Abstract
Resveratrol is a polyphenol, present in grapes, peanuts, and other plant sources, with a wide range of valuable biological activities. We established a Vitis amurensis cell culture accumulating high levels of resveratrol by introducing the rolB gene of Agrobacterium rhizogenes in the V. amurensis genome, and studied the stability of resveratrol accumulation during 27 months of continuous subculturing. This study demonstrates a decline in the high level of resveratrol production by the rolB transgenic cell line during its long-term cultivation. Elicitation of the rolB transgenic calli with methyl jasmonate and salicylic acid, which are known to stimulate the production of plant secondary metabolites, resulted in a recovery of resveratrol accumulation in the rolB transgenic cell culture, while the empty vector-transformed culture with trace starting content of resveratrol exhibited low inducibility to the treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants are an important source of a wide range of secondary metabolites which possess valuable biologically active properties. These metabolites are actively used in medicine, agrochemistry, cosmetology, and food technologies. Plant cell cultures show a promise as an economically feasible route for large-scale production of valuable secondary metabolites of high purity (Roberts and Shuler 1997; Rao and Ravishankar 2002). However, there are very few successful examples of utilization of plant cell cultures for industrial production of bioactive compounds (Verpoorte et al. 2000). Utilization of plant cell culture as an alternative source of bioactive compounds is often limited by slow growth and low productivity (Verpoorte et al. 2000). Another major problem is the difficulties in the long-term maintenance of consistent productive culture (Kolewe et al. 2008).
Plant cell cultures often show a high level of genetic and metabolic variability, resulting in highly unstable or repressed production of secondary metabolites over long-term subculturing. An inconsistent pattern or gradual loss of metabolite production during long-term subcultures has been reported for anthocyanins (Hirasuna et al. 1991; Callebaut et al. 1997; Qu et al. 2005), alkaloids (Deus-Neumann and Zenk 1984; Whitmer et al. 2003), and taxanes (Kim et al. 2004). Inherent heterogeneity of cell populations, genetic and epigenetic instability, environmental stress, and lack of tissue differentiation is generally regarded as the cause of biosynthetic instability in plant cell cultures (Kolewe et al. 2008). Although several investigations of biosynthetic capacity of plant cell cultures during months of continuous cultivation have been reported (Deus-Neumann and Zenk 1984; Hirasuna et al. 1991; Qu et al. 2005; Whitmer et al. 2003; Kim et al. 2004), there is a lack of data on cell cultures during months or years of cultivation. Little experimental evidence and mechanistic understanding of metabolic instability in plant cell culture have prevented widespread development of improved strategies for plant cell culture commercialization.
Resveratrol (3,4′,5-trihydroxy-stilbene) is a polyphenol produced in grapes, peanuts, and other plant sources in response to stress or pathogenic attack. Resveratrol exhibits a wide range of beneficial effects, including cancer prevention properties, cardioprotective activities, and anti-aging effects (Marques et al. 2009; Pervaiz and Holme 2009). In recent years, this compound has attracted substantial research interest, since it has multiple potential applications in food industry and medicine (Kiselev 2011). Extensive research work has been done to increase the levels of resveratrol production in plants, plant cell cultures, yeast, and bacteria (Donnez et al. 2009; Wang et al. 2010; Kiselev 2011). For example, it has been shown previously that transformation of a Vitis amurensis cell culture with the rolB gene of Agrobacterium rhizogenes significantly increased resveratrol accumulation in the transformed cells (Kiselev et al. 2007). rolB transgenic callus cultures of V. amurensis produced primarily resveratrol and only trace amounts of other polyphenolic compounds.
The purpose of the present study was to examine the effect of long-term subculturing on the accumulation of trans-resveratrol in the high-producing rolB transgenic cell cultures of V. amurensis. A systematic examination of resveratrol accumulation in the rolB transgenic and empty vector-transformed callus cultures of V. amurensis has been done for 27 months of continuous subculturing. A marked decrease in resveratrol accumulation has been observed in the high-producing rolB transgenic calli. We demonstrate that the decrease in biosynthetic capability of the rolB calli can be reversed using methyl jasmonate (MeJA) and salicylic acid (SA): elicitation of the rolB transgenic calli with MeJA and SA has led to a significant recovery of resveratrol accumulation in the rolB transgenic cell culture, while the empty vector control exhibited low inducibility to the compounds.
