Abstract
With the rapid adoption of robotics in colorectal surgery, there has been growing interest in the pace at which surgeons gain competency, as it may aid in self-assessment or credentialing. Therefore, we sought to evaluate the learning curve of an expert laparoscopic colorectal surgeon who performed a variety of colorectal procedures robotically. This is a retrospective review of a prospective database of 111 subsequent colorectal procedures performed by a single colorectal surgeon. The cumulative summation technique (CUSUM) was used to construct a learning curve for robotic proficiency by analyzing total operative and console times. Postoperative outcomes including length of stay, 30-day complications, and 30-day readmission rates were evaluated. Chi-square and one-way ANOVA (including Kruskal–Wallis) tests were used to evaluate categorical and continuous variables. Our patient cohort had a mean age of 62.4, mean BMI of 26.9, and mean ASA score of 2.41. There were two conversions to open surgery. The CUSUM graph for console time indicated an initial decrease at case 13 and another decrease at case 83, generating 3 distinct performance phases: learning (n = 13), competence (n = 70), and mastery (n = 28). An interphase comparison revealed no significant differences in age, gender, BMI, ASA score, types of procedures, or indications for surgery between the three phases. Over the course of the study, both mean surgeon console time and median length of stay decreased significantly (p = 0.00017 and p = 0.016, respectively). Although statistically insignificant, there was a downward trend in total operative time and postoperative complication rates. Learning curves for robotic colorectal surgery are commonly divided into three performance phases. Our findings contribute to the construction of a reliable learning curve for the transition of colorectal surgeons to robotics. Furthermore, they may help guide the stepwise training and credentialing of new robotic surgeons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, the adoption of the da Vinci robot (Intuitive Surgical, Inc., Santa Clara, CA, USA) for colorectal surgery has grown exponentially. Studies have shown that robotic surgery offers comparable short term and oncological outcomes to those of laparoscopic surgery, with lower conversion rates [1,2,3,4]. While there are abundant reports on outcomes of robotic colorectal surgery, there is limited data regarding the rate at which surgeons gain competency with this modality, especially in the colorectal field. Mapping the process of developing mastery in robotic surgery offers insight into how the transition from laparoscopic to robotic techniques in surgical departments can be facilitated.
A surgeon’s mastery is often measured by a surgical learning curve. The surgical learning curve is the time taken and/or the number of cases an average surgeon takes to attain surgical proficiency [5]. Existing learning curve studies for the use of robotics in colorectal surgery are limited to robotic-assisted rectal surgery, with much less data on learning curves for the broader range of robotic colorectal surgeries. Therefore, in our study, we aimed to evaluate the learning curve of a single expert laparoscopic surgeon’s experience with a variety of robotic colorectal procedures. The cumulative sums (CUSUM) of operative and surgical console times from consecutive robotic procedures were used to evaluate the learning curve of robotic colorectal procedures. Various preoperative, intraoperative, and postoperative factors were then compared between the different phases of the learning curve to identify and evaluate potential differences.
Methods
A retrospective review of a prospective colorectal surgery data registry was conducted. Non-emergent robotic colorectal surgery cases between February 2015 and July 2019 were examined. Surgeries were performed by a single double board-certified general and colorectal surgeon (YN). The surgeon completed a general surgery residency (2011) and colorectal fellowship (2012) during which he had advanced laparoscopic training though minimal exposure to robotic surgery. Prior to robotic surgery, he has performed over 250 laparoscopic colectomies (right, left, transverse, rectopexy, low anterior resection, abdominoperineal resections) that featured advance laparoscopic skills including intra-corporeal suturing and never with a hand-assist device. The surgeon’s robotics training consisted of multiport technology modules at Intuitive Surgical Headquarters followed by three proctored cases and two case observations. One of the proctored cases was on the Si system and the other two on the Xi system. The first case observation was on the Si (University of California-Irvine Medical Center, 8/14/2012) and the second on the Xi (Oklahoma Surgical Hospital, 3/28/2016). The second observation was an advanced 1-day course/observation of a very experienced colorectal surgeon on the Xi system. The surgeon performed various types of colorectal resections robotically over this study period.
