Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a highly infectious virus that belongs to the RNA virus family Coronaviridae, infects a variety of animal species, and replicates primarily in the lower and upper respiratory tract. A proportion of 90% of infections are uncomplicated, and 4–7% of people are hospitalized. The main focus is on people with comorbidities, which are risk factors for severe disease and lead to high rates of hospitalization and death. During the early stages of a pandemic, many diagnostic approaches such as biochemical, serological, and molecular investigations are used to detect SARS-CoV-2. This review emphasizes immunodiagnostic techniques, including virus neutralization assay, CRISPR, nicking endonuclease amplification reaction, lateral flow immunoassay, protein-peptide microarray, and chemiluminescence immunoassay; it also includes novel approaches such as next-generation sequencing. Nanotechnology is critical in the prevention, diagnosis, and treatment of SARS-CoV-2, with an in-depth review of its principles, usefulness, benefits, and drawbacks. The study sheds light on diagnostic methods that apply to future infectious viruses as well as SARS-CoV-2. Furthermore, the article explores diverse nano-based treatments, including vaccines, immunotherapy, and gene therapy, providing encouraging methods for the prevention and management of SARS-CoV-2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of the new 2019 coronavirus disease (COVID-19), which belongs to the large RNA viruses, i.e., the Coronaviridae family, which affects a variety of mammals (Nickbakhsh et al. 2020 and Bhattacharjee et al. 2023). The SARS-CoV-2 virus causes a wide spectrum of clinical symptoms, ranging from asymptomatic illness to severe pneumonia, short-lived flu-like symptoms, and acute respiratory distress syndrome. It is an extremely contagious virus that replicates mainly in the lower and upper respiratory tracts and is transmitted primarily by aerosols and droplets (Kang et al. 2020).
In general, 90% of infections are uncomplicated, have mild symptoms, or are oligosymptomatic, not requiring hospitalization. However, hospitalization is required in 5–10% of cases, including patients with comorbidities, such as hypertension, chronic heart or lung failure, diabetes mellitus, older age, and immunodeficiency (Salzberger et al. 2021 and Sahu et al. 2021). SARS-CoV-2 is structurally similar to SARS-CoV, middle respiratory syndrome coronavirus (MERS-CoV), and other coronaviruses of living organisms (Singh and Yi 2021).
Coronavirus consists of approximately 30,000 nucleotides and four structural proteins, namely the envelope protein (E), membrane protein (M), nucleocapsid protein (N), and spike protein (S) encoded by this gene, as well as many non-structural proteins (NSP) (Fig. 1). The viral capsid is a type of protein shell that contains a core capsid commonly referred to as a nuclear protein. It is linked to the positive single strand of the virus’s RNA, allowing it to enter living tissue and transform it into a viral casting unit (Boopathi et al. 2019). SARS-CoV-2 induces the angiotensin-converting enzyme 2 (ACE2) surface receptor to enter the living tissue of the host cell (Sarma et al. 2021). Table 1 provides information about the variants of SARS-CoV-2 and their structural differences.
The viruses and their characteristics were obtained from a variety of patient samples, including nasal and throat samples, mucus, saliva, plasma, and serum. Numerous techniques are used to analyze SARS-CoV-2 in the initial phase of the pandemic, such as monitoring body temperature (fever), as this is associated with SARS-CoV-2 symptoms. However, even individuals undergoing thermal screening are positive in confirmatory analytical procedures (Schultz et al. 2023). There are several diagnostic approaches to detect the presence of SARS-CoV-2 that can make the results more accurate. Biochemical testing is among the methods that can improve diagnosis. The tests include C-reactive protein (CRP), complete blood count, and cytokines; however, a major drawback is that they are not highly specific (Ye et al. 2022). To determine the target component of SARS-CoV-2, both molecular and serological diagnostic methods are used. The most widely used molecular diagnostic tests for the diagnosis of SARS-CoV-2 are polymerase chain reaction (PCR), real-time quantitative reverse transcription PCR (qRT-PCR), computed tomography (CT), and reverse transcription loop-mediated isothermal amplification (RT-LAMP). In contrast to SARS-CoV-2, the detection of viral antibodies (IgM/IgG) and antigens in serological tests, such as enzyme-linked immunosorbent assay (ELISA), lateral flow immunosorbent assay (LFIA), and biosensor-based diagnostics, as well as CRP in clinical samples, is used to determine the stage of the disease (Hazra and Patra 2023 and Xiao et al. 2023). The major drawback is that antibodies are slow to form in response to the SARS-CoV-2 virus (D. f. Li et al. 2023). In this regard, serological diagnostic systems provide not only extremely sensitive and specific reports but also faster reports, cost-effectiveness, simplicity, and ease of use, making them an excellent alternative for biosensor operations (Salahandish et al. 2023). After the onset of infection, IgM antibodies can be found in the acute phase. In secondary infection, higher levels of viral IgG and IgM antibodies can be detected, and the results of detecting the amount of IgM and IgG antibodies in the blood sample, along with viral RNA, can provide a minute indication of the extent of SARS-CoV-2 contamination and resistance in an individual. The diagnostic methods for SARS-CoV-2 are serologic and molecular systems using clinical specimens containing viral proteins or antigens, immunoglobulins/antibodies (IgM, IgG), and RNA unique to SARS-CoV-2 (Salahandish et al. 2023). Lymphopenia is an example of an immunological abnormality that can be used to predict the health status of a SARS-CoV-2-diagnosed individual. Table 2 provides an overview of existing methods for detecting SARS-CoV-2 and immunological techniques.
In addition, there are other promising techniques, such as CRISPR (clustered regularly interspaced short palindromic repeats), surface-enhanced Raman scattering, next-generation sequencing, vertical flow assay, amplicon-based metagenomic sequencing, nicking endonuclease amplification reaction (NEAR), virus neutralization test (VNT), and nano-based techniques, such as colorimetric assays, microfluidic devices, magnetic nanoparticle-based separation, gold nanoparticles, nucleic acid-based biosensors, antibody-based biosensors, and surface plasmon resonance (Truong et al. 2023 and Simon et al. 2023). Nano-based particles have been developed to target viral infections due to their unique physicochemical properties, such as nanoscale dimensions, easily accessible surface modifications, and greater surface proximity. Nano-based particles have been used as drug delivery systems for the therapy of coronavirus infections. Different types of nanoparticles (NPs) can be used as nanomedicines, such as nano-based vaccines, nano-based gene therapy, and nano-based immunotherapy against coronaviruses (McNamee et al. 2023 and Li et al. 2023). The current review mainly focuses on the various available diagnostic approaches, i.e., molecular, immunological, and platforms under development, as well as the future prospects with respect to techniques for the detection of SARS-CoV-2. In addition, a holistic overview of nano-based therapies against SARS-CoV-2 infections is provided.
The data for the current study were acquired from renowned academic databases, including Web of Science, Scopus, PubMed, and Google Scholar, and covers the publication period from 2020 to 2023. The following keywords used such as “SARS-CoV-2 and Diagnostic Tools”, “Nano-based Treatment”, and “Nano-based Vaccines” to get the most relevant articles. Initially, 692 articles were found based on our search keywords. We evaluated the information for preliminary assessment based on the abstract and title of each publication. We deducted all redundant and withdrawn articles, followed by the deduction of non-English-language, paid articles and research paper with no outcome. A considerable number of publications related to SARS-CoV-2 were found, and we limited ourselves to a selection of 200 articles.
Traditional laboratory diagnostic tools for SARS-CoV-2 recognition
Virus culture
Viral culture can be carried out using standard methods. Vero cells were used to inoculate nasopharyngeal and oropharyngeal samples, which were cultured at a temperature of 37 °C with 5% CO2 in Dulbecco’s modified Eagle medium in combination with 2% fetal serum (bovine) (Park et al. 2023a). The particular cytopathic effects were detected after 3 days of inoculation. Later, real-time RT-PCR was utilized to confirm these effects. By inoculating broncho-alveolar lavage (BAL) samples, researchers from Wuhan, China, were able to isolate 2019-nCoV from human airway Vero E6, Huh-7 cell lines, and epithelial cells (Dastoorpoor et al. 2023). Although the isolation of viruses from human airway epithelial cell cultures is a time-consuming process, it has been proven to be extremely promising for the analysis of human respiratory infections (Subramaniyan et al. 2023). Using Vero CCL-81 cells, an Indian team recently announced the first isolation of SARS-CoV-2 (Kumar et al. 2023). The inoculated cells were observed for particular cytopathic effects for SARS-CoV-2 using nasopharyngeal and oropharyngeal samples, then fixed, dried, and cut into segments for transmission electron microscopy using conventional methods. Coronavirus-specific morphology was discovered, as well as virus particle sizes ranging from 70 to 90 nm. The virus was also revealed to be present in a variety of intracellular organelles, including vesicles. The method’s decision to choose real specimens relying on the spread of virus loads seen throughout prognostic testing has both strengths and weaknesses, where the clinical features and infections were not taken into consideration while choosing samples, and it was likely that the specificities found for PCR-positive samples may have been overstated since PCR-positive and PCR-negative samples were assessed independently (Park et al. 2023a).
Reverse transcription polymerase chain reaction (RT-PCR)
Currently, available SARS-CoV-2 pandemic diagnostic approaches focus on nucleic acid, protein-based, and antibody detections; however, RT-PCR has been accepted as the ideal reference for pinpointing viral nucleic acids (Du and Wang 2023). Nucleic acid assays provide superior precision and accuracy relative to currently accessible serological investigations, enabling virus identification. In February 2020, the US Food and Drug Administration (FDA) authorized licensed laboratories to undertake SARS-CoV-2 diagnostic tests (World Health Organization 2020). The initial stage in the procedure is to isolate viral RNA and then convert it to cDNA (complementary DNA). The biological enzyme Taq DNA polymerase is used to expand the cDNA. The overall turnaround time can be as long as 2 days, with the danger of cross-contamination, which reduces specificity. In most cases, the tests are carried out in hospital laboratories (Islam et al. 2023). Variations in viral RNA sequencing can affect real-time RT-PCR findings, utilizing markers that target several virus-related genomic sections. Moreover, false-negative reports may occur as a result of virus progression (Bedoya-Joaqui et al. 2023). The challenges and weaknesses of RT-PCR techniques include specimen retention, inadequate extraction of nucleic acid, cost, and delayed results; even with these drawbacks, the RT-PCR technique is still the preferred tool for diagnosing SARS-CoV-2. Figure 2 shows a schematic workflow of the RT-PCR assay starting with the naso-/oropharyngeal swab, taken from the subject which can be stored at 2–8 °C for 3 days and followed by extraction and purification of RNA. The purified RNA is then transcribed reversibly to DNA using specific enzymes and markers in an RT-PCR machine. If a positive result from the amplification is obtained, then the SARS-CoV-2 virus is present (Cui et al. 2023).
Reverse transcription loop-mediated isothermal amplification (RT-LAMP)
Reverse transcription loop-mediated isothermal amplification (RT-LAMP) is an additional nucleic acid screening approach used to identify SARS-CoV-2. The RT-LAMP approach uses four to six oligos (oligonucleotides), DNA polymerase, and reverse transcriptase of a specific pattern to amplify nucleic acid in a single phase. Turbidity, fluorescence, and colorimetric measurements are employed to identify LAMP-based monitoring procedures. The methodology is lucid to construct and anticipate, and it produces negligible background distortion. Interpretation, experience, and reaction optimization are the key limits of LAMP testing (Moulahoum et al. 2021). The indication readout capabilities of EvaGreen were larger than those of SYBR® Green of the two fluorescent dyes examined (Zhu et al. 2020). RT-LAMP is a viral diagnostic system based on strips/paper incorporated as part of a microfluidic platform (Augustine et al. 2020). In the experiment, fluorescein was assigned to one primer set, and the reaction was catalyzed by labeled RT (Zhu et al. 2020). To develop a perceptible violet pigment with a leucocrystal violet dye, an alternate approach for LAMP reliably identified SARS-CoV-2, allowing for the detection of 100 duplicates per response. The limit of detection of the LAMP assay can be extended by deploying a closed-unit Penn-RAMP, which integrates RT-recombinant polymerase amplification and RT-LAMP in the same unit (Moulahoum et al. 2021 and Song et al. 2021). The strength of RT-LAMP is that LAMP is more proper than RT-PCR for analyzing a pandemic because it has certain intrinsic features over RT-PCR, such as proliferation at a static temperature, absence of a thermal cycler, a rapid test response, and perhaps a broader diagnostic capability. The RT-LAMP assay sequence is illustrated schematically in Fig. 3. The results of the first three phases of the RT-LAMP technique can be used as a model for LAMP system response. In phase (i) of Fig. 3, LAMP reagents, such as avian myeloblastosis virus (AMV) transcriptase, polymerase (Bst 2.0), and deoxyribose adenosine triphosphate (dATP), are employed to create the amplification solutions. The interaction of LAMP reagents with fluorescein isothiocyanate (FITC)-labeled open reading frame 1a/b (F1ab) forward loop primer (LF) (F1ab-LF*) and biotin-labeled nucleoprotein (np)-backward loop primer (LB)(np-LB*) initiates the isothermal amplification in phase (ii). In phase (iii), traceable SARS-COV-2RT-LAMP solutions are offered. FITC/biotin-labeled F1ab-LAMP and FITC/biotin-labeled np-LAMP amplicons, as well as the results of labeling np-LB* and np-LF* or F1ab-LB* and F1ab-LF* for biotin and digoxigenin, are displayed in phase (iii). The F1ab primer set, on the other hand, is tagged with FITC, and the np-RT–LAMP product is measured with biotin, as well as digoxigenin, whereas the F1ab-RT-LAMP result is tagged with biotin and FITC (Song et al. 2021).
