Abstract
As most gramineous plants, guinea grass (Panicum maximum) comprise cellulosic biomass, which may be used as a feedstock for bioenergy. In order to develop such potential energy plants on copper-polluted lands, the hydroponic experiments with Cu, Paclobutrazol (PP333, a kind of antigibberellin) and plant growth-promoting bacterial endophyte (PGPB) treatments were carried out in a greenhouse. The seedlings of two cultivars of guinea grass, GG1 (P. maximum var. Natsukomaki) and GG2 (P. maximum var. Natsukaze) in 3 weeks old were treated, respectively, with different Cu treatments [0(CK), 100, 200, 300, 400, 500 μM l−1 Cu] for estimating Cu toxicity. The results showed that elevated Cu restrained plant growth and reduced biomass. According to the EC50 value [the Cu concentration when the relative gain in fresh weight ratio was 50% of control] of two tested cultivars, the concentration of Cu for further experiments was decided as 300 μM l−1. Both pretreatments of PP333 (200, 400, 600 mg l−1) and PGPB (Pantoea sp.) significantly alleviated the negative affect caused by stress of 300 μM l−1 Cu. The pretreatment of 400 mg l−1 PP333 promoted both two cultivars in biomass, compared to 300 μM l−1 Cu treat. The inoculation of Pantoea sp. Jp3-3 increased shoot dry weight, compared to Cu treat. The results suggested that the main reason for both PP333 and Pantoea sp. Jp3-3 enhanced Cu tolerance in guinea grass was that their pretreatments significantly decreased Cu absorption and accumulation under excessive Cu stress. The present study has provided a new insight into the exploitation of energy plant in heavy metal polluted condition by the way of plant growth regulation for increasing heavy metal tolerance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metal mining and smelting, and the abuse of pesticides have caused severe heavy metal pollution to the environment (Bhuiyan et al. 2010), and then caused land degradation, which is a worldwide menace both for food security and human health (Nouairi et al. 2009). Among heavy metals, copper (Cu) is a required microelement for plant, but excess is highly toxic. A concentration of Cu in soils, that exceeding the maximum allowable concentration that required as nutrients or background levels, results in an accumulation of Cu in plants on deleterious levels (Srivastava et al. 2008). Excess copper could seriously inhibit the synthesis of chlorophyll and protein as well as carbohydrate and the uptake of phosphate and nitrogen (Xing et al. 2010). An important feature of copper toxicity is the formation of damaging oxygen radicals, and then causes increasing of MDA and proline contents, electrical conductivity and antioxidant enzyme activities (SOD, CAT, APX) (Li et al. 2009a).
By the crops from contaminated soils, excessive heavy metals enter the food chain and therefore do harm to human health (Lamb et al. 2009; Rahman et al. 2009). On the light of this, grain crops should be excluded from phytoremediation, although it is one of the most cost-effective and environment-friendly strategies of remediating metal-contaminated soils. Foregone hyperaccumulators, however, are known of their uneconomical usage, for almost all of them are at low biomass and slow growth velocity (Zhao et al. 2006). This shortcoming may also cause energy waste in disposal of the plant corpus. In recent years, developing energy plant in metal contaminated soils is considered as a corking solution to these problems (Shi et al. (2009). Energy plants can be used as producing biodiesel or bioethanol, which is a sustainable approach for the removal of metal pollutants by phytoremediation (Shi and Cai 2009). The tropical forage guinea grass (Panicum maximum var.), which has been widely used, is a common feed species (Ram 2009). Guinea grass behaves as high biomass, high growth rate and low humidity content, is a potential energy plant remain further study.
Paclobutrazol (PP333) is well-known as an antigibberellin plant growth regulator. Foliar applications of PP333 can reduce the top growth advantage apparently with few adverse effects on cropping (Blank et al. 2009; Li et al. 2009b). PP333 also increases the number of tillers, accelerates root growth, shortens internodes, promotes plant resistance to stress and improves crop yields (Mahoney et al. 1998; Sharma and Awasthi 2005; Thakur et al. 2006). Since invented in eighties of the last century, PP333 has been widely applied for both increasing in yield and promoting resistance (Chen et al. 2010).
Endophytic bacteria reside within plant hosts without causing disease symptoms (Taghavi et al. 2010). Endophytic bacteria have been found in virtually every plant studied, where they colonize the internal tissues of their host plant and form a range of different relationships (Rajkumar and Freitas 2008; Ryan et al. 2008). Among them endophytic plant growth-promoting bacteria (PGPB) could enhance host growth by phosphate solubilization activity (Rueda-Puente et al. 2010), osmotic adjustment, indole acetic acid production and the production of siderophore (Ma et al. 2009).