Case types in our study included right colectomy, transverse colectomy, left colectomy, low anterior resection, abdominoperineal resection, subtotal or total abdominal colectomy, proctocolectomy, and rectopexy. Demographics (age, sex, BMI, and ASA score), indications for surgery, types of operations, intraoperative data (console time, total operative time and blood loss), and 30-day postoperative outcomes (GI/overall complications, length of stay and readmission) were analyzed. All surgeries were performed using the Xi da Vinci multiport system. The model of the robotic system used was consistent throughout the study. Surgical console time corresponds to the total time the surgeon spends on the robotic console and total operative time is measured from the time of initial incision to the end of the closure of all incisions. Total operative time (at times significantly longer than total console time) includes time from first incision to the closing of the last incision. This includes: trocar placement, lysis of some adhesions laparoscopically in areas of trocars to facilitate trocar placement, docking and undocking of robot, specimen extraction, placing the anvil of an EEA stapler through the extraction site and performing colorectal anastomosis with an EEA stapler with laparoscopic guidance, placement of drains, injection of TAP (transversus abdominis plane) blocks, and any delays caused by nursing and anesthesia. Overall postoperative complications consist of surgical site infection, urinary retention, clostridium difficile colitis, stoma complications, deep venous thrombosis, pneumonia, urinary infection, acute renal failure, pulmonary embolus, myocardial infarction, ICU stay, reoperation, and death. Gastrointestinal (GI) complications include small bowel obstruction, ileus, anastomotic leak, and abdominal/pelvic abscess.
CUSUM
Cumulative sum (CUSUM) is a sequential analysis technique developed by Cambridge University to detect shifts in the process mean. The technique is commonly applied in medicine to approximate learning curves by determining the cumulative change between subsequent data points and the average. Upward and downward slopes demonstrate measurable changes and can signify a consistent trend over a series of data points. If there is no consistent change, the CUSUM graph would be sporadic around the zero line with no pronounced deviations.
For this study, CUSUM graphs were generated from console time and total operative time. Learning curve phases were determined from the console time CUSUM graphs as console time is typically more reflective of the surgeon’s independent improvement compared to total operative time, which may be confounded by other variables.
Statistical analysis
Continuous variables were expressed as means and standard deviations and were compared between phases using a two sample Student’s t test. Categorical variables were expressed as percentages and compared using the Chi-square test. Statistical significance was set at α = 0.05.
Results
One hundred and eleven consecutive patients (56 males, 50.4%) with a mean age of 62.4 and a mean BMI of 26.9 were studied. There were two conversions to open surgery: one due to uncontrolled bleeding from an aortic branch and the second for failure to progress due to locally advanced cancer to the abdominal wall, retroperitoneum, and bladder. These two cases were not included in the final analysis of 111 cases. For the entire cohort, the mean total operative time was 231.3 min and mean robotic console time was 131.0 min. Mean operative blood loss was 100.3 mL. Mean and median postoperative length of stay was 8.4 days (range 2–138) and 4 days respectively. Ten (9.01%) patients were readmitted within 30 days.
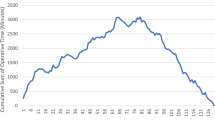
The console time CUSUM graph (Fig. 1) identified changes in slope at cases 13 and 83, which divided the learning curve into 3 distinct phases. The slope of the CUSUM curve is positive from cases 1–13, plateaus between cases 14–83, then is negative from cases 84–111. The three phases generated correspond to the learning, competency, and mastery phases delineated in previous literature on surgical learning curves. These trends were replicated in the CUSUM for total operative time (Fig. 2). The three phases of the total operative time mirror the three phases observed in the console time with the exception of being misaligned by a few cases.
Table 1 summarizes preoperative patient characteristics by phase. There were no significant differences in gender, BMI, or types of surgery performed across groups. However, patients in the initial learning phase were significantly older (p = 0.034) and had higher ASA scores (p = 0.039). The learning phase had significantly fewer patients with an ASA score of 2 (23.1%) compared to patients of the competency (53.3%, p = 0.04) and mastery phases (65.5%, p = 0.01). However, there were significantly more ASA score three patients in the learning phase (76.9%) than in the mastery phase (32.1%, p = 0.007). Table 1 also compares the preoperative indications for surgery across phases, which were found to be statistically insignificant (p value = 0.37).