Saliva testing
The presence of SARS-CoV-2 RNA in saliva studies, particularly in nasopharyngeal samples, is essentially irrelevant to the etiology of the disease. On the other hand, human saliva is becoming increasingly popular as a diagnostic tool for the diagnosis of infection (Ning et al. 2021). Sputum is a noninvasive and easy-to-use sampling method. Unfortunately, a weakness in the test is that 72% of SARS-COV-2 patients are unable to provide an adequate sample volume (Galderisi et al. 2023). Saliva is a valuable biological medium because it contains nucleic acids, proteins, hormones, and electrolytes derived from a variety of local or systemic sources. Saliva contains approximately 30% of the biomolecules that are also found in blood, which can be used in the diagnosis of diseases and infections caused by microbes and viruses (Miller et al. 2021). In addition, it has a benefit, as the saliva samples can be stored in emollient solutions and sent to the testing center many days later (Johannsen et al. 2021). The use of RT-PCR to detect respiratory infections in saliva, including two seasonal human coronaviruses, has resulted in matches (Bogdan et al. 2023). Figure 4 shows a schematic workflow of a saliva test. A sample of the subject’s saliva is collected. The saliva sample is processed to obtain gene material (RNA) tagging with biomarker for identification and then run for the test. A positive result from the test for viral RNA indicates the sample of saliva contains the SARS-CoV-2 virus.
Fecal test
When nasopharyngeal samples were virus-negative, a high prevalence and viral persistence in feces were reported (Xiao et al. 2020). It is worth noting that the viral strain can be determined in stool specimens up to one month after the commencement of the infection. The dangers of healthcare workers being exposed to the feces of diseased patients are well-established, especially in operations that generate a lot of aerosols. Whereas cough and fever are well-known symptoms, confirmed gastrointestinal symptoms suggest fecal–oral transmission pathways (Nobel et al. 2019). The European Centre for Disease Prevention has urged continued self-isolation based on the persistent virus detaching in feces and respiratory samples 14 days after release. According to studies, the live virus may also be isolated from feces samples, which suggests fecal–oral transmission. SARS-CoV-2 can be tracked through wastewater, which enables community monitoring and might be beneficial in tracking SARS-CoV-2 spread (Xiao et al. 2020).
Radiographic testing
The main diagnostics tool for SARS-CoV-2 is viral nucleic acid RT-PCR testing based on qualitative and quantitative analysis, although their sensitivity for nasal (63%) and oropharyngeal (32%) swab samples is still low (Wang et al. 2020a). As a result, suspected cases, whether or not they have been tested by RT-PCR, require further confirmation. The combinatorial radiographic and antigen-based molecular strategies are the leading methods of assessment of SARS-CoV-2 in research prospects (Hosseiny et al. 2019). Following the onset of breathing complications and the identification of SARS-CoV-2, a patient’s first examination generally includes a radiographic imaging assessment, which includes diagnostics techniques such as chest X-ray (CXR) (Liu et al. 2019). However, the imaging characteristics of a conventional CXR are frequently non-specific. The disease’s radiographic signs include extensive radiological spots on the lower-left corners and upper lobe of the lung. More well-defined radiographic characteristics appear as the illness progresses, increasing the authenticity of a SARS-CoV-2 diagnosis. Whereas a CXR is the most important test for confirming lung illness, it does not rule out other diseases, especially when SARS-CoV-2 symptoms are present, because it is not specific. Meta-analyses of individuals with lower respiratory infections, particularly those admitted to an intensive care unit, support the utility of CXR (Johannsen et al. 2021).
Computed tomography (CT) and magnetic resonance imaging (MRI)
SARS-CoV-2 supplemental diagnostic testing confirms viral infection and allows for ongoing surveillance. For initial identification of SARS-CoV-2-related lung illness, conventional CXR has a sensitivity of almost 60% (Hasan et al. 2023). The bilateral lower zone, hazy opacities, and peripherally prominent consolidation are among the CXR abnormalities (Wong et al. 2020). CT scans also reveal septal thickening, as well as a ‘reversed halo’ pattern (Bernheim et al. 2020). Consolidated pulmonary opacities and bilateral lung parenchymal ground glass with a sometimes-rounded shape and marginal lung dissemination are observed on CT scans. Individuals infected with SARS-CoV and MERS-CoV present with lung engrossment with a peripheral preponderance. A computed tomography scan of the chest with pulmonary ground-glass opacification and association is more likely to divulge SARS-CoV-2 virus infection (Wang et al. 2022a). Chest CT scans might be utilized as an auxiliary assessment technique in conjunction with recurring RT-PCR tests to diagnose patients with negative RT-PCR test results. High-resolution CT scanning is essential for confirmatory analysis and evaluation of disease severity in individuals with possible SARS-CoV-2 infection (Hosseiny et al. 2019). In a survey of 1014 individuals carried out at Tongji Hospital in Wuhan, 59% (i.e., 601 patients) had tested positive by RT-PCR, whereas 88% (i.e., 888 patients) had tested positive by chest CT scans. Even though despite chest CT scans showed a sensitivity of 97% for SARS-CoV-2, 75% (308 of 413 patients) had tested positive by chest CT scans despite negative results from RT-PCR (Liu et al. 2019). The immunological activation of SARS-CoV-2 causes the synthesis of chemokines and cytokines, which typically leave inflammatory cells visible on CT scans as yellow discolorations. Healthcare practitioners should restrict employing MRI in SARS-CoV-2 individuals, according to the American College of Radiology. The American College of Radiology’s implemented protocols suggest that other imaging methods can also be utilized to scan persons who have tested positive or are suspected to have contracted SARS-CoV-2 infection. The main drawback is that disinfecting MRI scanners is time-consuming and involves of several challenges. HEPA (high-efficiency particulate air) filter methods, which are commonly used to improve air interchange, are incompatible with MRI (Khalili et al. 2020).
Ultrasound
Patients with SARS-CoV-2 are also examined using pulmonary ultrasonography. Although lung ultrasound (LUS) does not appear to be specific for SARS-CoV-2 pneumonitis or pneumonia, it is recommended to identify the infection location. The merits of LUS are that it might be informative in the prompt detection of SARS-CoV-2 pneumonia as an expensive method of determining the site of infection; owing to its high sensitivity to positive terminal outflow, LUS results are more sensitive than CXR. LUS has several characteristics, including pulmonary consolidation in severe local illnesses. LUS revealed more apparent signs of SARS-CoV-2 pathogenesis in the posterior and inferior pulmonary regions in individuals with SARS-CoV-2 infection, similar to what was detected with CXR or chest CT. The infection usually spreads from the peripheral area to the center of the pulmonary tissues. LUS observations of pulmonary edema are frequently used by intensive care unit staff to position SARS-CoV-2 patients therapeutically (Poggiali et al. 2020).
Virus detection from breath
For the objective of monitoring SARS-CoV-2, the exploration of expelled breath may be a less intrusive form of analysis (Škapars et al. 2023 and Einoch Amor et al. 2023). However, screening for severe acute respiratory syndrome SARS-CoV-2 from expelled breath has proven to be quite complicated. Aspects of these investigations, which are especially significant, reveal that SARS-CoV-2 individuals breathe billions of severe acute respiratory syndrome coronavirus RNA molecules each hour (Ma et al. 2019). Expiratory breath exhibited a higher affirmative incidence (26.7%) than air (3.8%) and surface (5.4%) specimens, according to scientific interpretation. Moreover, it was indispensable to assemble the specimen for a prolonged period by adopting a distinctive methodology called expelled breath condensate (EBC) in an attempt to immediately pinpoint the virus from the expelled breath. Current investigations have shown that nonvolatile components, including viruses, bacteria, DNA, and RNA, may be readily detectable and observed by extracting and analyzing the aqueous fraction of the expelled breath (expelled breath aerosol (EBA) or EBC) (Lamote et al. 2020). Depending on these two factors, an EBC system can effectively capture various droplets: (1) the proportion of SARS-CoV-2 virus that is still alive after extraction and (2) the ratio of the percentage of particulates extracted to the total percentage of particulates in the air. Haick and colleagues conducted an introspective scientific analysis in Wuhan, China, for SARS-CoV-2 in March 2020, which included testing with a breath analyzer device that follows the principle underlying chemo-resistive sensors made up of gold nanoparticles in combination with machine intelligence algorithms (Škapars et al. 2023 and Shan et al. 2020). In another experiment, investigators explored preliminary markers of enhanced generation of reactive oxygen species in SARS-CoV-2 swab specimens (Miripour et al. 2020). In this approach, exposing swab specimens to an electrochemical sensor nanostructured with carbon nanotubes revealed 97% real affirmative recognition readings in 30 s.
Immunological/serological techniques for SARS-CoV-2 recognition
Considering molecular approaches have limitations, novel methodologies that employ antibodies or antigens in inpatient specimens to identify SARS-CoV-2 have been developed.
Antibody testing
Capillary blood sampling is the most common sample source used in antibody screening. The specimens are analyzed for patient antibodies, viz. IgA, IgM, and IgG, that are sensitive to a viral epitope to confirm the existence of SARS-CoV-2. The most prevalent antibody candidates for detection are IgM, IgA, and IgG antibodies. As a result of viral multiplication, the viral load in the samples fluctuates with time. As a consequence, the quantity of viral infection is a constraint of serological approaches. SARS-CoV-2 screening is now carried out using a variety of point-of-care (POC) diagnostic methods. These techniques include quick enzyme-linked immunosorbent assays (ELISA), proteome peptide microarrays (PPM), and lateral-flow diagnostic tests. The main drawback is that the predictive value of the test (false-negative report) is commonly reported (Kohmer et al. 2021).
Enzyme-linked immunosorbent assays (ELISA)
Antibodies, antigens, hormones, proteins, and glycoproteins are all ubiquitously identified and characterized using ELISA (Kohmer et al. 2021). Three distinct types of ELISA techniques are deployed to pinpoint SARS-CoV-2. These techniques comprise indirect ELISA, direct ELISA, and sandwich ELISA. Antibodies/antigens are exploited in both indirect and direct ELISA to pinpoint viral infection. Viral epitopes are spotted in sandwich ELISA after they adhere to detection and capture antibodies. The individual SARS-CoV-2 antibodies/antigens from human specimens are immobilized overnight on 96-microwell plates in all three procedures. An enzyme–substrate coupling enables these antibodies/antigens to adhere to the polystyrene surface (Sebbar and Choukri 2023). Antiviral antibodies from human specimens adhere to the surface when the pathogenic protein is expressed. A supplementary tracer antibody that gives a visual indication can be used to identify the antibody–protein aggregates (Kohmer et al. 2021). The median time required to obtain a response is 2–5 h. In contrast to IgA, IgM, and IgG antibodies, proinflammatory cytokines, such as interleukin-6 antibodies, are incorporated in ELISA to distinguish the existence of viral antibodies (Sebbar and Choukri 2023). The ELISA approach has significant advantages over nucleic acid analysis techniques to identifying SARS-CoV-2, which include high accuracy and sensitivity (Park et al. 2023b). Another feature of ELISA is that it takes less time to execute than other approaches to nucleic acid analysis. Furthermore, using gold nanoparticles (AuNP) with an ELISA assay enhances the efficiency of colorimetric antigen recognition for SARS-CoV-2 spike antigen (Bradley et al. 2023). An assessment of the several methods of ELISA-based techniques revealed that each has significant advantages in identifying the existence of viral antigens or antibodies in afflicted individuals. Direct ELISA minimizes the possibility of cross-reactivity with secondary antibodies because it only employs primary antibodies. Indirect ELISA is convenient, as it is very adaptable and may be used with various primary antibodies. Sandwich ELISA is the most sensitive of the three varieties (Yadav et al. 2023). Irrespective of its advantages, there are numerous pitfalls to adopting ELISA for viral antigen or antibody detection. For instance, some commercial diagnostic tools are designed for research study purposes and have never been used in a clinical context. Secondly, in an ELISA test, monoclonal antibodies are inadequate to capture the mutant SARS-CoV-2 virus (Żak et al. 2021).