Previous studies have shown that guinea grass was able to ensure high yield. However, no studies have examined their growth and biomass performance as potential energy plant under copper (Cu) stress. Thus, the aim of this study was to (1) test Cu tolerance of the two cultivars of guinea grass, find a better candidate which is more tolerant to Cu, (2) application of PP333 to enhance Cu tolerance of the two cultivars, investigate the physiological mechanism of the enhancement, (3) elucidate the effects of PGPB on the plant growth and the uptake of Cu by the two cultivars. This study will establish a meaningful base for developing bioenergy feedstock resources cultivated in the areas polluted by excess heavy metal Cu by exploring bio-regulate way.
Materials and methods
Materials and seedling cultivation
The seeds of two guinea grass cultivars, GG1 (P. maximum var. Natsukomaki) and GG2 (P. maximum var. Natsukaze) (Snow Brand Seed Co., Ltd., Japan) were, respectively, surface-sterilized in 0.1% HgCl2 for 15 min, rinsed with sterile water, soaked in 10% KNO3 for 12 h. Then the seeds were sowed in culture dishes (Diameter 9 cm) filled with 5 g sterile clean river sand. At 10 days after germination in 12 h dark at 20°C, 12 h light at 27°C, and humidity about 50 ± 20%, uniform seedlings were selected and cultured in plastic cup (350 ml) filled with 400 g river sand, which was sterilized with 5% H2O2 for 30 min, and full rinsed by sterile water, and then 170°C parched for 4 h in oven before used. And every six cups were gathered in a plastic pot submersed with 1.0 L Hoagland solution (pH 5.8) and placed in growth-chamber (52 × 51 × 102 cm). At 3 weeks after cultivation in the cup, when the fifth leaf of the seedling fully expanded, uniform seedlings were transplanted into hydroponics plastic pots (22 × 16 × 7.5 cm). After 3 days buffer period, different treatments were conducted.
All the treatments as below were set in triplicate repetitions with three pots filled with 2.0 l Hoagland solution (pH 5.8), and 15 uniform seedlings were allowed to grow in each pot, at a uniform spacing. The pot trials were conducted in a greenhouse (30 ± 5°C, humidity of 50 ± 20%).
The plant growth under several treatments (Table 1)
Copper treatments
Six treatments of Cu [0(CK), 100, 200, 300, 400, 500 mol l−1, supplied as CuSO4·5H2O were conducted for examining Cu tolerance of two grasses. The solution was refilled every 3 days. 12 days later, data were measured as described in “Measurements for plant growth, and physiological and chemical analysis”.
PP333 application before Cu stress
When the fifth leaves were fully unfolded, 0 (control), 200, 400, 600 mg l−1 PP333, supplied as 15% wettable powder (purchased from Jiangsu Academy of Agricultural Sciences, Nanjing, China) were foliar sprayed, respectively. Each pot was treated with 50 ml PP333 solution while 50 ml sterile water for the control. At 12 days after PP333 spraying, eight treatments, control (Cu: 0 μmol l−1, PP333: 0 mg l−1), Cu (Cu: 300 μmol l−1, PP333: 0 mg l−1), P200 (Cu: 0 μmol l−1, PP333: 200 mg l−1), P200-Cu (Cu: 300 μmol l−1, PP333: 200 mg l−1), P400 (Cu: 0 μmol l−1, PP333: 400 mg l−1), P400-Cu (Cu: 300 μmol l−1, PP333: 400 mg l−1), P600 (Cu: 300 μmol l−1, PP333: 600 mg l−1) and P600-Cu (Cu: 0 μmol l−1, PP333: 600 mg l−1) were conducted. The concentration of Cu (300 μmol l−1) was decided basted on the experimental results of series Cu treatments (“Copper treatments”). 12 days later, different parts of plant were sampled for testing as describe in “Measurements for plant growth, and physiological and chemical analysis”.
Endophytic PGPB pretreated before Cu stress
The Cu-resistance PGPB endophyte strain, Pantoea sp. Jp3-3 (accession number: EU781540) was screened and provided by Prof. Xia-fang Sheng, College of Life Sciences, Nanjing Agricultural University, China. The endophytic bacteria strain was separated from crabgrass (Digitaria sanguinalis (L.) Scop.), which grown on Cu contaminated soil in Tangshan Town, Jiangning District, Nanjing, China. Its 16S ribosomal RNA gene partial sequence is available on http://misuse.ncbi.nih.gov/. The bacteria was pre-cultured in liquid LB medium in 28°C for 24 h, centrifuged (7,000 rpm, 10 min), washed, and resuspended in phosphate buffer (pH 7), OD = 0.8 at 600 nm.
For PGPB endophytic pretreatment, germinated seeds were soaked in the bacterial suspension for 4 h while in sterile water was used as control. Then all the seeds were moved into plastic cups.
When the control group seedlings were 40 cm high (3 weeks after seeding), all the plants were transplanted in plastic pots. Four treatments, control (Cu: 0 μmol l−1, no PGPB pretreatment), Cu (Cu: 300 μmol l−1, no PGPB pretreatment), PGPB (Cu: 0 μmol l−1, PGPB pretreatment), PGPB-Cu (Cu: 300 μmol l−1, PGPB pretreatment). Twelve days later, data were measured as described in “Measurements for plant growth, and physiological and chemical analysis”.