Table 2 compares intraoperative data and postoperative outcomes across phases. There was a near significant decrease in operative time and a highly significant decrease in console times (p value = 0.051 and 0.0016, respectively). The initial learning phase had a mean total operative time of 282 min, which decreased to 220 in the competency phase, and slightly rose to 236 in the mastery phase. Mean console time decreased from 194 min to 128 to 107 across the three phases. Mean estimated blood loss also decreased, but was statistically insignificant (p value = 0.10). Mean and median postoperative length of stay decreased as the phases progressed and were statistically significant (p value = 0.039 and 0.016, respectively). There were no statistically significant trends identified in postoperative GI or overall complications.
Discussion
Given the rapid implementation of robotics in colorectal surgery, it is crucial to understand a surgeon’s capacity to transition to a robotic surgical approach. By evaluating a surgeon’s learning curve, the relationship between the mastery of robotic surgery and the number of consecutive robotic cases performed can be better understood.
In our study, we observed three phases: learning, competency, and mastery phases based on the console time learning curve. In the initial phase, we see a consistent positive slope until case 13, after which the curve plateaus. During this period, the surgeon was gaining familiarity with the technical aspects of the da Vinci robotic system such as docking, ergonomics, and instrumentations. Consequently, this phase was associated with the longest console and operative times, as well as greater adverse postoperative events (most notably length of stay). As the surgeon became increasingly comfortable with the logistics of operating the robot, he entered the competency phase and his performance outcome gradually improved and eventually leveled off. The plateau persists until case 83, suggesting a stable performance at around average time. A consistent negative slope is observed from case 83 onwards, representing a decrease in total operative time and a significant decrease in console time compared to his average. This continuous downward trend suggests gradual improvement as the surgeon became more experienced and entered the mastery phase. A comparison of the different phases demonstrated a significant decrease in mean and median length of stay. As of case 111, the learning curve still has not plateaued, suggesting that the surgeon may still be improving. The first and third change in slope for the total operative time learning curve generally corresponds with the trends in the slope of the console time learning curve, albeit a few cases delayed for the total operative time. The difference between the console time and total operative time curves may be due to the variation in the surgical team. Total operative time is reflective of not only the surgeon’s performance but also of the scrub technologist, nurses, and anesthesiologist. Therefore, a surgeon’s improvement must be more substantial for it to be reflected in the total operative time.
Table 3 summarizes the number of patients and types of procedures included in previous robotic learning curve studies. Our results are similar to those found in these studies. Park et al. [6] evaluated the learning curve of an experienced surgeon who performed 130 robotic low anterior resections for rectal cancer. In their CUSUM analysis, they observed a three-phase curve including an initial learning phase (cases 1–44), competency phase (cases 45–78), and challenge phase (cases 79–130). The authors found a decreasing trend in mean operative and console times across these three phases. However, they did not find any significant differences in perioperative outcomes, postoperative complications, length of stay, or pathological outcomes across the three learning phases.
Sng et al. also analyzed the learning curve of an experienced laparoscopic surgeon performing robotic-assisted rectal procedures. They included a broader range of rectal procedures and, to our knowledge, was the largest study, consisting of 197 patients whose procedures were performed by a single surgeon. A CUSUM analysis of the robotic console time showed a three-phase learning curve. Phase 2 and 3 were shown to have a significant decrease in console time compared to phase 1. Unlike our results, they observed a significant increase in length of stay across the three phases. The authors attributed this finding to the increasing complexity of cases the surgeon took on during the later phases [7]. Despite the advantage of having a large study size, the procedures investigated were limited to rectal procedures. This limitation applies to the majority of existing studies, as shown in Table 3.