Chemiluminescence immunoassay (CLIA)
CLIA is a method for identifying the prevalence of antigens or antibodies in human blood samples. Presently, protein-coated and magnetic microparticles are adopted in this methodology. Chemiluminescence immunoassays are identical to direct ELISA in terms of concept. The primary variation is that the outcomes are interpreted using luminescence. The CLIA methodology has numerous advantages. First, because of the strong signal magnitude and lack of interference emission, CLIA can achieve better specificity and sensitivity to SARS-CoV-2 than other serological procedures, such as ELISA and LFIA. CLIA has a cumulative sensitivity of 97.8% quantifying IgG/IgM antibodies. LFIA has a sensitivity of 66%, whereas ELISA has a sensitivity of 84.3% (Rahayu et al. 2023). The introduction of protein-coated and magnetic microparticles to CLIA improves the sensitivity of the technique. The high cost and requirements for a sophisticated apparatus and highly skilled specialists to detect SARS-CoV-2 are a few of the key constraints of CLIA (Liu et al. 2020a).
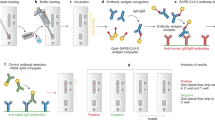
Lateral flow immunoassay (LFIA)
The lateral flow immunoassay is a subjective separation technique used to detect the involvement of a specific biomarker in an unknown specimen. This procedure often uses fluids from nasal swabs, capillary blood sampling, urine, and saliva as specimen materials. These specimens are investigated the occurrence of the SARS-CoV-2 virus by screening the human antibodies (IgA, IgM, and IgG) that are sensitive to pathogenic antigens in several target sites. Spike (S) glycoproteins (receptor-binding area S1 and S2 subunits) and nucleocapsid (N) proteins are among the antigens that have been identified as targets (Wu et al. 2020). LFIA is usually designed as a band with an absorbent pad, a conjugate pad, a membrane zone, a sample pad, and a visualizing zone (Fig. 5). The mechanism of LFIA is dependent on capillary forces transferring a sample fluid across multiple regions of a band. A pathogenic antigen is coupled to fluorescent or gold particles to identify SARS-CoV-2-associated antibodies. The pathogenic antigen is effectively stabilized on the conjugate pad, and the antibodies in the human specimen adhere to it. SARS-CoV-2-targeting antibodies interact with the gold-conjugated antigen of SARS-CoV-2 if they are expressed in a specimen. Then, the antibody–antigen complexes flow to the device-capturing zone. The antibody–antigen complexes interact with additional antibodies in the capturing zone, triggering color development. The LFIA concept is used by a variety of sophisticated laboratories and organizations to manufacture kits. These systems exist in a variety of packages, including casings and cassettes that are analogous to those employed in pregnancy testing (Ma et al. 2019). The LFIA implementation of antigen/antibody screening to discern the overall status of SARS-CoV-2 has significant potential. First, LFIA is equipped to generate results promptly. Generally, SARS-CoV-2 is identified by LFIA, which takes around 10–30 min. Secondly, two weeks after exposure to infection, LFIA accuracy and sensitivity for detection of SARS-CoV-2 improved. Thirdly, LFIA is low-cost, and the findings are accessible to the human eye (Kim et al. 2021).
LFIA for anti-SARS-CoV-2 antibody detection. The specimen material circulates horizontally down the band, which contains several zones, including as a sample pad, an absorbent pad, adhesive pad, conjugate pad, and membrane pad. The conjugate pad comprises antibodies specific to the targeted molecule, as well as antibodies complexed with signaling markers, i.e., gold and fluorescent particles. The screening columns (IgM and IgG columns) and the reference column are positioned on the nitrocellulose membrane. The final region is the absorption pad, which hinders liquid reflux
Antigen testing
Antigen testing is a method used to detect SARS-CoV-2 infection. In this method, serological approaches and antigen tests primarily use nucleocapsid proteins (N) and spike glycoprotein (S). When identifying the SARS-CoV-2 spike (S) glycoprotein with the LFIA technique, Baker et al. suggest that glycans are used as the capture moiety (Baker et al. 2020). Nasopharyngeal and nasal swabs are the most commonly used sample sources. These samples are tested for specific antigens at different target sites (nucleocapsid protein and spike glycoprotein) to confirm SARS-CoV-2 infection. Antigen testing has several advantages, including high efficiency and sensitivity, immediate results, high specificity, and low cost (Mitchell and Ventura 2020).
Proteome peptide microarrays (PPMs)
The proteome peptide microarray approach is extensively applied to explore antibody–protein relationships in the amino acid domain (Holenya et al. 2021). This approach provides insights into affinity mapping and viral protein motif recognition for antibody interaction toward SARS-CoV-2 domains. High-density proteome peptide microarrays have recently been exploited to investigate the interactions between antibodies in a patient’s serum. This approach can aid in defining infection indications by aiding in the comprehension of antibody responsiveness to the SARS-CoV-2 protein. The underlying feature of the PPM approach is that it offers an exhaustive overview of antibody sensitivities to the viral protein (Wang et al. 2020b). The advantages and limitations of the above-mentioned methodology in the diagnosis of SARS-CoV-2 are depicted in Table 3.
Impending techniques for SARS-CoV-2 detection
Clustered regularly interspaced short palindromic repeats (CRISPR/Cas)
In contrast to established PCR approaches, the nucleic acid screening strategy based on CRISPR/Cas was designed using the attributes of affordability, accuracy, quickness, and sensitivity (Hillary et al. 2021). Wang et al. designed a technique for diagnosis with as few as ten duplicates of SARS-CoV-2 within 45 min that does not require specific equipment and demonstrated high accuracy with the qPCR technique (Wang et al. 2020d). Technologies based on CRISPR diagnostics (CRISPR-Dx) provide intriguing solutions for the detection of viruses. The CRISPR bacterial technology can distinguish and digest extraneous genetic substances. To support target-binding CRISPR RNA (crRNA), the CRISPR-associated (Cas) proteins, namely Cas13 and Cas12, exclusively bind to RNA and DNA strands (Casati et al. 2022). DNA endonuclease-targeted CRISPR trans reporter (DETECTOR) and specific high-sensitivity enzymatic reporter unlocking are the names of the Cas12- and Cas13-based detection systems (SHERLOCK), respectively. Utilizing SHERLOCK in lateral-flow configurations, information can be interpreted in less than 60 min to find viruses, pathogens, and tumors. Loop-mediated isothermal amplification (LAMP) and recombinase polymerase amplification (RPA), which do not necessitate advanced technology, have been coupled with Cas-mediated nucleic acid identification to obtain considerable accuracy (Kevadiya et al. 2021).
Wang et al. successfully generated SARS-CoV-2 readily bounded, interspaced brief palindromic repetitions of RNAs, a single-stranded DNA reader, and Cas12a proteins. They further tagged the single-stranded DNA reader with a quenching green fluorescence marker readily fragmented by proteins of Cas12a under the existence of nucleic acid of SARS-CoV-2 in the monitoring device, ensuring that the green illumination would be visible to the naked human eye by using a 485-nm wavelength light, facilitating on-site detection (Wang et al. 2020d).
An RNA-targeting molecule called Cas13 provocatively cleaves non-target nucleic acids in samples. In order to determine femtomolar quantities, the enzyme cleaves specific nucleotide molecules, such as various RNA molecules (collateral cleavage). A thermostatic pre-amplification stage has been employed in Cas13 and detected by the SHERLOCK method. This SHERLOCK CRISPR SARS-CoV-2 kit has been granted emergency use authorization (EUA) for the purpose of detecting SARS-CoV-2 and the test is having merits where currently, more than 160 viral variants can be detected using SHERLOCK-based multiplexed diagnostics. By using the CRISPR-Cas12a/guide RNA complex and a fluorescent sensor with RT-PCR or isothermal recombinase polymerase amplification, the targeted amplicons are obtained by employing viral F1ab and nucleocapsid area markers that capture two RNA variants. A negative RT-PCR report can provide a positive result in fluorescent detection based on CRISPR (Kevadiya et al. 2021).
The fabrication and validation of nucleic acid are based on Cas-13 techniques that use freeze-dried chemicals and swift specimen ablation at normal temperatures. The screenings, namely SHINEv.2 (for “streamlined emphasizing of diseases to manage epidemiological emergencies, version 2”), expedite the previously published RNA-extraction-free SHINEv.1 technique by doing away with the heating process and reagent storage (cold condition). By utilizing lateral-flow techniques and incubating in a thermal bath at 37 °C, SHINEv.2 can pinpoint SARS-CoV-2 in nasopharyngeal specimens in less than 1 h 30 min with 90.5% vulnerability and 100% accuracy (as compared to RT-qPCR). Additionally, SHINEv.2 enables the morphological distinction of the SARS-CoV-2 subtypes omicron (O), delta (δ), gamma (γ), beta (β), and alpha (α) and may be used without efficiency shortfalls by utilizing body temperature (Arizti-Sanz et al. 2022).
Earlier research, known as PAC-MAN (prophylactic antiviral CRISPR in humans), evidenced that virus-targeting crRNAs and Cas13d may restrict influenza-A virus (IAV) and SARS-CoV-2 sequencing. SARS-CoV-2 gene mutation escape has been demonstrated to be restrained by Cas13b, whereas Cas13a was recently found to block the influenza virus or SARS-CoV-2 in laboratory animals. The ability of CRISPR to attack numerous newly emerging SARS-CoV-2 and related coronavirus variants as a broad-spectrum antiviral is still uncertain, but it can be used as a pandemic preparation tool for future strains of concern (Kevadiya et al. 2021).
Nicking endonuclease amplification reaction
Nicking endonuclease amplification reaction (NEAR) is used to exponentially amplify short oligonucleotides. Both nicking endonuclease enzymes and strand-displacement DNA polymerase (e.g., Bst polymerase) are used (Cao et al. 2022). The first step consists of combining a sample with nicking primers P1 and P2, each containing a restriction or stabilization site, a binding sequence, and a nicking site. The displacement extension, nicking, and primer-binding activities result in double-stranded DNA with restriction sites at both cleavages. Second, the restriction sites of the duplex are then cleaved by nicking enzymes, resulting in two free-ending templates that are unstable at 55 °C and ready for separation (NguyenVan et al. 2021). The advantage of this method is the amplification of the products, which is achieved by repeating the polymerization and single-strand cleavage on each template. These products also hybridize to the primers and support bidirectional amplification until the reaction mixture components are depleted. Thousands of copies can be made with a single restriction site, making NEAR a unique amplification technology with maximum efficiency. A key drawback of NEAR is the generation of non-specific yields that lower detectability and increase the diagnostic approach. Identifying the ideal reaction conditions (e.g., nicking enzyme content, Mg2+ content, and generated heat of reactivity) could help to solve this problem (Cao et al. 2022). ID NOW tests for influenza and group A streptococci are already on the market, favoring the rapid market introduction of ID NOW SARS-CoV-2. This assay was created to identify an array of RNA-dependent RNA polymerase (RdRp) segments in the SARS-CoV-2 genome, and the LOD result was 0.125 copies/mL; the assay has received FDA-EUA approval for SARS-CoV-2 (Nie et al. 2020).