Measurements for plant growth, and physiological and chemical analysis
The hydroponic plants were sampled after Cu treatment for 12 days. Plant height and root length were measured before the samplings were separated into shoot and roots, and washed by distilled water, dried at 75°C in oven for 1 day and weighed before analysis of Cu concentrations. Also biomass per plant was measured by weighing using an electronic balance.
The chlorophyll content of the first full expanded leaf (youngest and count from top to bottom) in vivo was measured by chlorophyll meter (SPAD-502, KONICA MINOLTA, Tokyo, Japan) (Uddling et al. 2007).
Root activity was measured by triphenyltetrazolium chloride (TTC) deoxidization intensity (Lin et al. 2001). All the absorbencies were measured using a spectrophotometer (UV-2450, SHIMADZU, Tokyo, Japan).
The calorific values of the dry plant samples were measured using a SXHW-III microcomputer automatic calorimeter (Qitian, China) at 20°C, after porphyrization, compression and weighed.
Dry plant samples (0.2 g) were digested in concentrated HNO3–HClO4 (Guarantee reagent, 85: 15, v/v) using a microwave laboratory system (Milestone Ethos T, USA). After defining volumes to 10 ml using deionized water, concentrations of heavy metals were determined using an atomic absorption spectrophotometer (AAS novAA 400, Analytic-Jena, Germany).
Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA) and Tukey’s HSD using SPSS 17.0. Differences were considered to be significant at p < 0.05 or p < 0.01.
Results and analysis
Effect of Cu stress on the growth of two guniea grass cultivars
Growth parameters of GG1 and GG2 seedlings under Cu concentration gradient
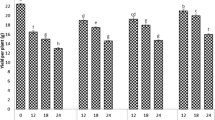
The growth of GG1 and GG2 was suppressed, both their length and biomass of shoot and root decreased when the treated Cu concentration increased (Figs. 1, 2). Plants exposed to Cu in the concentration of 100 and 200 μM l−1 did not show significant inhibition in the shoot length compared to control, but root length was significantly inhibited under the Cu concentration of 100 μM l−1. It was also shown that roots were more sensitive than shoots under heavy metal stress because of the immediate contact (Hernandez and Pastor 2008).
Biomass of GG1 (Panicum maximum var. Natsukomaki) and GG2 (Panicum maximum var. Natsukaze) when exposed to different concentrations of Cu for 12 days. The data are mean ± SE (n = 9). Different letters mean significant differences (p < 0.05) according to the Tukey HSD test. FW fresh weight, DW dry weight
The relative gain in fresh weight ratio (RGW, similar to the tolerance index; Wilkins 1957) was used to characterize the degree of inhibition of plant growth by Cu stress, which was expressed as:
T treatment groups, C control groups, W final weight, W0 initial weight.
The Cu concentrations (X) were related to fresh weight (Y) by regression analysis. The EC50, defined as the Cu concentration when the RGW was 50%, was used to characterize the tolerance to Cu stress (Table 2). According to the results shown, the EC50 values were 245.89 μM l−1 Cu for GG1 and 308.59 μM l−1 Cu for GG2, which suggest that GG2 was more tolerant to Cu than GG1.
Plants exposed to Cu treats in 100 and 200 μM l−1 did not show obvious inhibition in the root dry weight, compared to control. However, the root biomass was reduced and no significant difference was found among treats of 300, 400, 500 μM l−1 Cu. The stimulation in shoot dry weight of GG2 was found at 100 and 200 μM l−1 Cu concentrations. As for shoot, GG1 was significantly reduced in 100 μM l−1 Cu, and continuous decreased in higher Cu concentrations. As an essential element, lower Cu concentration often stimulates plant growth, this is also observed in hypocotyls and radicals length (Lou et al. 2004). GG2, which was more tolerant according to fresh weight, performed better in shoot dry weight. Low Cu concentrations (100, 200 μM l−1) even increased GG2 shoot dry weight by 13.41 and 12.87%, respectively, and high Cu concentrations decreased it gently. And then, 300 μM l−1 Cu was selected as a representative concentration for further treatments.
Physiological indexes of guinea grass under Cu stress
Root activity and chlorophyll content of GG1 and GG2 were shown in Fig. 3. These two physiological characters were positively related with the biomass. Root activity was significantly inhibited in 100 μM l−1 Cu, and showed a gradual trend to decrease in higher Cu concentrations (p < 0.05). Chlorophyll content also showed a continuous decreasing, and the youngest leaves (count from top to bottom) were more sensitive to Cu than the old leaves.