Of the existing robotic learning curve studies, only Bokhari et al. examined a greater variety of colorectal cases performed by one experienced laparoscopic surgeon. Their study included all pelvic colorectal cases with the addition of rectopexy to abdominoperineal resection, anterior resection, and low anterior resection. However, colorectal operations that were completely outside of the pelvis were excluded. All 50 cases were robotic-assisted laparoscopic. CUSUM analysis of the console time also yielded 3 phases: initial learning (first 15 cases), increased competency (middle 10 cases), and mastery (remaining 25 cases) [5]. In comparison to the initial phases, the mastery phase had significant decreases in docking time and estimated blood loss, but increases in console and operative times. Bokhari et al. [8] attributed the increase in time to a greater number of difficult cases during that period. There were no significant differences in other intraoperative parameters and postoperative outcomes across the phases.
More recent robotic colorectal learning curve studies have all noted three distinct performance phases. Many of these studies, like Bokhari et al., indicated that the surgeons began accepting more difficult cases as they progressed. In the Foo and Law study of 39 robotic-assisted resections performed by a single surgeon, the surgeon advanced in the mastery phase to total robotic cases from the initial robotic-assisted procedures [9]. Shaw et al. [10] assigned and tracked complexity levels of their cases and determined that a greater number of their later cases were of the highest level complexity. Thus, many studies have attributed an increase in console time during the mastery phase to an increase in difficult cases. However, given their smaller study size, the cut-off point for the true mastery phase may not have been achieved yet.
In our study, there were no significant differences amongst the types or complexity of procedures performed on patients across our three identified phases. Thus, the decreasing operative and console times observed as our surgeon progressed to mastery are a product of his increasing competence, rather than a function of case complexity. We believe this allows for a more accurate and representative learning curve for colorectal surgeons performing total robotic procedures.
Our study has several strengths. To our knowledge, it is the biggest study of the robotic learning curve for an expert laparoscopic colorectal surgeon who performs a variety of robotic colorectal surgeries. It specifically addresses robotic learning curve in a surgeon well versed and skilled in total laparoscopic surgery which included intra-corporeal suturing. We believe that inclusion of variety of cases is more reflective of real-life adoption of robotic surgery by a surgeon and presents a more accurate analysis of the learning curve. Most surgeons introducing robotic surgery into their practice are likely to incorporate it into several types of operations. The cases included were consecutive and inclusive of all robotic surgeries performed by the surgeon. Learning curve of a specific operation can be skewed as it does not take into account proficiency gained on the robot during other procedures interspersed between the specific procedure being studied. Although, we believe that since the skills used in various colorectal procedures overlap, we are accounting for overall competency on the robot gained by performing a variety of procedures. We were unable to perform a subgroup analysis of the procedures because the numbers in each group would be too small. Moreover, our study consists of almost exclusively total robotic cases while previous studies only examined robotic-assisted cases.
However, there are some limitations to our study. Significant differences in the age and ASA scores of the patient groups could have confounded results, such as postoperative outcomes and length of stay. Nevertheless, since these demographics do not directly correlate with the technical difficulty of the surgery, we do not believe that these differences resulted in unreliable measures of console time. Additionally, case variety in our analysis may be a weakness. Heterogeneity of the cases potentially confounds our results as each case type has a unique set of steps, instrument use, and certain skill sets, such as intra-corporeal suturing. Nevertheless, the case mix was not significantly different across the three phases and represents a more realistic adoption of robotics by a colorectal surgeon. We were unable to account for other potential confounding variables such as experiences of the operating room staff members, which may have affected operative outcomes. Experienced staff facilitates various tasks, such as docking of the robot or handling instrument exchanges. Finally, we believe that this study is worth revisiting in the future with a larger number of patients to determine if the last phase of the curve eventually plateaus.
Our study contributes to the growing pool of research on robotic learning curves. Elucidating approximate checkpoints for competency and mastery of cases would potentially help establish credentialing guidelines and aid in evaluating the progress of transitioning or newly trained robotic surgeons. This study serves as a framework for evaluating the true outcomes and efficacy of robotic surgery, especially when comparing its advantages to laparoscopic procedures.