Recombinase polymerase amplification
The principle of analogous DNA recombination is used to replicate double-stranded DNA in recombinase polymerase amplification (RPA) (Bai et al. 2022). These compounds look for homologous structures in the focused DNA and subsequently occupy appropriate locations. After the restriction enzyme has unpacked the nucleoprotein-bonded strand, the DNA polymerase displaces strand extension. Activated recombinases develop to produce fresh nucleoprotein strands for subsequent cycles, and the changed strand is maintained by uni-stranded binding proteins during this phase. Upon completion of this procedure, the targeted double-stranded DNA is extensively replicated (Jiang et al. 2022). Xia et al. described RPA primers targeting regions of the N gene to detect SARS-CoV-2. To enhance SARS-CoV-2 one-spot RNA reverse transcription, standard RPA solution was combined with transcriptase and RNase inhibitors. Available fluorescent or lateral-flow probing tools were used to identify the enhanced targets. At an LOD of 0.2 copies per microliter, the response takes approximately half an hour to complete. However, instead of studying extracted viral RNA samples, the outcomes remain confined to utilizing artificial RNA (Xia and Chen 2020).
Virus neutralization test
The viral neutralization test (VNT) is a key indicator of a person's evolving antibody status against a target virus. An advanced kind of immunoassay used to identify antibodies capable of preventing viral reproduction in vitro is the live virus neutralization test (VNT). The live VNT is regarded as the gold-standard technique for evaluating neutralized antibodies (nAbs) and was utilized to establish a lower serum threshold value to screen against SARS-CoV-2 infection (Harcourt et al. 2020). Several types of neutralization tests are available, where studies have been performed with SARS-CoV-2. In the plaque reduction neutralization test (PRNT), colony-forming cells are quantified on an agar or carboxymethyl cellulose-coated cell layer, whereas neutralizing antibody titers are calculated using immuno-colorimetric analysis in the focus reduction neutralization test (FRNT). A recent study evaluated the efficacy of FRNT and PRNT testing for specified RBD IgG reactions produced by SARS-CoV-2 individuals 6 days after PCR analysis and discovered a substantial association among the tests (Suthar et al. 2020). Additionally, pseudo-virus-based neutralization assays (PBNAs) have been developed by several researchers, employing pseudo-virus (PSV) as a preferable (biosafety level 2) alternative for the detection of SARS-CoV-2 (Wang et al. 2020e). PSV was created by integrating the S protein of SARS-CoV-2 in the envelope of a pseudo-type of vesicular stomatitis virus, further revealing that neutralizing antibody titers peaked 10–15 days following the initiation of the illness and then remained steady (Mitchell and Ventura 2020). In a study, almost 30% of recuperated subjects (n = 175) had minute concentrations of neutralizing antibodies; this finding could have consequences for how serologic tests are applied and interpreted to detect previous SARS-CoV-2 infection. The main strength of this test is that it acts as a good indicator, and also helps to comprehend the prominence of protective immune response in asymptomatic and symptomatic cases (Guo et al. 2022).
Next-generation sequencing
Next-generation sequencing is a technology that, in conjunction with existing methods, is intended to simultaneously establish the pattern of numerous microscopic DNA extracts (Chiara et al. 2021). Next-generation sequencing has proven effective in identifying and studying infections (SARS-CoV-2) when integrated with bioinformatics approaches (Hillary et al. 2021). This technology facilitates the retrieval of knowledge from a viral genome while also recognizing the occurrence of a pathogen in a specimen. This potentially efficient methodology was designed by Illumina (Carter et al. 2020). This technology having the advantage of indicating the appearance of several coronaviruses (SARS-CoV-2) variants in a specimen, as well as the presence of diverse pathogenic entities (Wang et al. 2020d).
Amplicon-based metagenomic sequencing
Amplicon-based metagenomic sequencing is an additional high-throughput sequencing approach for recognizing SARS-CoV-2. This approach, analogous to next-generation sequencing, recognizes and characterizes the microbiota in nasopharyngeal samples by integrating the base amplicon with metagenomic sequencing. By enhancing viral evolutionary analyses, genomic epidemiological investigations, and contact mapping, this amplicon-based sequencing approach advances the identification of SARS-CoV-2 infection. This approach is having the strength to be utilized in determining the extent to which a genomic expression has deviated (Moore et al. 2020).
Vertical flow assay
A vertical flow assay is an innovative technique for identifying SARS-CoV-2. This method is identical to a lateral flow assay, although devoid of a horizontal flow sequence, instead adopting a vertical flow sequence. These techniques encompass the development of antibody–antigen interaction, the adsorption of the capturing antibodies into a readout surface, and the adsorption of the tagged detecting antibodies to create a visual indication (Lei et al. 2022). Vertical flow assays furnish multiple features, including the utilization of extrinsic factors, viz. capillary and gravitational forces, and the ability to quickly duplicate the analysis. Furthermore, the detection advantage of a vertical flow assay is more rapid than that of a lateral flow assay, and the assay insights can be interpreted by inexperienced personnel (Kim et al. 2021).
Detection of SARS-CoV-2 by nano-based diagnostic techniques
Colorimetric assay
When the rate of SARS-CoV-2 infection is intense, a simple, rapid, and accurate “naked-eye” colorimetric SARS-CoV-2 diagnostic assay is required. Colorimetric assays make use of gold nanoparticle (AuNP) optical features and target antisense oligonucleotides (ASOs), aiming at the phosphoprotein of SARS-CoV-2 altered with conjugated thiol (Duan et al. 2021). After RNA isolation, the detection is completed within 10 min. When the targeted SARS-CoV-2 RNA sequence is present, the ASO-modified thiol binds the AuNP combination, resulting in a shift in the surface plasmonic resonance, as shown in Fig. 6. The RNA strand is broken from the DNA-RNA fusion when RNase H is added, followed by the fabrication of a visible deposition aided by AuNP accumulation. In the manifestation of RNA of viral MERS-CoV, the LOD utilizing this approach was 0.18 ng/L of RNA with a SARS-CoV-2 viral load. A colorimetric analysis using a comparable method was also used to identify the occurrence of the Middle East respiratory syndrome coronavirus (MERS-CoV) (Moitra et al. 2020).
Microfluidic devices
Microfluidic devices offer an alternative way to conduct a proof-of-concept study where the chip is only the size of a palm inscribed with micrometer-sized networks and response compartments composed of paper, polydimethylsulfoxide, or glass, with benefits such as small size, rapid identification time, and low sample volume. The essential premise of these microfluidic chips is that they use capillary action and electrokinetic capabilities to mix and separate liquid samples (Kumar et al. 2022). Multiple antibodies toward three sexually transmitted diseases can be identified of using microfluidic devices and a smartphone presentation connection, exhibiting 87% exactitude and 100% intensity. These devices can be adapted to detect the presence of RNA (SARS-CoV-2) or proteins, owing to their convenience and durability (Kumar et al. 2021a). Several modalities of study utilizing a microfluidic channel are shown in Fig. 7.
Gold nanoparticles (AuNPs)
The use of AuNPs in biopharmaceutical and clinical contexts has advanced significantly. AuNP innovations have the advantages of being sensitive, expeditious, simple, and versatile, in addition to permitting quantitative analysis with outstanding combinatorial abilities. The sensitivity ranges of AuNP-based technologies for the recognition of antibodies and nucleic acid are equivalent to or superior to those of commercial ELISA kits that are not based on AuNP and RT-PCR. As a result, AuNP-based SARS-CoV-2 screening is a plausible substitute for RT-PCR, presenting opportunities to address this major unmet biological need, particularly in venues with inadequate facilities (Wang et al. 2022b). Research conducted in vivo and in vitro revealed that the respiratory syncytial virus was irreparably deformed by AuNPs with extended chains of mercaptoethanesulfonic acid (MES) and sulfonate undecanesulfonic acid (MUS). A molecular interaction exposes the combination of AuNPs and MUS, which is connected to the virus in a multivalent association. The structure of the capsid also disintegrates as a result. This multivalent bonding consequently offers a compelling plan of approach for COVID-19 treatment. In one study, AuNP was also used against an RNA virus, which may be used to treat SARS-CoV-2. Based on the immunochromatography of gold nanoparticles, a commercialized SARS-CoV-2 antibody testing kit was developed using the colloidal gold method. Although the test has to be validated on entire viral RNA sequences from human specimens, it may ameliorate the present stress on PCR-based diagnostics (Asdaq et al. 2021).
Magnetic nanoparticle-based separation
Molecular analysis for SARS-CoV-2 detection begins with the isolation of nucleic acids from a medical specimen, which is a slow and tedious process. Magnetic nanoparticles encapsulated in carboxyl polymer (pcMNP) were produced to facilitate the extraction of viral RNA for the diagnosis of SARS-CoV-2 RNA (Zhao et al. 2020). RT-PCR can also be performed with pcMNP–RNA complexes, providing a number of benefits over standard column-based extraction methods, including the ability to adhere viral RNA, as well as enhanced sensitivity. In addition, the pcMNP–RNA complex generated during MNP extraction can be used with a number of isothermal amplifying methods, such as LAMP. As a result, this technology may be utilized to create proof-of-concept systems (Khizar et al. 2022).
Surface-enhanced Raman scattering (SERS)
Surface-enhanced Raman scattering spectroscopy takes place in a robust investigative platform with the aim of molecular characterization (especially detection of viral antigens and DNA sequences), which can be extremely convenient for diagnosing applications when integrated with the intrinsic chemical and optical attributes of nanoparticles (Cha et al. 2022). SERS improves conventional fluorescence-based screening techniques with respect to specificity, sensitivity, and screening of distinct constituents in a blend, which is becoming increasingly relevant for therapeutic diagnosis. However, no outcomes have been reported to date with respect to the ability of SERS to recognize SARS-CoV-2 (Berry et al. 2021).
Surface plasmon resonance (SPR)
Surface plasmon resonance is a versatile visual platform that is often used to analyze the refraction index alteration of plasmonic components in real-time surfaces (Bahl et al. 2021). The term LSPR (localized surface plasmon resonance) refers to a photon-driven combined alternation of transmission band electrons on the planes of relatively small plasmonic sensible configurations (such as metal NPs). The plasmonic field exhibits remarkable reactivity, with an alteration in refractive index and molecular adherence of the LSPR sensing systems in the proximity of tiny structures (nanoscale range). As a result, LSPR may be used as a typical system for label-free, real-time diagnosis of very small amounts of samples (Qiu et al. 2020). A rapid and effective multi-use biosensor based on LSPR was devised recently by combining plasmonic sensing attributes and photothermal abilities to identify the viral SARS-CoV-2 nucleic acid (SC-NA) on a singular plasmonic nanoabsorber chip made of gold nanoislands (AuNI) (Jiang et al. 2021a). Ultimately, this setup allows for the label-free and real-time exposure of any nucleic acid sequence, especially E genes, RdRp-COVID, and ORF1ab COVID from SARS-CoV-2. Moreover, this biosensor of LSPR can determine the availability of samples at quantities greater than 0.22 pM and provides a simply realizable diagnostics phase, which, when combined, could increase diagnostic accuracy and minimize reliance on PCR-based assays (Qiu et al. 2020).
Biosensors
Biosensors represent a rapid, high-efficiency diagnostic technology. Qiu et al. designed a double plasmonic biosensor that identifies SARS-CoV-2 by integrating the localized surface plasmonic resonance with the plasmonic photothermal effect (Qiu et al. 2020). The researchers employed a biosensor based on a field-effect transistor that can recognize SARS-CoV-2 from a human specimen (Seo et al. 2020).
Nucleic acid-based biosensors
A double-function plasmonic biosensor was created for the sensitive detection of SARS-CoV-2 nucleic acid. Plasmonic photothermal (PPT) response and local surface plasmon resonance sensing transduction are combined in this biosensor. A cDNA receptor for the E gene, ORF1ab, or RdRp is immobilized via gold-thiol linkage on an assimilated chip derived from two-dimensional gold nanoislands (AuNIs). This approach applies the concept of RNA conjugation with a LOD of 0.22 pM to ascertain SARS-CoV-2 RNA. The device’s increased sensitivity is due to an improvement in the hybridization kinetics of the complementary strands resulting from the heat-generating capability of the local PPT of the AuNI plasmonic chips (Qiu et al. 2020). The action of biosensors in clinical specimens is still being investigated. Another test combines a nanoparticle-based LFIA with multiplex analysis, LAMP amplification, and reverse transcription (SARS-CoV-2 RT-LAMP-LFB). With no cross-reactivity, this LOD platform was estimated to yield 12 replicas per response. The SARS-CoV-2 N genome, two LAMP primer groups, and ORF1 antibodies were all amplified and identified simultaneously using coated polymer of streptavidin nanoparticles (Pouresmaieli et al. 2021).