Root activity and chlorophyll content of GG1 (Panicum maximum var. Natsukomaki) and GG2 (Panicum maximum var. Natsukaze) when exposed to different concentrations of Cu for 12 days. The first leaf was the youngest. The data are mean ± SE (n = 9). Different letters mean significant differences (p < 0.05) according to the Tukey HSD test
Alleviate effects of PP333 pretreatments on Cu toxicity to GG1 and GG2
Effect of PP333 on the growth of GG1 and GG2
It is showed that onefold treatment of PP333 and pretreatments of PP333 before Cu stress promoted growth of GG1 and GG2, according to their growth parameters, length and biomass of root and shoot in Tables 3 and 4, respectively. Especially, after 12 days different Cu treatment, such promoting effects in pretreatments of PP333 before Cu stress were more significant (p < 0.05) in shoot length and shoot weight than in root length and root weight, especially in GG1. Anyway, pretreatments of PP333 had alleviated effects on Cu toxicity. Among the three pretreatments of PP333, under comprehensive consideration in contain economic efficiency; P200 was the most effective dosage.
In addition, there existed different effects of Cu and PP333 to growth of GG1 and GG2. In P200 and P200-Cu, Cu reduced GG1 shoot fresh weight by 10.3% while GG2 by 19.4%. In P400 and P400-Cu, these reduce rates were 14.8 and 35.8%, and 18.4 and 46.2% in P600 and P600-Cu. These results strongly indicated that PP333 was more effective to GG1 than GG2, although GG2 biomass was still much higher than GG1 (Table 4).
Physiological effects of the PP333 pretreatment on copper stress
According to the results of regulating root activity by PP333 (Fig. 4a, b), all the pretreatments of PP333 before Cu stress (P200-Cu, P400-Cu and P600-Cu) showed their protective effects, but such effects were significant only in the treats at P200-Cu and P400-Cu for GG1. These results also strongly indicated that PP333 was more effective to GG1 than GG2. No significant effects of pretreatments of PP333 on chlorophyll contents in top three leaves were showed in comparing with Cu treat alone (Fig. 4c, d).
Effects of PP333 pretreatment on root activity and chlorophyll content of guinea grass under Cu stress for 12 days. GG1 (Panicum maximum var. Natsukomaki) and GG2 (Panicum maximum var. Natsukaze). The data are mean ± SE (n = 9). Different letters mean significant differences (p < 0.05) according to the Tukey HSD test. Control (Cu 0 μM l−1, PP333 0 mg l−1), Cu (Cu 300 μM l−1, PP333 0 mg l−1), P200 (Cu 0 μM l−1, PP333 200 mg l−1), P200-Cu (Cu 300 μM l−1, PP333 200 mg l−1), P400 (Cu 0 μM l−1, PP333 400 mg l−1), P400-Cu (Cu 300 μM l−1, PP333 400 mg l−1), P600 (Cu 300 μM l−1, PP333 600 mg l−1), P600-Cu (Cu 0 μM l−1, PP333 60 0 mg l−1)
As shown in Table 5, under onefold treatment of PP333 (P400), the Cu contents in shoot and root were significant lower than control (except in shoot of GG2), while no such significant difference found in P200 and P600. Furthermore, under the pretreatments of PP333 before Cu stress (P200-Cu, P400-Cu and P600-Cu), the Cu contents both in shoot and root in two cultivars were significantly reduced (p < 0.05), compared to 300 μM l−1 Cu treat. And the Cu contents of roots in GG1 and GG2 decreased to 31.9 and 49.2%, respectively, in P400-Cu, which may be the most effective PP333 dosage. For the Cu contents of shoot in GG1, no significant differences among P200-Cu, P400-Cu and P600-Cu were found. The Cu content in shoot of GG2 also decreased compared to Cu treat, but the least content was observed in P200-Cu. Therefore, the pretreatments of PP333 may be a feasible choice for reducing the Cu content of the plant tissues.
It suggested that the main reason for PP333 enhanced Cu tolerance in guinea grass was that the pretreatments of PP333 significantly decreased Cu absorption and accumulation under excessive Cu stress. Although PP333’s effects on the distribution and accumulation of Cu in different parts of the plant, and the mechanisms, are still unknown, it can be surely confirmed that PP333 is an effectual regulator for reducing Cu uptake by plants, and finally alleviating Cu toxicity to the plant. .
Influence of Pantoea sp. Jp3-3 on GG1 and GG2 growth under Cu stress
After inoculating Pantoea sp. Jp3-3 (PGPB), the fresh weights of shoot and root increased significantly (Fig. 5). Jp3-3 enhanced the fresh weights of shoot and roots by 217 and 202.2% in GG1, and by 240.1 and 228.8% in GG2, respectively, compared to the control. Under the pretreatment of inoculating Pantoea sp. Jp3-3 before Cu treat (PGPB-Cu), both the fresh weights and dry weights of shoot and root in GG1 and GG2 increased significantly, not only comparing with Cu treat, but also with control. These results indicated that Jp3-3 was an effective PGPB for GG1 and GG2, not only at normal condition, but also under Cu stress. In addition, such PGPB’s effects alleviating Cu toxicity was more impactful to GG2 than GG1 (Fig. 5).