Materials and methods
Grape cell cultures
A callus culture (V2) of wild-growing grape Vitis amurensis Rupr. (Vitaceae) was established in 2002 as described (Kiselev et al. 2007; Kiselev and Dubrovina 2010). The VV callus culture was obtained in the last quarter of 2004 by co-cultivation of the V2 cell suspension with A. tumefaciens GV3101/pMP90RK strains containing pPCV002 plasmid vector, which contained only the nptII (kanamycin resistance) gene (Kiselev et al. 2007). The VB2 callus culture was obtained in the last quarter of 2004 by transformation of the V2 callus culture with pPCV002-CaMVB/pMP90RK (Spena et al. 1987) as described previously (Kiselev et al. 2007; Kiselev and Dubrovina 2010).
The transformed VV and VB2 callus cultures were cultivated with 35-day intervals in the dark at 24–25°C, in test tubes with 15 ml of solid Murashige and Skoog modified WB/A medium (Kiselev et al. 2009) supplemented with 0.5 mg/l 6-benzylaminopurine (BA) and 2.0 mg/l α-naphthaleneacetic acid (NAA). Reagents for cell culture medium were purchased from Sigma Chemical Co (MO, USA), Serva (Heidelberg, Germany) and Labtech (Moscow, Russia). Tissue samples were harvested from 35-day cultures (linear phase of growth and the highest resveratrol content). To determine fresh weight (FW), we placed the cell mass of the calluses on a preweighed dry filter paper, and weighed it. To avoid tissue desiccation, we opened the culture tubes only when we were ready to process it. For dry weight (DW) determination, the cell mass was air dried under hot air flow (60°C for 2 h) and then weighed and used for resveratrol determination. FW and DW were calculated in grams per liter of the solid culture medium.
Elicitor treatments
Sterile solutions of MeJA (Sigma) were added to the autoclaved culture medium aseptically in desired concentrations. Stock solution of MeJA in DMSO (ICN Pharmaceuticals, Eschwege, Germany) was prepared at a concentration of 100 mg/ml. SA (Serva, Heidelberg, Germany) was dissolved in water (1 mg/ml), titrated with 5% KOH to pH 5.6, and added to the cell culture medium in desired concentrations before pH measurement as described (Kiselev et al. 2007). Samples for resveratrol determination were harvested after 35 days of cultivation (linear phase of growth and the highest resveratrol content) in the presence of MeJa and SA.
High-performance liquid chromatography (HPLC) and resveratrol identification
The dried and powdered callus culture samples (100 mg) were extracted two times using 96% ethanol with 0.1% HCl (3 ml) for 2 h at 60°C. The analytical HPLC was carried out using an Agilent Technologies 1100 Series HPLC system equipped with VWD detector (Agilent Technologies, Germany) as described (Kiselev et al. 2007). The data were analyzed with the ChemStation® program var. 09 (Agilent Technologies, Germany). After 2007, the analytical HPLC was carried out using a Shimadzu 10 series HPLC system equipped with UV-vis detector (Japan) as described (Dubrovina et al. 2010). The data were analyzed with the Lc Solution version 1.11 SP1 program (Shimadzu Corporation). Resveratrol was identified by 1H and 13C NMR using a Bruker NMR Avance DPX-500 instrument as described (Kiselev et al. 2007). To test resveratrol stability under 60°C, we incubated a 96% ethanolic solution of pure trans-resveratrol (3,4′,5-trihydroxy-trans-stilbene approximately 99% GC; SIGMA-ALDRICH; St. Louis, MI, USA) at 60°C for 2 and 4 h, and then analyzed the effect using HPLC. No significant difference has been found.
Statistical analysis
Statistical analyses were carried out using the Statistica 9.0 program. The data are presented as the mean value ± standard deviation (SD), and were tested for statistical significance using the paired Student’s t test. The 0.05 level was selected as the point of minimal statistical significance in all analyses. Appropriate numbers of replications and tests used are indicated in the descriptions to the tables, and in the figure.
