobreaux S (2001a) Structure and differential expression of the four members of the Arabidopsis thaliana ferritin gene family. Biochem J 359:575–582
Petit JM, van Wuytswinkel O, Briat JF, Lobreaux S (2001b) Characterization of an iron-dependent regulatory sequence involved in the transcriptional control of AtFer1 and ZmFer1 plant ferritin genes by iron. J Biol Chem 276:5584–5590
Premchand (1981) Presence of feeding deterrent in velvetbean (Mucuna cochinchinensis). Indian J Entomol 43:217–219
Puig S, Andres-Colas N, Garcia-molina A, Penarrubia L (2007) Copper and iron homeostasis in Arabidopsis: responses to metal deficiencies, interactions and biotechnological applications. Plant Cell Environ 30:271–290
Ravet K, Touraine B, Boucherez J, Briat JF, Gayard F, Cellier F (2009) Ferritins control interaction between iron homeostasis and oxidative stress in Arabidopsis. Plant J 57:400–412
Rehr SS, Janzen DH, Feeny PP (1973) L-DOPA in legume seeds: a chemical barrier to insect attack. Science 181:81–82
Soares AR, Ferrarese MLL, Siqueira RC, Bohm FMLZ, Ferrarese-Filho O (2007) L-DOPA increases lignification associated to Glycine max root growth-inhibition. J Chem Ecol 33:265–275
Stacey MG, Patel A, McClain WE, Mathieu M, Remley M, Rogers EE, Gassmann W, Blevins DG, Stacey G (2008) The Arabidopsis AtOPT3 protein functions in metal homeostasis and movement of iron to developing seeds. Plant Physiol 146:589–601
Thimm O, Essigmann B, Kloska S, Altmann T, Buckhout TJ (2001) Response of Arabidopsis to iron deficiency stress as revealed by microarray analysis. Plant Physiol 127:1030–1043
Wang HY, Klatte M, Jakoby M, Baumlein H, Weisshaar B, Bauer P (2007) Iron deficiency-mediated stress regulation of four subgroup Ib BHLH genes in Arabidopsis thaliana. Planta 226:897–908
Wintz H, Fox T, Wu YY, Feng V, Chen W, Chang HS, Zhu T, Vulpe C (2003) Expression profiles of Arabidopsis thaliana in mineral deficiencies reveal novel transporters involved in metal homeostasis. J Biol Chem 278:47644–47653
Wu H, Li L, Du J, Yuan Y, Cheng X, Ling HQ (2005) Molecular and biochemical characterization of the Fe(III) chelate reductase gene family in Arabidopsis thaliana. Plant Cell Physiol 46:1505–1514
Acknowledgments
This work was partially supported by the Japanese Society of Promotion of Science (JSPS fellowship award to Anna Golisz, Id. No. P 05643) and by the projects as follows: Research and Development Program for Resolving Critical Issues (project title, “Risk assessment of alien plants and their control in the field”), and by the Bio-oriented Technology Research Advancement Institution (BRAIN), Promotion of Basic Research Activities for Innovative Biosciences (project title, “Screening of allelochemicals and development of innovative bioactive substances”).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Golisz, A., Sugano, M., Hiradate, S. et al. Microarray analysis of Arabidopsis plants in response to allelochemical l-DOPA. Planta 233, 231–240 (2011). https://doi.org/10.1007/s00425-010-1294-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-010-1294-7