Effects of PGPB pretreatment on biomass of guinea grass under Cu stress for 12 days. The data are mean ± SE (n = 9). Different letters mean significant differences (p < 0.05) according to the Tukey HSD test. Control (Cu 0 μM l−1, no PGPB pretreatment), Cu (Cu 300 μM l−1, no PGPB pretreatment), PGPB (Cu 0 μM l−1, PGPB pretreatment), PGPB-Cu (Cu 300 μM l−1, PGPB pretreatment). FW fresh weight, DW dry weight. GG1 (Panicum maximum var. Natsukomaki) and GG2 (Panicum maximum var. Natsukaze)
Under the pretreatment of inoculating Pantoea sp. Jp3-3 before Cu treat (PGPB-Cu), the Cu contents both in the root and shoot decreased significantly both in GG1 and GG2 (Fig. 6), compared to Cu treat. Cu content in PGPB-Cu group was 36.2% of the Cu treat in GG1 roots, and 36.8% in GG2 roots. As one kind of PGPB, Jp3-3 exhibited an obvious protection from absorbing excessive Cu by root firstly and transferring into shoots secondly. It also suggested that the main reason for PGPB enhanced Cu tolerance in guinea grass was that the pretreatments of PGPB significantly decreased Cu absorption and accumulation under excessive Cu stress.
Effects of PGPB pretreatment on Cu content of guinea grass under Cu stress for 12 days. The data are mean ± SE (n = 9). Control (Cu 0 μM l−1, no PGPB pretreatment), Cu (Cu 300 μM l−1, no PGPB pretreatment), PGPB (Cu 0 μM l−1, PGPB pretreatment), PGPB-Cu (Cu 300 μM l−1, PGPB pretreatment). GG1 (Panicum maximum var. Natsukomaki) and GG2 (Panicum maximum var. Natsukaze)
Discussion
Energy plant is considered of multitudinous purposes such as being transformed into energy for heat and electric power production through direct combustion, used as a feedstock for liquid transportation fuel, or gasified and liquefied to produce syn-gas or bio-oil. Several perennial herbaceous grasses have been studied in recent years as potential dedicated cellulosic biomass crops (Bals et al. 2010; Keshwani and Cheng 2009). Switchgrass (P. virgatum) has been legalized as one of the most important bioenergy plants over the world, especially in USA (Parrish and Fike 2005). Guinea grass has similar characteristics to switchgrass, such as tolerant to abiotic stress, high biomass, low humidity content, abundant cellulose and low requirements for agricultural inputs (Ram 2009; Carvalho et al. 2006). Previous studies were focused on its high productivity, but an important prerequisite for high productivity is the suitable soil conditions (Aylott et al. 2008). However, the use of arable land for bioenergy production has been criticized as diverting material away from the human food chain, with resulting of food shortages and price rise (Escamilla-Trevino et al. 2010). Meanwhile, the continued industrialization of countries has led to extensive environmental problems. A wide variety of chemicals (e.g. heavy metals, pesticides, chlorinated solvents, etc.) have been detected in soil and water (Rajkumar and Freitas 2008; Zhao et al. 2007). Among the pollutants, heavy metal is an important reason contributing to land deterioration, especially in mine-affected agricultural soils.
Generally, elevated levels of Cu are known to exhibit harmful effects on plant growth. At high concentrations, Cu inhibits seed germination, reduces plant growth, decreases enzyme activity of metabolism in plants and even causes plant death (Brewin et al. 2003). The present results showed that both GG1 and GG2 were tolerant to low concentration of Cu, especially GG2. Cu at the level of 200 μM l−1 did not significantly inhibit the shoot length of GG1 and GG2 in comparison with the control. However, as the Cu concentrations in the solution increased continually, plant growth was inhibited, according to the results of length and biomass of shoot and root in GG1 and GG2. Therefore, it is necessary to find a way to increase the Cu tolerance of guinea grass.
Paclobutrazol (PP333) is generally applied as root and foliar treatments (Wang et al. 1986). Previous researches were focused on its production increasing and water and salt stress alleviation effects (Dubey et al. 2009; Lin et al. 2006). It was found that PP333 provided an average reduction of banana in pseudostem height of 26%, thus promoted the yield of bananas (Maia et al. 2009). In another research, PP333 significantly increased the root and stem length, total leaf area, fresh weight, dry weight and activities of antioxidant enzymes, thus ameliorated the adverse effects of NaCl stress in V. unguiculata plants (Manivannan et al. 2008). Under water stress, PP333 exhibited a significant promotion of groundnut (Arachis hypogaea L.) growth by increasing the antioxidant levels and activities of scavenging enzymes such as SOD, APX and CAT (Sankar et al. 2007). Besides, other studies also proved that PP333 could increase plant growth under stress (Hajihashemi and Kiarostami 2007; Jaleel et al. 2007). Our studies provided a new regulate function of PP333 here. PP333 significantly increased biomass and root activities of two cultivars of guinea grass (GG1 and GG2) even under stress of 300 μM l−1 Cu, compared with the control. Although the cellula interaction mechanism of PP333 and Cu was still unknown, it was clear that PP333 exhibited a definite positive effect to the plant under heavy metal stress. Besides, there was littlereports on the effect of PP333 to plant heavy metal contents. PP333 reduced the potassium and magnesium contents of potato tubers (Tekalign and Hammes 2005), decreased Cu content in peach trees (Blanco et al. 2002). In our study, all the pretreatments of PP333 significantly reduced Cu contents of shoots and roots in 300 μM l−1 Cu. These results indicated that PP333 could decrease root uptake of Cu, thus decrease the shoot Cu content, and finally caused to alleviate stress of high concentration of Cu.