Results
Resveratrol accumulation in the empty vector-transformed and rolB transgenic callus cultures of V. amurensis during long-term subcultures
The control callus culture (VV), transformed with an empty vector, and two rolB transgenic callus cultures of V. amurensis (VB1 and VB2) were established in the last quarter of 2004 (Kiselev et al. 2007). Shortly after transformation, the VB2 cell line was shown to produce dramatically higher levels of resveratrol compared to that in the empty vector-transformed cell line (Kiselev et al. 2007). Also, the VB2 cell line was shown to possess a markedly higher level of rolB gene expression and resveratrol accumulation compared to that in the VB1 cell line.
Using HPLC, we analyzed dynamics of resveratrol accumulation in the VV and VB2 cell lines over 27 months of continuous subculturing. The measurements have not been done in the first quarter of 2005 (Year 1), since at that time, the rolB-transformed calli showed high variability in morphology and growth (Kiselev et al. 2007). The HPLC determinations revealed that the VV cell calli produced trans-resveratrol in low amounts throughout the period of cultivation (Table 1). Trace amounts of other phenolic substances were observed (data not shown). In the VV calli, the content of resveratrol did not exceed 0.04% DW, and the level of resveratrol production did not exceed 3.19 mg/l of the solid medium or 19.27 μg/g FW (Table 1).
VB2 calli also produced trans-resveratrol as a predominant phenolic compound throughout the period of cultivation (Table 1). Until second quarter of Year 2, the content of resveratrol in the VB2 calli was stable and did not significantly vary (Table 1). The highest content of resveratrol (3.04% DW) and resveratrol production (153.82 mg/l or 1,878.86 μg/g FW) was observed in the first quarter of Year 2. However, a significant reduction (up to 0.65% DW) of resveratrol accumulation in the VB2 calli was observed 1.5 years after transformation (Table 1). Although there was a short-term increase in the culture productivity in the first quarter of Year 3 (Table 1), it has dropped to 0.14–0.21% DW in Year 3, 2 years after transformation (Table 1), and has never reached the high levels that were initially observed. In Year 4, Year 6, and Year 7, sporadic HPLC analyses were done to control the VB2 culture productivity. According to the analyses, resveratrol content in the VB2 calli varied from 0.21 (third quarter of Year 6) to 0.84% DW (third quarter of Year 4). The most recent measurement (first quarter of Year 7) has shown 0.39% DW of resveratrol in the VB2 calli. Thus, although we observed a considerable reduction of trans-resveratrol accumulation in the rolB transgenic cell line 2 years after transformation, the content of resveratrol was always higher in the rolB transgenic cell culture compared to the empty vector-transformed cell line (Table 1).
Biomass accumulation in the empty vector-transformed and rolB transgenic callus cultures of V. amurensis
Throughout the 27 months of repeated subcultures, the VB2 cells produced compact slowly growing calli, while the empty vector-transformed VV cells produced friable vigorously growing calli. Importantly, V2, the untransformed parent cell line of the VV and VB2 cell cultures, also produced friable vigorously growing calli (Kiselev et al. 2007). It has been shown previously that the growth of the rolB transgenic culture VB2 was reduced compared to that of the empty vector control (Kiselev et al. 2007). Since the rate of biomass accumulation is important for commercialization of plant cell culture-based bioprocesses, we analyzed dynamics of fresh and dry biomass accumulation in the VV and VB2 cell cultures over long-term subculturing (Table 2). The rate of fresh biomass accumulation in the VB2 cell culture was significantly slower than that in the VV cell culture, throughout the period of cultivation (Table 2). However, the difference in the rate of dry biomass accumulation was less apparent between the cell cultures (Table 2).
Treatment of the empty vector-transformed and rolB transgenic calli of V. amurensis with MeJA and SA
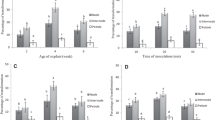
MeJA and SA treatments were shown to increase accumulation of trans-resveratrol in the untransformed callus culture of V. amurensis (V2) (Kiselev et al. 2007). Elicitation of the rolB transgenic cell culture with MeJA and SA was applied to test whether it is possible to reverse the decrease in resveratrol production observed in the third quarter of the Year 2 when the average resveratrol content was as low as 0.65% DW (Table 1; Fig. 1). The addition of MeJA and SA to the culture medium increased the content of resveratrol in the VB2 cell culture, and did not change it in the empty vector control (Fig. 1a, b).