Antibody-based biosensors
A novel biosensor based on antibodies was purportedly applied to diagnose the spike protein of SARS-CoV-2. As antigens, nasopharyngeal samples were taken from clinical patients and used to encapsulate the respective viral antibody on graphene sheets of a field-effect transistor (FET) (Kang et al. 2021). The biosensor-developed limit of detection (LOD) was reported to be 100 and 1 fg/mL in a basic transport vehicle and saline solution. The virus was furthermore discovered in a growing medium, showing a detectable limit of 1.6 × 101 pfu/mL according to this sensor. Recent research found that with a limit of detection of 2.42 × 102 copies/mL, the SARS-COV-2 FET sensor can categorize diseased and fit individuals (Chiara et al. 2021). An ELISA kit based on recombinant S and N proteins was used to detect IgM and IgG antibodies, which were produced and tested on patients. A positive rate of 80.4 and 82.2% for N protein and S protein, respectively, were detected by these kits (Liu et al. 2020c).
Commercial kits based on the above techniques
Various COVID-19 commercial testing kits from different countries have been summarized based on their techniques or principles of detection like RT-PCR, RT-LAMP, biosensor, CRISPR, antigen testing, antibody testing, LFIA, ELISA, and CLIA by also highlighting the various target proteins which are responsible in aiding in the detection of SARS-CoV-2 and are being listed in Table 4.
Nano-based vaccine candidates against coronaviruses
Vaccination is a well-established biomedical approach to life-threatening infectious conditions; however, rapid and complex genetic alterations in SARS-CoV-2 viruses have made vaccine research and deployment challenging (Kurup and Schnell 2021). To mitigate these challenges, a plethora of attempts have been made to design vaccines that elicit a robust immunological response. Considering breakthroughs in vaccine innovation, vaccination prevalence remains constrained. Through the continuing advancement of nano-sciences, the concept of nanomaterials for vaccine development appears enticing, offering phenomenal potential, owing to the inimitable attributes of nanoparticles that render them excellent vectors for vaccine conveyance by safeguarding vaccines from immature deterioration while promoting the vaccine’s optimum consistency, augmenting cellular transport via endocytosis mechanisms, improving depot response, and eliciting both cellular and humoral immunity. SARS-CoV-2 viral components (proteins and RNA) can undergo structural analysis and analogs to form virus-like particles, which can be utilized as nano-vaccines. The viral components can also be separated and inactivated. The inactivated components are incorporated into nanoparticles (NPs) to form vaccine-loaded NPs. Additionally, the inactivated components can directly be used as a vaccine, which is depicted in Fig. 8 (Butkovich et al. 2021 and Gale et al. 2021). Importantly, NPs serve as adjuvants or immunostimulatory molecules, significantly improving the sensitivity of antigens (SARS-CoV-2). It has also been demonstrated that certain nano-vaccines promote increased polyclonal antibody response (Gale et al. 2021). Table 5 provides a comprehensive list of nano-vaccines in the pipeline.
DNA-based vaccines
Entos Pharmaceuticals, a Canadian healthcare research organization, introduced a DNA vaccination utilizing its Fusogenix nanomedicine system (Muthiah et al. 2022). The Fusogenix system uses a proteolipid vehicle that integrates a neutral lipid biomaterial containing fusion technology of tiny permeability protein molecules to enable optimal merging and effectual transport of genomic cargo immediately to the cytoplasm of targeted cells. Entos Pharmaceutical intends to introduce a pancoronavirus vaccine design that could encrypt numerous autoantigens of SARS-main CoV-2 immunogenic molecules, enabling a substantial and formidable defensive immunological response against the virus’s complex architectural subunits. In vivo preclinical investigations were undertaken, leading to the advancement of Covigenix (Fusogenix DNA) vaccines targeting SARS-CoV-2, as shown in Fig. 9A, which exhibited significantly higher neutralizing antibody responses, regulated CD4 T cell immunity, high sensitivity, and effectiveness. Subsequent confirmations of this vaccine candidate, i.e., its immunogenicity, safety, and effectiveness, have been accomplished satisfactorily in phase 1 and 2 human clinical studies, with the vaccine candidate advancing to phase 3 investigations (Muthiah et al. 2022).
Diagram of the several COVID-19 vaccination proposals based on DNA and RNA. 1 The mRNA -1273 (Moderna) vaccine is a comprehensive prefusion-stabilized spike protein of SARS-CoV-2 that is encoded by an mRNA-based vaccination that is embedded in lipid nanoparticles. 2 Covigenix (Entos Pharmaceuticals) is a plasmid DNA vaccine that manifests key antigenic determinants from SARS-CoV-2
RNA-based vaccines
The vaccine potential, i.e., mRNA-1273, for the therapeutics of SARS-CoV-2 designed by Moderna in coordination with the National Institute of Allergy and Infectious Diseases (NIAID), a division of the National Institutes of Health (NIH) in the United State of America, is a marketed accessible SARS-CoV-2 booster and was the first to be evaluated in individuals (Fig. 9B) (Szabó et al. 2022). An mRNA version of the novel SARS-CoV-2 consists of prefusion-stabilized spike protein embedded within an experimental lipid nanoparticle matrix consisting of the exclusive ionizable SM-102, lipid, and three conventionally provided lipids, i.e., polyethylene glycol 2000 dimyristoyl glycerol, distearoyl phosphatidylcholine, and cholesterol. Moderna successfully developed an mRNA candidate vaccine entrapped in cationic phospholipid NPS targeting the SARS-CoV-2 viruses using an identical approach. The vaccines feature entire epitope viral (S/S1/S2) proteins from a virus that created a significant concentration of neutralizing antibodies in rodents (Szabó et al. 2022).
Virus-like particle (VLP) vaccines
Virus-like particles are an intriguing, innovative strategy for vaccine design, as they imitate the original hierarchy of viruses and suggest a pathway to the immunological response for rapid identification and consequent modulation. Medicago, a biopharmaceutical firm based in Canada, has accomplished the preliminary process of researching a vaccine candidate by efficiently manufacturing VLPs of the SARS-CoV-2 virus, utilizing its plant-based VLP synthesis method (Kang et al. 2021). Nicotiana benthamiana plant are exploited by the firm as mini biofactories to establish virus-like particles and restorative molecules for vaccine manufacturing. The vaccine has been in phase 3 clinical testing since 20 March 2021 (England et al. 2023).
Nano-based immunotherapy against coronaviruses
Immunomodulatory drugs in nanomaterial form have achieved encouraging results in the context of regulating immune system activity and minimizing immunomodulation-related damage (Bonam et al. 2021). Importantly, nanomaterials have the competence to assimilate numerous antigens on their interface for more robust immune system stimulation. As a result, nanoparticles can perform as prospective immunological adjuvants, as well as pharmaceutical vehicles. Carbon nanotubes, dendrimers, inorganic nanoparticles, liposomes, and polymer-based materials have all been explored as promising immunological venues to date (Andresen and Fenton 2021). Nanoparticles, including liposomes and poly (lactic-co-glycolic acid), can trigger CD4+/CD8+ T lymphocyte cells and facilitate antigen cross arrangement, resulting in efficacious antigen conveyance (Shinn et al. 2022). However, inorganic nanoparticles, especially gold nanoparticles, can connect with dendritic cells, elevating the performance of proinflammatory markers (viz., tissue necrotic factor-α, interleukin-6, interleukin-1, interleukin-12, and interferon-α) while suppressing the expression of anti-inflammatory mediators (viz., interleukin-10 and tissue growth factor-β1) (Dykman et al. 2020). AuNPs also triggered immunological activation in T cells and augmented dendritic cell phagocytic action. Despite breakthroughs in the clinical use of NPs in immunotherapy, basic research on the use of nanoparticle-based immunotherapy targeting SARS-CoV-2 is still limited (Yang et al. 2020).
Nano-Based Gene Therapy for coronaviruses
Small interfering RNA (siRNA) is efficacious in inhibiting the replication of RNA viruses, i.e., coronaviruses (Liu et al. 2022). The success of siRNA-based therapeutics is entirely reliant on the precise tailoring of the virion genetic pattern and the delivery of restorative siRNA to the target cells (Nooraei et al. 2021). From this perspective, nontoxic, biomaterial nanoparticles made of nanohydrogels, iron oxide NPs, dendrimers, lipids, silica, AuNPs, polymers, or lipid/polymer hybrid NPs are seen as potential siRNA delivery carriers. By hindering enzymatic breakdown, these nanocomposites can improve siRNA longevity (Idris et al. 2021). Lipids, polylactic-co-glycolic acid (PLGA), and polymer nanocomposites are appropriate for delivering antiviral siRNA in respirable form and aerosol-based cardiopulmonary administration of antiviral siRNA (Wu et al. 2021). Moreover, cholesterol-loaded lipid NPS were recently reported to achieve good performance in conveying SARS-CoV-2 vaccines relying on mRNA (Le et al. 2020). Nanocomposites based on spermine-liposome conjugate and histidine-lysine copolymer have also been validated for delivery of siRNA to appropriate sites in the SARS-CoV gene (Eygeris et al. 2020).
Nanosponges
A new method of developing treatments focuses on the impaired recipient tissues instead of the pathogenetic component. Cellular nanosponges were designed as therapeutic protection against coronavirus infection. These nanosponges are made from membranes extracted from mammalian cells that are inherently SARS-CoV-2 targets. Two categories of cellular nanosponges are generated using plasma cell membranes: either human lung epithelial type-II cells or human macrophages. The same protein receptors, both known and unknown, that are required for SARS-CoV-2 to infiltrate cells are present in each of these nanosponges.
We have learned that SARS-CoV-2 is a distinct virus that is responsible for the recent worldwide epidemic, and details about it are being discovered day after day. The virus has been proven to be rapidly evolving since the initial case that was identified at the end of 2019. The advancement of medicines and preventative measures is facing significant difficulties as a result of this high rate of mutation. Both Epithelial-NS and Mɸ-NS revealed a concentration-dependent neutralization of SARS-CoV-2. The nanosponge platform has a distinct advantage over other SARS-CoV-2 treatments that are under research since the nanosponges are possibly viral and mutation-insensitive. Once primed with the nanosponges, SARS-CoV-2 is neutralized and rendered incapable of infiltrating tissue. Importantly, the nanosponge platform is resistant to viral modifications and potential viral species, as the virus continues to attack its targeted host cell, and the nanosponges are ultimately capable of ejecting it. With regard to mutation and other recently developed coronaviruses, microsponges offer a wide-ranging defense system that is mutation-resistant. Additional testing in suitable animal studies is necessary to confirm the effectiveness of cellular nanosponges for the management of SARS-CoV-2 infections. Such testing is currently being carried out and will eventually lead to clinical studies. The antiviral effectiveness of such nanosponges can be enhanced by optimizing the core composition (Zhang et al. 2020).
Nanodecoys
Angiotensin-converting enzyme 2 (ACE2), a carboxypeptidase that is prevalent in all body tissue and is located in a variety of cells, is essential for viral entry into infected cells. Through the association of the spike protein with ACE2, SARS-CoV-2 selectively targets type II pneumocytes that exhibit ACE2 in the pulmonary tract and goblet secretory cells in the nasopharynx membrane (Ziegler et al. 2020). Resident lung epithelial and mesenchymal cells, with both type I and II pneumocytes, make up lung spheroid cells (LCS); these cells generate ACE2 as resident lung cells, and nanovesicles of the LSC membrane are created as ACE2 nanodecoys. Therefore, by serving as cell mimetics, these nanodecoys adhere to the spike (S) proteins of SARS-CoV-2 and cause a phagocytic reaction from macrophages, which leads to eradication of the virus (Jiang et al. 2021b). A recent study reported that nebulized treatment was used to provide such LSC nanodecoys to mice, where they lasted in the respiratory organs for more than 72 h after treatment. Moreover, SARS-CoV-2 analogs were rapidly eliminated from the lungs after inhalation of LSC nanodecoys, and no cytotoxicity was indicated. Four doses of these nanodecoys administered through inhalation enhanced viral elimination and lessened lung damage in cynomolgus macaques exposed to live SARS-CoV-2. The findings imply that LSC nanodecoys could be used as a medicinal therapy to combat SARS-CoV-2 (Li et al. 2021).