The endophytic PGPB promoted the growth of two cultivars of guinea grass (GG1 and GG2) not only under Cu stress, but also in non-Cu treat. There were no significant differences in dry biomass between PGPB and PGPB-Cu (Fig. 5). However, as for PP33, which markedly increased tiller number of both cultivars (Fig. 7, data not shown), this might be a major reason for PP33 pretreatment to biomass increasing under Cu stress. As for endophytic PGPB, which promote plant growth caused by a number of similar mechanisms. These include phosphate solubilization activity indole acetic acid production and the production of a siderophore (Verma et al. 2001). Pantoea sp. is an endophytic nitrogen-fixing bacterium isolated from sugarcane tissues (Loiret et al. 2004). Pantoea sp. showed nitrogenase activity in 5 mmol L−1 of serine, asparagine, threonine, alanine, proline, tyrosine, valine, methionine, lysine, phenylalanine, cysteine, tryptophan, citrulline and ornithine (Loiret et al. 2009). The increase in amino acid contents as result of Pantoea sp. inoculation could be related to biological N fixation, metabolic response of the plant or bioactive molecules secreted from the bacteria. Pantoea sp. 18-2 was also found to effectively reduce the acetylene in cultivated rice (Oryza sativa cv. Nipponbare) and wild rice (O. officinalis) (Zakria et al. 2008). Another research reported that Pantoea agglomerans could help in biocontrol of banana pathogens. Our results firstly showed PGPB activities of Pantoea sp. in heavy metal resistance. Besides, certain endophytic bacteria can surely improve host plant’s resistance to heavy metals and application of endophytic bacteria was found to enhance uptake of minerals and concentration of heavy metals (Rajkumar et al. 2009). However, in our present study, Pantoea sp. Jp3-3 significantly reduced Cu uptake by guinea grass, and the efficiency of reduction was similar to PP333 (Fig. 7). Since excessive metals in plant cells inhibited enzyme activities, thus put adverse effects on metabolism (Tandy et al. 2009), therefore, we can concluded that the main regulating function both for PP333 and PGPB alleviated the stress caused by excessive Cu to the plants of guinea grass may be owing to inhibition of Cu uptake caused by pretreatment PP333 or PGPB.
References
Aylott MJ, Casella E, Tubby I, Street NR, Smith P, Taylor G (2008) Yield and spatial supply of bioenergy poplar and willow short-rotation coppice in the UK. New Phytol 178:358–370
Bals B, Rogers C, Jin M, Balan V, Dale B (2010) Evaluation of ammonia fibre expansion (AFEX) pretreatment for enzymatic hydrolysis of switchgrass harvested in different seasons and locations. Biotechnol Biofuels 3:1
Bhuiyan MA, Parvez L, Islam MA, Dampare SB, Suzuki S (2010) Heavy metal pollution of coal mine-affected agricultural soils in the northern part of Bangladesh. J Hazard Mater 173:384–392
Blanco A, Monge E, Val J (2002) Effects of paclobutrazol and crop-load on mineral element concentration in different organs of “Catherine” peach trees. J Plant Nutr 25:1667–1683
Blank AF, de Paula JWA, Arrigoni-Blank MD, Moreira MA (2009) Utilization of paclobutrazol in vetiver for plantlet production and its effect over plants in the field. Hortic Bras 27:425–430
Brewin LE, Mehra A, Lynch PT, Farago ME (2003) Mechanisms of copper tolerance by Armeria maritima in Dolfrwyong Bog, north Wales–initial studies. Environ Geochem Health 25:147–156
Carvalho DD, Irving LJ, Carnevalli RA, Hodgson J, Matthew C (2006) Distribution of current photosynthate in two guinea grass (Panicum maximum Jacq.) cultivars. J Exp Bot 57:2015–2024
Chen SY, Chien CT, Baskin JM, Baskin CC (2010) Storage behavior and changes in concentrations of abscisic acid and gibberellins during dormancy break and germination in seeds of Phellodendron amurense var wilsonii (Rutaceae). Tree Physiol 30:275–284
Wilkins DA (1957) A technique for the measurement of lead tolerance in plants. Nature 180:37
Dubey AK, Srivastav M, Singh AK, Pandey RN (2009) Growth and physiological response of salt-sensitive and salt-tolerant rootstocks of citrus to paclobutrazol under salt stress. Indian J Agric Sci 79:595–599