Effect of SA (a) and MeJA (b) on growth and trans-resveratrol production in the control VV and rolB transgenic VB2 callus cultures of V. amurensis. Mean growth values ± SD based on ten replicate samples were obtained in a single experiment. Average values of resveratrol content were received from dried calli of the ten test tubes
Interestingly, the effect of SA treatment on the resveratrol accumulation in the VB2 calli was higher than that of MeJA treatment (Fig. 1a, b). For example, 150 μM SA increased resveratrol content to 2% DW (in 3.7 times), while 50 μM MeJA—to 0.83% DW (in 1.7 times). It is noteworthy that such high concentrations of SA as 150 and 300 μM did not alter the dry growth of both control and rolB transgenic cell cultures, while MeJA treatments slightly reduced it (Fig. 1a, b).
Discussion
Many efforts have been made to elicit stilbene biosynthesis in plant cell cultures. The production of resveratrol or resveratrol derivatives by plant cell cultures can be significantly increased when using cyclodextrins (Bru and Pedreno 2006; Lijavetzky et al. 2008), sucrose (Ferri et al. 2011), MeJA (Tassoni et al. 2005; Santamaria et al. 2011), ethephon combined with high hydrostatic pressure (Cai et al. 2011a), or insect saliva (Cai et al. 2011b). In the present study, we report high resveratrol production by a callus culture of V. amurensis transformed with the rolB gene of A. rhizogenes during 15 months of continuous subculturing. During this time, resveratrol content in the rolB transgenic cell line was significantly higher than that in cultivated or genetically engineered plants (Iriti et al. 2004; Delaunois et al. 2009), or in some plant cell cultures (Tassoni et al. 2005; Arora et al. 2009; Santamaria et al. 2011). Importantly, experiments including monitoring resveratrol accumulation in cell cultures of various plants were limited by one subculture period. In the present study, we show that resveratrol content in a high-producing cell culture of V. amurensis can markedly decrease during long-term cultivation. We observed a decrease in resveratrol accumulation in a rolB transgenic V. amurensis cell line, producing high amounts of resveratrol, after 2 years of continuous subculturing. This result shows an agreement with the data on dynamics of anthocyanin accumulation in cell cultures of V. vinifera and Ajuga reptans (Callebaut et al. 1997; Qu et al. 2005), and alkaloid accumulation in cell cultures of Catharanthus roseus (Deus-Neumann and Zenk 1984; Whitmer et al. 2003). The authors show that anthocyanin and indole alkaloid biosynthetic capacity of the cell cultures was lost at various rates during long-term subculturing.
The origin of the gradual decrease in resveratrol production by the rolB transgenic callus culture of V. amurensis is not clear. Expression of the rolB gene was detected in the rolB transgenic culture during periods of both high and low levels of resveratrol production (data not shown), and it was present in the rolB transgenic calli 5 years after transformation (Kiselev et al. 2009). Therefore, the decrease in resveratrol content cannot be explained by the absence of transgene expression. After the selection of the rolB-transformed cells for kanamycin resistance, we observed cell aggregates with different growth properties (Kiselev et al. 2007). Perhaps, the rolB transgenic cell culture contained cell types with different levels of rolB gene expression. And during long-term subcultures, the number of cells with lower rolB gene expression has increased.
Treatment of the rolB transgenic calli with SA and MeJA resulted in a recovery of high resveratrol content in the calli (Fig. 1). The data suggest that after long-term cultivation, the rolB-transformed cells produce the RolB protein which enables the increased capability of the cells for resveratrol production and their higher inducibility to MeJA and SA treatments compared to the empty vector-transformed cells. A regular addition of the inductors to the culture media could be a method to maintain high levels of resveratrol accumulation in grape cell cultures. However, further analysis is needed to investigate how long grape cells can synthesize high amounts of trans-resveratrol in response to MeJA or SA elicitation.
Zeng et al. (2010) have shown that the level of expression of extraneous genes in in vitro micropropagated clones of transgenic birch decreased with increasing subculture number. The authors have shown that this effect was associated with DNA methylation. It is possible that rolB gene expression was silenced in certain groups of cells in the rolB transgenic callus culture of V. amurensis, during its long-term cultivation, due to an increase in the number of methylation sites (GC or GNC) in the 35S-CaMV promoter or in the body of the rolB gene. Besides mutagenesis of the rolB transgene or 35S-CaMV promoter, it is possible that accumulation of various mutations in the genes responsible for efficient resveratrol biosynthesis, or in the regulatory regions of the genes, contributed to the loss of high resveratrol levels in the rolB transgenic calli of V. amurensis.