Exosomes
Exosomes are a form of extracellular vesicular particles that are produced spontaneously in the body system, making them intrinsic and perfect for delivery of vesicles for drug targeting (Stefańska et al. 2023 and Popowski et al. 2020). Particles are more effective in attacking similar recipient cell tissues because they possess and assert the proteins, RNAs, and lipids of their original cell, as well as the original cell’s surface receptors and proteins (García-Fernández and Fuente Freire 2023). A study reported a vaccine comprising a modified SARS-CoV-2 receptor-binding domain (RBD) coupled with exosomes derived from the lungs, which, in comparison with liposomes, improved the accumulation of RBD in all the pulmonary airways lined with mucus and in the lung tissue. After a trial, the vaccine effectively eliminated SARS-CoV-2 pseudo-virus in mice by eliciting RBD-specific antibodies (IgG), mucosal IgA reaction, and CD8 + and CD4 + T cells with a Th1-like cytokine production pattern in the mouse airway. Upon treatment with active SARS-CoV-2, double doses of the vaccination in hamsters suppressed inflammation and lessened chronic pneumonia. VLPs that are inhalable, durable at room temperature, with long shelf lives, and potential cost savings as a result of reduced shipping make the modified vaccine more widely available and a potentially viable vaccination alternative (Wang et al. 2022c).
Future perspectives
An increased focus on the novel, moveable diagnostic technology has been sparked by the plethora of the SARS-CoV-2 outbreak and its effects on testing accessibility. It is important to have diagnostic testing tools with high accuracy, fast developing, cost-effective antigen and serological tests, which are up to global standards and with the employment of new biosensors, nano-based therapy, electrospinning, and electrospun nanofibers which can prevent and reduce the spread of the virus.
High accuracy
Under ideal conditions and when performed by trained clinicians, most nucleic acid amplification tests have a diagnostic sensitivity and specificity of approximately 95% or greater. However, in practice, sensitivity drops to 60–70%, requiring retesting, which wastes time in symptomatic patients. To improve accuracy, several countries have introduced dual testing of pharyngeal/sputum and nasopharyngeal samples. Further studies are needed to assess the efficiency of swab testing and RNA yield (Serrano et al. 2020).
Developing fast, cost-effective antigen tests
For several reasons, including reducing the need for time-consuming RT-PCR, rapid antigen-based SARS-CoV-2 assays are needed. The development of nucleic acid amplification tests (NAATs) is a plausible and reasonable option, given their high analytical sensitivity and rapid development time. On the other hand, NAATs are often highly processed, susceptible to spoilage, and exorbitant (Chen et al. 2020). Antigen-based screening could represent a specialized option for cost-effective identification in outpatient clinics. Similar methods have already been developed for certain infections, such as influenza (Gentilotti et al. 2022). A SARS-CoV-2 antigen test can be performed as a triaging method to reduce the need for molecular testing.
Establishing effective serological tests
Current serological tests have many variations with generally low reactivity and accuracy. When comparing different test kits, virtually all of which are based on the lateral flow assay design, some perform significantly better than others as a result of the affinity compounds used. To increase the accuracy of diagnosis, researchers must first identify and synthesize the most immunogenic, high-affinity viral antigens (Whitman et al. 2020). Investigation of interference problems is also necessary to determine how medications, drugs, and coagulation states affect serologic test results. The demand for serologic testing will increase as SARS-CoV-2 enters its flattening phase. These tests can be used by those who are cured or asymptomatic to generate information for public pronouncements; community-wide serologic surveillance could allow governments to discover the true prevalence of the disease (Lassaunière et al. 2020).
Setting up global standards
The analytical validity of novel SARS-CoV-2 tests has led to their acceptance, as tested against the manufacturers’ simulated samples. Independent testing has revealed significant differences in performance. Global reference standards (e.g., viral antigen, antibodies, viral nucleic acids, and pseudo-viruses) need to be developed to allow for objective comparison between tests (Uğur and Özdemir 2023). Recommendations (e.g., on cost, sensor specifications, and accuracy) for various test targets need to be developed; detection of acute infections in healthcare facilities and long-term care facilities is required, as well as home-based surveillance with demographic surveys. These activities will help both the clinical and scientific sectors and drive technological advances (Ghaffari et al. 2021).
New biosensors
New diagnostic procedures should be developed more quickly. Novel sensor techniques, namely optical resonators, nanoplasmonics, and ion-gate transistors, offer exceptionally high sensitivity and could be utilized to directly detect viruses. A transistor based on graphene with a LOD o 2.4 × 102 viruses/mL and a plasmonic photothermal device that recognizes the RdRp target down to 0.22 pM has been reported. These techniques must be further explored to overcome present NAATs and provide rapid on-site diagnoses (Qiu et al. 2020 and Seo et al. 2020).
Nano-based therapy
The search is currently on for a vaccine against SARS-CoV-2 that is trustworthy, stable, effective, and long-lasting. Nanotechnology-based vaccines have been shown to elicit significantly more robust immunoreactivity than various forms of coronavirus vaccines, as previous research has demonstrated. Therefore, further analysis of the use of nanotechnology in the analysis of SARS-CoV-2 is needed to develop an inventive, nano-based vaccine in appropriate animal studies to potentially induce long-term immunity (Gale et al. 2021). Promising alternatives for dissemination of effective drugs against coronavirus include magnetic nanoparticles (MNPs), AuNPs, and silver nanoparticles (AgNPs), as well as their associated compounds (Dykman et al. 2020). Several nanoparticle vaccines have demonstrated the ability to induce a strong autoimmune response. In addition, further research on the interface of virions with recipient cells is needed before smart nanoparticles can be used to target altered variants of the highly infectious SARS-CoV-2 (Bonam et al. 2021).
Electrospinning and electrospun nanofibers
Continuously non-woven nanofibers can be synthesized from a variety of polymeric composites using the basic, adaptable, and affordable method of electrospinning. With the help of polymeric compositions and electrospinning variables, the length and shape of nanofibers can be altered. As a result, electrospun polymer membranes are typically preferable for air-filtering processes and can be used as materials for protective masks against viruses or bacteria. Furthermore, the electrospinning method has been used for drug delivery, and scientists are attempting to design scaffolds that can deliver drugs against SARS-CoV-2 while regulating the amount of a drug absorbed by the body system, which is essential for usage as a quick absorbent. Moreover, this novel formulated nanotechnology has still not been advanced for application with potentially developed therapeutics (Castillo-Henríquez et al. 2020 and Ding et al. 2020b).
Conclusions
The identification of SARS-CoV-2 RNA in the upper respiratory tract, particularly nasopharyngeal specimens, is commonly achieved through polymerase chain reaction assays. Although SARS-CoV-2 immunity testing techniques are developing, precautions while interpreting results are advised because very few test kits on the market have been validated. Rapid testing and ELISA antibody analysis show a typical antibody response, with IgM production occurring initially, then IgA and IgG. On the other hand, limited information is known about how long protective immunity lasts. T cell response assessment test kits are presently unavailable for diagnostic use. Numerous diagnostic techniques are available, each with unique benefits. These techniques include chemiluminescence immunoassay, lateral flow immunoassay, antigen testing, and protein-peptide microarray. Innovative techniques such as CRISPR, AuNPs, and nano-based technologies have the potential to identify more infectious viruses in addition to SARS-CoV-2. NPs based on immunotherapy are becoming an exceedingly prominent treatment alternative; however, there are still issues with maximizing benefits and reducing side effects. Effective immunotherapy requires a thorough understanding of immune responses and immunity regulation. When compared to traditional antiviral medications and immunizations, nano-based therapeutic agents particularly siRNA antiviral treatment offer benefit in terms of rapid response, effectiveness, and low RNA consumption.
References
Abduljalil JM (2020) Laboratory diagnosis of SARS-CoV-2: available approaches and limitations. New Microb New Infect 36:100713
Acevedo ML, Alonso-Palomares L, Bustamante A, Gaggero A, Paredes F, Cortés CP, Valiente-Echeverría F, Soto-Rifo R (2021) Infectivity and immune escape of the new SARS-CoV-2 variant of interest lambda. Medrxiv. https://doi.org/10.1101/2021.06.28.21259673.abstract
Adams ER, Ainsworth M, Anand R, Andersson MI, Auckland K, Baillie JK, Barnes E, Beer S, Bell JI, Berry T (2020) Antibody testing for COVID-19: a report from the national COVID scientific advisory panel. Welcome Open Res 5:139
Andresen JL, Fenton OS (2021) Nucleic acid delivery and nanoparticle design for COVID vaccines. Mrs Bull 46:1–8
Annavajhala MK, Mohri H, Wang P, Nair M, Zucker JE, Sheng Z, Gomez-Simmonds A, Kelley AL, Tagliavia M, Huang Y, Bedford T (2021) A novel and expanding SARS-CoV-2 variant, B. 1.526, identified in New York. MedRxiv. https://doi.org/10.1101/2021.02.23.21252259.abstract
Arizti-Sanz J, Bradley AD, Zhang YB, Boehm CK, Freije CA, Grunberg ME, Kosoko-Thoroddsen T-SF, Welch NL, Pillai PP, Mantena S (2022) Simplified Cas13-based assays for the fast identification of SARS-CoV-2 and its variants. Nature Biomed Eng 6:932–943
Artik Y, Coşğun AB, Cesur NP, Hızel N, Uyar Y, Sur H, Ayan A (2022) Comparison of COVID-19 laboratory diagnosis by commercial kits: effectivity of RT-PCR to the RT-LAMP. J Med Virol 94:1998–2007
Asdaq SMB, Ikbal AMA, Sahu RK, Bhattacharjee B, Paul T, Deka B, Fattepur S, Widyowati R, Vijaya J, Al Mohaini M (2021) Nanotechnology integration for SARS-CoV-2 diagnosis and treatment: an approach to preventing pandemic. Nanomaterials 11:1841