Escamilla-Trevino LL, Shen H, Uppalapati SR, Ray T, Tang Y, Hernandez T, Yin Y, Xu Y, Dixon RA (2010) Switchgrass (Panicum virgatum) possesses a divergent family of cinnamoyl CoA reductases with distinct biochemical properties. New Phytol 185:143–155
Hajihashemi S, Kiarostami K (2007) Effects of paclobutrazol and salt stress on growth and ionic contents in two cultivars of wheat. Pak J Biol Sci 10:41–48
Hernandez AJ, Pastor J (2008) Relationship between plant biodiversity and heavy metal bioavailability in grasslands overlying an abandoned mine. Environ Geochem Health 30:127–133
Jaleel CA, Manivannan P, Gomathinayagam M, Sridharan R, Panneerselvam R (2007) Responses of antioxidant potentials in Dioscorea rotundata Poir following paclobutrazol drenching. C R Biol 330:798–805
Keshwani DR, Cheng JJ (2009) Switchgrass for bioethanol and other value-added applications: a review. Bioresour Technol 100:1515–1523
Lamb DT, Ming H, Megharaj M, Naidu R (2009) Heavy metal (Cu, Zn, Cd and Pb) partitioning and bioaccessibility in uncontaminated and long-term contaminated soils. J Hazard Mater 171:1150–1158
Li QS, Deng M, Chen JJ, Henny RJ (2009a) Effects of light intensity and paclobutrazol on growth and interior performance of Pachira aquatica Aubl. Hortscience 44:1291–1295
Li Y, Song YP, Shi GJ, Wang JJ, Hou XL (2009b) Response of antioxidant activity to excess copper in two cultivars of Brassica campestris ssp chinensis Makino. Acta Physiol Plant 31:155–162
Lin CH, Chen BS, Yu CW, Chiang SW (2001) A water-based triphenyltetrazolium chloride method for the evaluation of green plant tissue viability. Phytochem Anal 12:211–213
Lin KH, Tsou CC, Hwang SY, Chen LF, Lo HF (2006) Paclobutrazol pre-treatment enhanced flooding tolerance of sweet potato. J Plant Physiol 163:750–760
Loiret FG, Ortega E, Kleiner D, Ortega-Rodes P, Rodes R, Dong Z (2004) A putative new endophytic nitrogen-fixing bacterium Pantoea sp from sugarcane. J Appl Microbiol 97:504–511
Loiret FG, Grimm B, Hajirezaei MR, Kleiner D, Ortega E (2009) Inoculation of sugarcane with Pantoea sp increases amino acid contents in shoot tissues; serine, alanine, glutamine and asparagine permit concomitantly ammonium excretion and nitrogenase activity of the bacterium. J Plant Physiol 166:1152–1161
Lou LQ, Shen ZG, Li XD (2004) The copper tolerance and mechanisms of Elsholtzia haichowensis, a plant from copper-enriched soils. Environ Exp Bot 51:111–120
Ma Y, Rajkumar M, Freitas H (2009) Improvement of plant growth and nickel uptake by nickel resistant-plant-growth promoting bacteria. J Hazard Mater 166:1154–1161
Mahoney SR, Ghosh S, Peirson D, Dumbroff EB (1998) Paclobutrazol affects the resistance of black spruce to high light and thermal stress. Tree Physiol 18:121–127
Maia E, Siqueira DL, Salomao LC, Peternelli LA, Ventrella MC, Cavatte RP (2009) Development of the banana plants ‘Prata Ana’ and ‘FHIA-01’ under the effect of paclobutrazol applied on the soil. An Acad Bras Cienc 81:257–263
Manivannan P, Jaleel CA, Kishorekumar A, Sankar B, Somasundaram R, Panneerselvam R (2008) Protection of Vigna unguiculata (L.) Walp. plants from salt stress by paclobutrazol. Colloids Surf B Biointerfaces 61:315–318
Nouairi I, Ben Ammar W, Ben Youssef N, Ben Miled DD, Ghorbal M, Zarrouk M (2009) Antioxidant defense system in leaves of Indian mustard (Brassica juncea) and rape (Brassica napus) under cadmium stress. Acta Physiol Plant 31:237–247
Parrish DJ, Fike JH (2005) The biology and agronomy of switchgrass for biofuels. Crit Rev Plant Sci 24:423–459
Rahman MM, Chen Z, Naidu R (2009) Extraction of arsenic species in soils using microwave-assisted extraction detected by ion chromatography coupled to inductively coupled plasma mass spectrometry. Environ Geochem Health 31(1):93–102
Rajkumar M, Freitas H (2008) Influence of metal resistant-plant growth-promoting bacteria on the growth of Ricinus communis in soil contaminated with heavy metals. Chemosphere 71:834–842
Rajkumar K, Sivakumar S, Senthilkumar P, Prabha D, Subbhuraam CV, Song YC (2009) Effects of selected heavy metals (Pb, Cu, Ni, and Cd) in the aquatic medium on the restoration potential and accumulation in the stem cuttings of the terrestrial plant, Talinum triangulare Linn. Ecotoxicology 18:952–960
Ram SN (2009) Production potential, biological feasibility and economics of guinea grass (Stylosanthes hamata) intercropping systems under various fertility levels in rainfed conditions. Indian J Agric Sci 79:871–875