Genetic instability of plant cell cultures and plants regenerated from the cultures is well established (Rani and Raina 2000; Kaeppler et al. 2000). This genetic variation, which is referred to somaclonal variation, includes changes in ploidy, chromosome rearrangements, single base substitutions or changed DNA methylation patterns (Rani and Raina 2000; Kaeppler et al. 2000). Since high rates of mutagenesis and other genetic alterations impose limitations on the subsequent commercial utilization of plant cell cultures as alternative sources of biologically active compounds, the mechanisms producing genetic or epigenetic variations that could contribute to the gradual decline in resveratrol production by grape cell cultures need further investigation.
Abbreviations
- MeJA:
-
Methyl jasmonate
- SA:
-
Salicylic acid
- DW:
-
Dry weight
- FW:
-
Fresh weight
- HPLC:
-
High-performance liquid chromatography
References
Arora J, Roat C, Goyal S, Ramawat KG (2009) High stilbenes accumulation in root cultures of Cayratia trifolia (L.) Domin grown in shake flasks. Acta Physiol Plant 31:1307–1312
Bru MR, Pedreno GMLDE (2006) Method for the production of resveratrol in cell cultures. US 2006/0205049 A1
Cai Z, Riedel H, Saw N, Mewis I, Reineke K, Knorr D, Smetanska I (2011a) Effects of elicitors and high hydrostatic pressure on secondary metabolism of Vitis vinifera suspension culture. Process Biochem 6:1411–1416
Cai Z, Knorr D, Smetanska I (2011b) Enhanced anthocyanins and resveratrol production in Vitis vinifera cell suspension culture by indanoyl-isoleucine, N-linolenoyl-l-glutamine and insect saliva. Enzyme Microb Technol. doi:10.1016/j.enzmictec.2011.09.001
Callebaut A, Terahara N, de Haan M, Decleire M (1997) Stability of anthocyanin composition in Ajuga reptans callus and cell suspension cultures. Plant Cell Tiss Organ Cult 50:195–201
Delaunois B, Cordelier S, Conreux A, Clément C, Jeandet P (2009) Molecular engineering of resveratrol in plants. Plant Biotechnol J 7:2–12
Deus-Neumann B, Zenk MH (1984) Instability of indole alkaloid production in Catharanthus roseus cell suspension cultures. Planta Med 50:427–431
Donnez D, Jeandet P, Clement C, Courot E (2009) Bioproduction of resveratrol and stilbene derivatives by plant cells and microorganisms. Trends Biotechnol 27:706–713
Dubrovina AS, Manyakhin AY, Zhuravlev YN, Kiselev KV (2010) Resveratrol content and expression of phenylalanine ammonia-lyase and stilbene synthase genes in rolC transgenic cell cultures of Vitis amurensis. Appl Microbiol Biotechnol 88:727–736
Ferri M, Righetti L, Tassoni A (2011) Increasing sucrose concentrations promote phenylpropanoid biosynthesis in grapevine cell cultures. J Plant Physiol 168:189–195
Hirasuna TJ, Shuler ML, Lackney VK, Spanswick RM (1991) Enhanced anthocyanin production in grape cell-cultures. Plant Sci 78:107–120
Iriti M, Rossoni M, Borgo M, Faoro F (2004) Benzothiadiazole enhances resveratrol and anthocyanin biosynthesis in grapevine, meanwhile improving resistance to Botrytis cinerea. J Agric Food Chem 52(14):4406–4413
Kaeppler SM, Kaeppler HF, Rhee Y (2000) Epigenetic aspects of somaclonal variation in plants. Plant Mol Biol 43:179–188
Kim BJ, Gibson DM, Shuler ML (2004) Effect of subculture and elicitation on instability of taxol production in Taxus sp. suspension cultures. Biotechnol Prog 20:1666–1673
Kiselev KV (2011) Perspectives for production and application of resveratrol. Appl Microbiol Biotechnol 90:417–425
Kiselev KV, Dubrovina AS (2010) A new method for analysing gene expression based on frequency analysis of RT-PCR products obtained with degenerate primers. Acta Physiol Plant 32:495–502