Augustine R, Hasan A, Das S, Ahmed R, Mori Y, Notomi T, Kevadiya BD, Thakor AS (2020) Loop-mediated isothermal amplification (LAMP): a rapid, sensitive, specific, and cost-effective point-of-care test for coronaviruses in the context of COVID-19 pandemic. Biology 9:182
Bahl S, Bagha AK, Rab S, Javaid M, Haleem A, Singh RP (2021) Advancements in biosensor technologies for medical field and COVID-19 pandemic. J Ind Integr Manag 6:175–191
Bai Y, Ji J, Ji F, Wu S, Tian Y, Jin B, Li Z (2022) Recombinase polymerase amplification integrated with microfluidics for nucleic acid testing at point of care. Talanta 240:123209
Baker AN, Richards S-J, Guy CS, Congdon TR, Hasan M, Zwetsloot AJ, Gallo A, Lewandowski JR, Stansfeld PJ, Straube A (2020) The SARS-COV-2 spike protein binds sialic acids and enables rapid detection in a lateral flow point of care diagnostic device. ACS Cent Sci 6:2046–2052
Bastos ML, Tavaziva G, Abidi SK, CampbelHaraoui JRLP, Johnston JC, Lan Z, Law S, MacLean E, Trajman A, Menzies D (2020) Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis. BMJ. https://doi.org/10.1136/bmj.m2516
Bedoya-Joaqui V, Gutiérrez-López MI, Caicedo PA, Villegas-Torres MF, Albornoz-Tovar LL, Vélez JD, Hidalgo-Cardona A, Tobón GJ, Cañas CA (2023) Persistent and fatal severe acute respiratory syndrome coronavirus 2 infection in a patient with severe hypogammaglobulinemia: a case report. J Med Case Rep 17:194
Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, Diao K, Lin B, Zhu X, Li K (2020) Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology 295:685–691
Berry ME, Kearns H, Graham D, Faulds K (2021) Correction: Surface enhanced Raman scattering for the multiplexed detection of pathogenic microorganisms: towards point-of-use applications. Analyst 146:6335–6336
Bhattacharjee B, Ikbal AM, Farooqui A, Sahu RK, Ruhi S, Syed A, Miatmoko A, Khan D, Khan J (2023) Superior possibilities and upcoming horizons for nanoscience in COVID-19: noteworthy approach for effective diagnostics and management of SARS-CoV-2 outbreak. Chem Papers 77(8):4107–4130
Bogdan I, Gadela T, Bratosin F, Dumitru C, Popescu A, Horhat FG, Negrean RA, Horhat RM, Mot IC, Bota AV (2023) The assessment of multiplex PCR in identifying bacterial infections in patients hospitalized with SARS-CoV-2 Infection: a systematic review. Antibiotics 12:465
Bonam SR, Kotla NG, Bohara RA, Rochev Y, Webster TJ, Bayry J (2021) Potential immuno-nanomedicine strategies to fight COVID-19 like pulmonary infections. Nano Today 36:101051
Boopathi S, Poma AB, Kolandaivel P, Novel, (2019) coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J Biomol Struct Dyn 39(2021):3409–3418
Bradley Z, Coleman PA, Courtney MA, Fishlock S, McGrath J, Uniacke-Lowe T, Bhalla N, McLaughlin JA, Hogan J, Hanrahan JP (2023) Effect of selenium nanoparticle size on IL-6 detection sensitivity in a lateral flow device. ACS Omega 8:8407–8414
Butkovich N, Li E, Ramirez A, Burkhardt AM, Wang SW (2021) Advancements in protein nanoparticle vaccine platforms to combat infectious disease. Wiley Interdiscip Rev: Nanomed Nanobiotechnol 13:e1681
Cao S, Tang X, Chen T, Chen G (2022) Types and applications of nicking enzyme-combined isothermal amplification. Int J Mol Sci 23:4620
Carter LJ, Garner LV, Smoot JW, Li Y, Zhou Q, Saveson CJ, Sasso JM, Gregg AC, Soares DJ, Beskid TR (2020) Assay techniques and test development for COVID-19 diagnosis. ACS Pub 6:591–605
Casati B, Verdi JP, Hempelmann A, Kittel M, Klaebisch AG, Meister B, Welker S, Asthana S, Di Giorgio S, Boskovic P (2022) Rapid, adaptable and sensitive Cas13-based COVID-19 diagnostics using ADESSO. Nat Commun 13:3308
M. Cascella, M. Rajnik, A. Aleem, S.C. Dulebohn, R. Di Napoli, (2022) Features, evaluation, and treatment of coronavirus (COVID-19), Statpearls [internet]
Castillo-Henríquez L, Brenes-Acuña M, Castro-Rojas A, Cordero-Salmerón R, Lopretti-Correa M, Vega-Baudrit JR (2020) Biosensors for the detection of bacterial and viral clinical pathogens. Sensors 20:6926
Cha H, Kim H, Joung Y, Kang H, Moon J, Jang H, Park S, Kwon H-J, Lee I-C, Kim S (2022) Surface-enhanced Raman scattering-based immunoassay for severe acute respiratory syndrome coronavirus 2. Biosens Bioelectron 202:114008
Chan JF-W, Yip CC-Y, To KK-W, Tang TH-C, Wong SC-Y, Leung K-H, Fung AY-F, Ng AC-K, Zou Z, Tsoi H-W (2020) Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens. J Clin Microbiol 58:e00310-00320
Chen L, Liu W, Zhang Q, Xu K, Ye G, Wu W, Sun Z, Liu F, Wu K, Zhong B (2019) RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in, Wuhan outbreak. Emerg Microb Infect 9:313–319
S. Chenchula, K.C. Amerneni, M.K. Ghanta, R. Padmavathi, M.B. Chandra, M.B. Adusumilli, S. Mudda, M. Chavan, R. Gupta, B. Lakhawat, (2022) Clinical Virology and Effect of Vaccination and Monoclonal Antibodies against SARS-CoV-2 Omicron Sub variant BF. 7 (BA. 5.2. 1.7): A systematic review, medRxiv, 2022.2012. 2025.22283940.
Chiara M, D’Erchia AM, Gissi C, Manzari C, Parisi A, Resta N, Zambelli F, Picardi E, Pavesi G, Horner DS (2021) Next generation sequencing of SARS-CoV-2 genomes: challenges, applications and opportunities. Brief Bioinform 22:616–630
Cui H, Song W, Ru X, Fu W, Ji L, Zhou W, Zhao Z, Qu G, Yu X-F, Jiang G (2023) A simplified viral RNA extraction method based on magnetic nanoparticles for fast and high-throughput detection of SARS-CoV-2. Talanta 258:124479
Dankova Z, Novakova E, Skerenova M, Holubekova V, Lucansky V, Dvorska D, Brany D, Kolkova Z, Strnadel J, Mersakova S (2021) Comparison of SARS-CoV-2 detection by rapid antigen and by three commercial RT-qPCR tests: a study from Martin university hospital in Slovakia. Int J Environ Res Public Health 18:7037
Dastoorpoor M, Borsi SH, Khodadadi N, Hanafi MG, Ahmadzadeh S, Zarei J (2023) Epidemiological, clinical and imaging features of 2019 novel coronavirus diseases (COVID-19) in Southwestern Iran: a descriptive study. J Clin Res Paramed Sci 12:e122581
Davies NG, Jarvis CI, Edmunds WJ, Jewell NP, Diaz-Ordaz K, Keogh RH (2021) Increased mortality in community-tested cases of SARS-CoV-2 lineage B. 1.1. 7. Nature 593:270–274
Deng X, Garcia-Knight MA, Khalid MM, Servellita V, Wang C, Morris MK, Sotomayor-González A, Glasner DR, Reyes KR, Gliwa AS, Reddy NP (2021) Transmission, infectivity, and neutralization of a spike L452R SARS-CoV-2 variant. Cell 184(13):3426–3437
Ding X, Yin K, Li Z, Liu C (2020a) All-in-one dual CRISPR-Cas12a (AIOD-CRISPR) assay: a case for rapid, ultrasensitive and visual detection of novel coronavirus SARS-CoV-2 and HIV virus. BioRxiv. https://doi.org/10.1101/2020.03.19.998724
Ding Y, Dou C, Chang S, Xie Z, Yu D-G, Liu Y, Shao J (2020b) Core–shell eudragit s100 nanofibers prepared via triaxial electrospinning to provide a colon-targeted extended drug release. Polymers 12:2034
Du T, Wang Z (2023) High positive rate after consecutive negative tests of SARS-CoV-2. Medicine 102:e33333
Duan Y, Wang S, Zhang Q, Gao W, Zhang L (2021) Nanoparticle approaches against SARS-CoV-2 infection. Curr Opin Solid State Mater Sci 25:100964
Dykman LA, Staroverov SA, Fomin AS, Gabalov KP (2020) The potential of gold nanoparticles for coronavirus diagnosis and prophylaxis, Saratov fall meeting, optical and nanotechnologies for biology and medicine. SPIE 2021:277–284
Einoch Amor R, Levy J, Broza YY, Vangravs R, Rapoport S, Zhang M, Wu W, Leja M, Behar JA, Haick H (2023) Liquid biopsy-based volatile organic compounds from blood and urine and their combined data sets for highly accurate detection of cancer. ACS Sens 8:1450–1461
El-Sherif DM, Abouzid M, Gaballah MS, Ahmed AA, Adeel M, Sheta SM (2022) New approach in SARS-CoV-2 surveillance using biosensor technology: a review. Environ Sci Pollut Res 29:1677–1695
England C, TrejoMartinez J, PerezSanchez P, Karki U, Xu J (2023) Plants as biofactories for therapeutic proteins and antiviral compounds to combat COVID-19. Life 13:617
Eygeris Y, Patel S, Jozic A, Sahay G (2020) Deconvoluting lipid nanoparticle structure for messenger RNA delivery. Nano Lett 20:4543–4549
Ferrareze PA, Franceschi VB, de Menezes MA, Caldana GD, Zimerman RA, Thompson CE (2021) E484K as an innovative phylogenetic event for viral evolution: genomic analysis of the E484K spike mutation in SARS-CoV-2 lineages from Brazil. Infect Genet Evolut 93:104941
Galderisi A, Lista G, Cavigioli F, Trevisanuto D (2023) Clinical features of neonatal COVID-19, Seminars in fetal and neonatal medicine. Elsevier, Amsterdam, p 101430
E.C. Gale, L.J. Lahey, V. Böhnert, A.E. Powell, B.S. Ou, J.A. Carozza, L. Li, E.A. Appel, (2021) A cGAMP-containing hydrogel for prolonged SARS-CoV-2 RBD subunit vaccine exposure induces a broad and potent humoral response, bioRxiv, 2021.2007. 2003.451025.
Galloway SE, Paul P, MacCannell DR, Johansson MA, Brooks JT, MacNeil A, Slayton RB, Tong S, Silk BJ, Armstrong GL, Biggerstaff M (2021) Emergence of SARS-CoV-2 b 1.1. 7 lineage-United States, December 29, 2020-January 12, 2021. Morb Mortal Wkly Rep 70(3):95
García-Fernández J, de la Fuente Freire M (2023) Exosome-like systems: nanotechnology to overcome challenges for targeted cancer therapies. Cancer Lett 561:216151
Garg A, Ghoshal U, Patel SS, Singh D, Arya AK, Vasanth S, Pandey A, Srivastava N (2021) Evaluation of seven commercial RT-PCR kits for COVID-19 testing in pooled clinical specimens. J Med Virol 93:2281–2286
Gentilotti E, De Nardo P, Cremonini E, Górska A, Mazzaferri F, Canziani LM, Hellou MM, Olchowski Y, Poran I, Leeflang M (2022) Diagnostic accuracy of point-of-care tests in acute community-acquired lower respiratory tract infections. A Syst Rev Meta-Anal, Clin Microbiol Infect 28:13–22
Ghaffari A, Meurant R, Ardakani A (2021) COVID-19 point-of-care diagnostics that satisfy global target product profiles. Diagnostics 11:115
Guo L, Wang G, Wang Y, Zhang Q, Ren L, Gu X, Huang T, Zhong J, Wang Y, Wang X (2022) SARS-CoV-2-specific antibody and T-cell responses 1 year after infection in people recovered from COVID-19: a longitudinal cohort study. Lancet Microb 3:e348–e356
Harcourt J, Tamin A, Lu X, Kamili S, Sakthivel SK, Murray J, Queen K, Tao Y, Paden CR, Zhang J (2020) Severe acute respiratory syndrome coronavirus 2 from patient with coronavirus disease, United States. Emerg Infect Dis 26:1266
Hasan S, Dwivedi M, Mukhopadhyay S, Gupta N (2023) Landscape determinants of infectivity and insights into vaccine development and effectiveness-novel coronavirus. Lett Drug Des Discov 20:119–143
S. Hazra, S. Patra, (2023) Carbon nanomaterials in biosensor applications for infectious disease diagnostics, carbon nanostructures in biomedical applications, Springer. pp. 257–283
Hillary VE, Ignacimuthu S, Ceasar SA (2021) Potential of CRISPR/Cas system in the diagnosis of COVID-19 infection. Expert Rev Mol Diagn 21:1179–1189