Rueda-Puente EO, Murillo-Amador B, Castellanos-Cervantes T, Garcia-Hernandez JL, Tarazon-Herrera MA, Moreno Medina S, Gerlach Barrera LE (2010) Effects of plant growth promoting bacteria and mycorrhizal on Capsicum annuum L. var. aviculare ([Dierbach] D’Arcy and Eshbaugh) germination under stressing abiotic conditions. Plant Physiol Biochem 48:724–730
Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN (2008) Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett 278:1–9
Sankar B, Jaleel CA, Manivannan P, Kishorekumar A, Somasundaram R, Panneerselvam R (2007) Effect of paclobutrazol on water stress amelioration through antioxidants and free radical scavenging enzymes in Arachis hypogaea L. Colloids Surf B Biointerfaces 60:229–235
Sharma D, Awasthi MD (2005) Uptake of soil applied paclobutrazol in mango (Mangifera indica L.) and its persistence in fruit and soil. Chemosphere 60:164–169
Shi G, Cai Q (2009) Cadmium tolerance and accumulation in eight potential energy crops. Biotechnol Adv 27:555–561
Shi GR, Cai QS, Liu QQ, Wu L (2009) Salicylic acid-mediated alleviation of cadmium toxicity in hemp plants in relation to cadmium uptake, photosynthesis, and antioxidant enzymes. Acta Physiol Plant 31(5):969–977
Srivastava J, Kayastha S, Jamil S, Srivastava V (2008) Environmental perspectives of Vetiveria zizanioides (L.) Nash. Acta Physiol Plant 30:413–417
Taghavi S, van der Lelie D, Hoffman A, Zhang YB, Walla MD, Vangronsveld J, Newman L, Monchy S (2010) Genome sequence of the plant growth promoting endophytic bacterium Enterobacter sp. 638. PLoS Genet 6:e1000943
Tandy S, Healey JR, Nason MA, Williamson JC, Jones DL (2009) Heavy metal fractionation during the co-composting of biosolids, deinking paper fibre and green waste. Bioresour Technol 100:4220–4226
Tekalign T, Hammes PS (2005) Growth responses of potato (Solanum tuberosum) grown in a hot tropical lowland to applied paclobutrazol: 2. Tuber attributes. N Z J Crop Hortic Sci 33:43–51
Thakur R, Sood A, Nagar PK, Pandey S, Sobti RC, Ahuja PS (2006) Regulation of growth of Lilium plantlets in liquid medium by application of paclobutrazol or ancymidol, for its amenability in a bioreactor system: growth parameters. Plant Cell Rep 25:382–391
Uddling J, Gelang-Alfredsson J, Piikki K, Pleijel H (2007) Evaluating the relationship between leaf chlorophyll concentration and SPAD-502 chlorophyll meter readings. Photosynth Res 91:37–46
Verma SC, Ladha JK, Tripathi AK (2001) Evaluation of plant growth promoting and colonization ability of endophytic diazotrophs from deep water rice. J Biotechnol 91:127–141
Wang SY, Sun T, Faust M (1986) Translocation of paclobutrazol, a gibberellin biosynthesis inhibitor, in apple seedlings. Plant Physiol 82:11–14
Xing W, Huang WM, Liu GH (2010) Effect of excess iron and copper on physiology of aquatic plant Spirodela polyrrhiza (L.) Schleid. Environ Toxicol 25:103–112
Zakria M, Udonishi K, Ogawa T, Yamamoto A, Saeki Y, Akao S (2008) Influence of inoculation technique on the endophytic colonization of rice by Pantoea sp isolated from sweet potato and by Enterobacter sp isolated from sugarcane. Soil Sci Plant Nutr 54:224–236
Zhao FJ, Jiang RF, Dunham SJ, McGrath SP (2006) Cadmium uptake, translocation and tolerance in the hyperaccumulator Arabidopsis halleri. New Phytol 172:646–654
Zhao FJ, McGrath SP, Merrington G (2007) Estimates of ambient background concentrations of trace metals in soils for risk assessment. Environ Pollut 148:221–229
Acknowledgments
We thank the financial support from Natural Science Foundation of China (40971296) and Nanjing Agricultural University Student Research Training (SRT) Fund (0906A23). The authors thank Dr. Mike Zhang for his sincere advice during preparation and correction of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B. Barna.
Rights and permissions
About this article
Cite this article
Huo, W., Zhuang, Ch., Cao, Y. et al. Paclobutrazol and plant-growth promoting bacterial endophyte Pantoea sp. enhance copper tolerance of guinea grass (Panicum maximum) in hydroponic culture. Acta Physiol Plant 34, 139–150 (2012). https://doi.org/10.1007/s11738-011-0812-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-011-0812-y