Kiselev KV, Dubrovina AS, Veselova MV, Bulgakov VP, Fedoreyev SA, Zhuravlev YN (2007) The rolB gene-induced overproduction of resveratrol in Vitis amurensis transformed cells. J Biotechnol 128:681–692
Kiselev KV, Dubrovina AS, Bulgakov VP (2009) Phenylalanine ammonia-lyase and stilbene synthase gene expression in rolB transgenic cell cultures of Vitis amurensis. Appl Microbiol Biotechnol 82:647–655
Kolewe ME, Gaurav V, Roberts SC (2008) Pharmaceutically active natural product synthesis and supply via plant cell culture technology. Mol Pharm 5:243–256
Lijavetzky D, Almagro L, Belchi-Navarro S, Martinez-Zapater JM, Bru R, Pedreno MA (2008) Synergistic effect of methyljasmonate and cyclodextrin on stilbene biosynthesis pathway gene expression and resveratrol production in Monastrell grapevine cell cultures. BMC Res Notes 1:132
Marques FZ, Markus MA, Morris BJ (2009) Resveratrol: cellular actions of a potent natural chemical that confers a diversity of health benefits. Int J Biochem Cell Biol 41:2125–2128
Pervaiz S, Holme AL (2009) Resveratrol: its biologic targets and functional activity. Antioxid Redox Signal 11:2851–2897
Qu JG, Zhang W, Yu XJ, Jin MF (2005) Instability of anthocyanin accumulation in Vitis vinifera L. var. Gamay Freaux suspension cultures. Biotechnol Bioprocess Eng 10:155–161
Rani V, Raina SN (2000) Genetic fidelity of organized meristem-derived micropropagated plants: A critical reappraisal. In Vitro Cell Dev Biol-Plant 36:319–330
Rao SR, Ravishankar GA (2002) Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv 20:101–153
Roberts SC, Shuler ML (1997) Large-scale plant cell culture. Curr Opin Biotechnol 8:154–159
Santamaria AR, Mulinacci N, Valletta A, Innocenti M, Pasqua G (2011) Effects of elicitors on the production of resveratrol and viniferins in cell cultures of Vitis vinifera L. cv Italia. J Agric Food Chem 59:9094–9101
Spena A, Schmulling T, Koncz C, Schell JS (1987) Independent and synergistic activity of rol A, B and C loci in stimulating abnormal growth in plants. EMBO J 6:3891–3899
Tassoni A, Fornale S, Franceschetti M, Musiani F, Michael AJ, Perry B, Bagni N (2005) Jasmonates and Na-orthovanadate promote resveratrol production in Vitis vinifera cv. Barbera cell cultures. New Phytol 166:895–905
Verpoorte R, van der Heijden R, Memelink J (2000) Engineering the plant cell factory for secondary metabolite production. Transgenic Res 9:323–343
Wang Y, Chen H, Yu O (2010) Metabolic engineering of resveratrol and other longevity boosting compounds. Biofactors 36:394–400
Whitmer S, Canel C, van der Heijden R, Verpoorte R (2003) Long-term instability of alkaloid production by stably transformed cell lines of Catharanthus roseus. Plant Cell Tiss Organ Cult 74:73–80
Zeng F, Qian J, Luo W, Zhan Y, Xin Y, Yang C (2010) Stability of transgenes in long-term micropropagation of plants of transgenic birch (Betula platyphylla). Biotechnol Lett 32:151–156
Acknowledgments
We thank Dr. Marina V. Veselova and Dr. Artem Y. Manyakhin for assistance with HPLC analyses. This work was supported by a grant of the Russian Foundation for Basic Research (10-04-00189-a) and by the grant program ‘‘Molecular and Cell Biology’’of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. V. Jorrin-Novo.
Rights and permissions
About this article
Cite this article
Dubrovina, A.S., Kiselev, K.V. Effect of long-term cultivation on resveratrol accumulation in a high-producing cell culture of Vitis amurensis . Acta Physiol Plant 34, 1101–1106 (2012). https://doi.org/10.1007/s11738-011-0907-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-011-0907-5