Conclusion
The learning curve for total robotic colorectal surgery can be divided into three phases. The initial learning phase was comprised of the first 13 cases, while mastery was achieved after 83 cases. This graph can potentially be used as a reference when training and credentialing new robotic surgeons.
Data availability
Not applicable.
Code availability
Not applicable.
References
Zelhart M, Kaiser AM (2018) Robotic versus laparoscopic versus open colorectal surgery: towards defining criteria to the right choice. Surg Endosc 32(1):24–38. https://doi.org/10.1007/s00464-017-5796-2
Catanzarite T, Tan-Kim J, Whitcomb E, Menefee S (2018) Ergonomics in surgery: a review. Female Pelvic Med Reconstr Surg 24(1):1–12. https://doi.org/10.1097/SPV.0000000000000456
Köckerling F (2014) Robotic vs. standard laparoscopic technique—What is better? Front Surg 1:15. https://doi.org/10.3389/fsurg.2014.00015
Bhama AR, Obias V, Welch KB et al (2016) A comparison of laparoscopic and robotic colorectal surgery outcomes using the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database. Surg Endosc 30(4):1576–1584. https://doi.org/10.1007/s00464-015-4381-9
Guend H, Widmar M, Patel S et al (2017) Developing a robotic colorectal cancer surgery program: understanding institutional and individual learning curves. Surg Endosc 31(7):2820–2828. https://doi.org/10.1007/s00464-016-5292-0
Park EJ, Kim CH, Cho MS et al (2014) Multidimensional analyses of the learning curve of robotic low anterior resection for rectal cancer: 3-phase learning process comparison. Surg Endosc 28(10):2821–2831. https://doi.org/10.1007/s00464-014-3569-8
Sng KK, Hara M, Shin J et al (2013) The multiphasic learning curve for robot-assisted rectal surgery. Surg Endosc 27(9):3297–3307. https://doi.org/10.1007/s00464-013-2909-4
Bokhari M, Patel C, Ramos-Valadez D, Ragupathi M, Haas E (2011) Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg Endosc 25(3):855–860. https://doi.org/10.1007/s00464-010-1281-x
Foo C, Law WL (2016) The learning curve of robotic-assisted low rectal resection of a novice rectal surgeon. World J Surg 40(2):456–462. https://doi.org/10.1007/s00268-015-3251-x
Shaw D, Wright M, Taylor L, Bertelson N et al (2018) Robotic colorectal surgery learning curve and case complexity. J Laparoendosc Adv Surg Tech 28(10):1163–1168. https://doi.org/10.1089/lap.2016.0411
Parisi A, Scrucca L, Desiderio J, Gemini A, Guarino S et al (2017) Robotic right hemicolectomy: analysis of 108 consecutive procedures and multidimensional assessment of the learning curve. Surg Oncol 26(1):28–36. https://doi.org/10.1016/j.suronc.2016.12.005
Yamaguchi T, Kinugasa Y, Shiomi A et al (2015) Learning curve for robotic-assisted surgery for rectal cancer: use of the cumulative sum method. Surg Endosc 29(7):1679–1685. https://doi.org/10.1007/s00464-014-3855-5
Jiménez-Rodríguez R, Díaz-Pavón JM, Portilla de Juan F et al (2013) Learning curve for robotic-assisted laparoscopic rectal cancer surgery. Int J Colorectal Dis 28(6):815–821. https://doi.org/10.1007/s00384-012-1620-6
Funding
No relevant funding to disclose.
Author information
Authors and Affiliations
Contributions
Not applicable.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Yosef Nasseri is a key opinion leader for Intuitive. Isabella Stettler, Wesley Shen, Ruoyan Zhu, Arman Alizadeh, Anderson Lee, and Drs. Jason Cohen and Moshe Barnajian have no conflicts of interest to disclose.
Ethical approval
This research study was conducted retrospectively from data obtained for clinical purposes. We consulted extensively with the IRB of Cedars-Sinai Medical Center who approved the study.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nasseri, Y., Stettler, I., Shen, W. et al. Learning curve in robotic colorectal surgery. J Robotic Surg 15, 489–495 (2021). https://doi.org/10.1007/s11701-020-01131-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-020-01131-1