Holenya P, Lange PJ, Reimer U, Woltersdorf W, Panterodt T, Glas M, Wasner M, Eckey M, Drosch M, Hollidt JM (2021) Peptide microarray-based analysis of antibody responses to SARS-CoV-2 identifies unique epitopes with potential for diagnostic test development. Eur J Immunol 51:1839–1849
Hosseiny M, Kooraki S, Gholamrezanezhad A, Reddy S, Myers L (2019) Radiology perspective of coronavirus disease, (COVID-19): lessons from severe acute respiratory syndrome and Middle East respiratory syndrome. Ajr Am J Roentgenol 214(2020):1078–1082
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X (2020) Clinical features of patients infected with, novel coronavirus in Wuhan, China. Lancet 395:497–506
Idris A, Davis A, Supramaniam A, Acharya D, Kelly G, Tayyar Y, West N, Zhang P, McMillan CL, Soemardy C (2021) A SARS-CoV-2 targeted siRNA-nanoparticle therapy for COVID-19. Mol Ther 29:2219–2226
Islam MA, Hossen F, Rahman MA, Sultana KF, Hasan MN, Haque MA, Sosa-Hernández JE, Oyervides-Muñoz MA, Parra-Saldívar R, Ahmed T (2023) An opinion on wastewater-based epidemiological monitoring (WBEM) with clinical diagnostic test (CDT) for detecting high-prevalence areas of community COVID-19 infections. Curr Opin Environ Sci Health 31:100396
Jiang M, Pan W, Arasthfer A, Fang W, Ling L, Fang H, Daneshnia F, Yu J, Liao W, Pei H (2020) Development and validation of a rapid, single-step reverse transcriptase loop-mediated isothermal amplification (RT-LAMP) system potentially to be used for reliable and high-throughput screening of COVID-19. Front Cell Infect Microbiol 10:331
Jiang K, Wu J, Qiu Y, Go YY, Ban K, Park HJ, Lee J-H (2021a) Plasmonic colorimetric PCR for rapid molecular diagnostic assays. Sens Actuators, B Chem 337:129762
Jiang S, Zhang X, Du L (2021b) Therapeutic antibodies and fusion inhibitors targeting the spike protein of SARS-CoV-2. Exp Opin Ther Targets 25:415–421
Jiang X, Zhu L, Zhan D (2022) Development of a recombinase polymerase amplification assay for rapid detection of Streptococcus suis type 2 in nasopharyngeal swab samples. Diagn Microbiol Infect Dis 102:115594
Johannsen B, Karpíšek M, Baumgartner D, Klein V, Bostanci N, Paust N, Früh SM, Zengerle R, Mitsakakis K (2021) One-step, wash-free, bead-based immunoassay employing bound-free phase detection. Anal Chim Acta 1153:338280
Kang M, Wei J, Yuan J, Guo J, Zhang Y, Hang J, Qu Y, Qian H, Zhuang Y, Chen X (2020) Probable evidence of fecal aerosol transmission of SARS-CoV-2 in a high-rise building. Ann Intern Med 173:974–980
Kang H, Wang X, Guo M, Dai C, Chen R, Yang L, Wu Y, Ying T, Zhu Z, Wei D (2021) Ultrasensitive detection of SARS-CoV-2 antibody by graphene field-effect transistors. Nano Lett 21:7897–7904
Kannan S, Shaik Syed Ali P, Sheeza A (2021) Omicron (B 1.1. 529)-variant of concern-molecular profile and epidemiology: a mini review. Eur Rev Med Pharmacol Sci 25:8019–8022
Kevadiya BD, Machhi J, Herskovitz J, Oleynikov MD, Blomberg WR, Bajwa N, Soni D, Das S, Hasan M, Patel M (2021) Diagnostics for SARS-CoV-2 infections. Nat Mater 20:593–605
Khalili N, Haseli S, Bahrami-Motlagh H, Keshavarz E, Khalili N, Langroudi TF, Khameneh A, Taheri MS (2020) Neurologic involvement in COVID-19: radiologists’ perspective. Acad Radiol 27:1051
Khizar S, Al-Dossary AA, Zine N, Jaffrezic-Renault N, Errachid A, Elaissari A (2022) Contribution of magnetic particles in molecular diagnosis of human viruses. Talanta 241:123243
Kim S, Hao Y, Miller EA, Tay DM, Yee E, Kongsuphol P, Jia H, McBee M, Preiser PR, Sikes HD (2021) Vertical flow cellulose-based assays for SARS-CoV-2 antibody detection in human serum. ACS Sens 6:1891–1898
Kohmer N, Rühl C, Ciesek S, Rabenau HF (2021) Utility of different surrogate enzyme-linked immunosorbent assays (sELISAs) for detection of SARS-CoV-2 neutralizing antibodies. J Clin Med 10:2128
Kumar S, Nehra M, Khurana S, Dilbaghi N, Kumar V, Kaushik A, Kim K-H (2021a) Aspects of point-of-care diagnostics for personalized health wellness. Int J Nanomed 16:383
Kumar A, Dowling WE, Román RG, Chaudhari A, Gurry C, Le TT, Tollefson S, Clark CE, Bernasconi V, Kristiansen PA (2021b) Status report on COVID-19 vaccines development. Curr Infect Dis Rep 23:1–12
Kumar A, Parihar A, Panda U, Parihar DS (2022) Microfluidics-based point-of-care testing (POCT) devices in dealing with waves of COVID-19 pandemic: the emerging solution. ACS Appl Bio Mater 5:2046–2068
Kumar N, Santhoshkumar R, Prasad P, George AK, Aiyar J, Joshi S, Narayanappa G, Desai AS, Ravi V, Venkataswamy MM (2023) An ultrastructural and genomic study on the SARS-CoV-2 variant B. 1.210 circulating during the first wave of COVID-19 pandemic in India. Indian J Med Microbiol 41:45–52
Kurup D, Schnell MJ (2021) SARS-CoV-2 vaccines-the biggest medical research project of the 21st century. Curr Opin Virol 49:52–57
Kyriakidis NC, López-Cortés A, González EV, Grimaldos AB, Prado EO (2021) SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. Npj Vacc 6(1):28
Lamote K, Janssens E, Schillebeeckx E, Lapperre TS, De Winter BY, Van Meerbeeck J (2020) The scent of COVID-19: viral (semi-) volatiles as fast diagnostic biomarkers? J Breath Res 14:042001
R. Lassaunière, A. Frische, Z.B. Harboe, A.C. Nielsen, A. Fomsgaard, K.A. Krogfelt, C.S. Jørgensen, (2020) Evaluation of nine commercial SARS-CoV-2 immunoassays, MedRxiv.
Le TT, Andreadakis Z, Kumar A, Román RG, Tollefsen S, Saville M, Mayhew S (2020) The COVID-19 vaccine development landscape. Nat Rev Drug Discov 19:305–306
Lei R, Wang D, Arain H, Mohan C (2022) Design of gold nanoparticle vertical flow assays for point-of-care testing. Diagnostics 12:1107
Li Z, Yi Y, Luo X, Xiong N, Liu Y, Li S, Sun R, Wang Y, Hu B, Chen W (2020a) Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol 92:1518–1524
Li M, Jin R, Peng Y, Wang C, Ren W, Lv F, Gong S, Fang F, Wang Q, Li J, Shen T (2020b) Generation of antibodies against COVID-19 virus for development of diagnostic tools. MedRxiv. https://doi.org/10.1101/2020.02.20.20025999.abstract
Li Z, Wang Z, Dinh P-UC, Zhu D, Popowski KD, Lutz H, Hu S, Lewis MG, Cook A, Andersen H (2021) Cell-mimicking nanodecoys neutralize SARS-CoV-2 and mitigate lung injury in a non-human primate model of COVID-19. Nat Nanotechnol 16:942–951
Li M, Du J, Liu W, Li Z, Lv F, Hu C, Dai Y, Zhang X, Zhang Z, Liu G (2023) Comparative susceptibility of SARS-CoV-2, SARS-CoV, and MERS-CoV across mammals. ISME J 17:549–560
Li DF, Liu QS, Yang MF, Xu HM, Zhu MZ, Zhang Y, Xu J, Tian CM, Yao J, Wang LS, Liang YJ (2023) Nanomaterials for mRNA-based therapeutics: challenges and opportunities. Bioeng Transl Med. https://doi.org/10.1002/btm2.10492
Liu J, Yu H, Zhang S (2019) The indispensable role of chest CT in the detection of coronavirus disease, COVID-19. Eur J Nucl Med Mol Imaging 47(2020):1638–1639
Liu W, Kou G, Dong Y, Zheng Y, Ding Y, Ni W, Wu W, Tang S, Xiong Z, Zhang Y (2020a) Clinical application of chemiluminescence microparticle immunoassay for SARS-CoV-2 infection diagnosis. J Clin Virol 130:104576
Liu R, Han H, Liu F, Lv Z, Wu K, Liu Y, Feng Y, Zhu C (2020b) Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin Chim Acta 505:172–175
Liu W, Liu L, Kou G, Zheng Y, Ding Y, Ni W, Wang Q, Tan L, Wu W, Tang S (2020c) Evaluation of nucleocapsid and spike protein-based enzyme-linked immunosorbent assays for detecting antibodies against SARS-CoV-2. J Clin Microbiol 58:e00461-e1420
Liu Z, Yan J, Tong L, Liu S, Zhang Y (2022) The role of exosomes from BALF in lung disease. J Cell Physiol 237:161–168
Lucia C, Federico PB, Alejandra GC (2020) An ultrasensitive, rapid, and portable coronavirus SARS-CoV-2 sequence detection method based on CRISPR-Cas12. BioRxiv. https://doi.org/10.1101/2020.02.29.971127v1.abstract
Ma J, Qi X, Chen H, Li X, Zhang Z, Wang H, Sun L, Zhang L, Guo J, Morawska L (2019) Coronavirus disease patients in earlier stages exhaled millions of severe acute respiratory syndrome coronavirus 2 per hour. Clin Infect Dis 72(2021):e652–e654
Maharjan PM, Choe S (2021) Plant-based COVID-19 vaccines: current status, design, and development strategies of candidate vaccines. Vaccines 9:992
Mathuria JP, Yadav R (2020) Laboratory diagnosis of SARS-CoV-2-A review of current methods. J Infect Public Health 13:901–905
McNamee M, Wong S, Guy O, Sharma S (2023) Microneedle technology for potential SARS-CoV-2 vaccine delivery. Exp Opinion Drug Deliv. https://doi.org/10.1080/17425247.2023.2209718
Miller EH, Annavajhala MK, Chong AM, Park H, Nobel YR, Soroush A, Blackett JW, Krigel A, Phipps MM, Freedberg DE (2021) Oral microbiome alterations and SARS-CoV-2 saliva viral load in patients with COVID-19. Microbiol Spectr. https://doi.org/10.1128/Spectrum.00055-21
Miripour ZS, Sarrami-Forooshani R, Sanati H, Makarem J, Taheri MS, Shojaeian F, Eskafi AH, Abbasvandi F, Namdar N, Ghafari H (2020) Real-time diagnosis of reactive oxygen species (ROS) in fresh sputum by electrochemical tracing; correlation between COVID-19 and viral-induced ROS in lung/respiratory epithelium during this pandemic. Biosens Bioelectron 165:112435
Mitchell SL, Ventura SE (2020) Evaluation and comparison of the hologic aptima SARS-CoV-2 assay and the CDC 2019-nCoV real-time reverse transcription-PCR diagnostic panel using a four-sample pooling approach. J Clin Microbiol 58:e02241-e12220
Moitra P, Alafeef M, Dighe K, Frieman MB, Pan D (2020) Selective naked-eye detection of SARS-CoV-2 mediated by N gene targeted antisense oligonucleotide capped plasmonic nanoparticles. ACS Nano 14:7617–7627
Moore SC, Penrice-Randal R, Alruwaili M, Dong X, Pullan ST, Carter DP, Bewley K, Zhao Q, Sun Y, Hartley C (2020) Amplicon based MinION sequencing of SARS-CoV-2 and metagenomic characterisation of nasopharyngeal swabs from patients with COVID-19. MedRxiv. https://doi.org/10.1101/2020.03.05.20032011.abstract
Moulahoum H, Ghorbanizamani F, Zihnioglu F, Turhan K, Timur S (2021) How should diagnostic kits development adapt quickly in COVID 19-like pandemic models? Pros and cons of sensory platforms used in COVID-19 sensing. Talanta 222:121534
Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Raabe V, Bailey R, Swanson KA (2020) Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 586:589–593
Muthiah G, Sarkar A, Roy S, Singh P, Kumar P, Bhardwaj K, Jaiswal A (2022) Nanotechnology toolkit for combating COVID-19 and beyond. ChemNanoMat 8:e202100505
Nasiri H, Alavi SA (2022) A novel framework based on deep learning and ANOVA feature selection method for diagnosis of COVID-19 cases from chest X-ray images. Comput Intell Neurosci. https://doi.org/10.1155/2022/4694567
Nguyen NN, McCarthy C, Lantigua D, Camci-Unal G (2020) Development of diagnostic tests for detection of SARS-CoV-2. Diagnostics 10:905
NguyenVan J-C, Gerlier C, Pilmis B, Mizrahi A, de Ponfilly GP, Khaterchi A, Enouf V, Ganansia O, Le Monnier A (2021) Prospective evaluation of ID NOW COVID-19 assay used as point-of-care test in an emergency department. J Clin Virol 145:105021
Nickbakhsh S, Ho A, Marques DF, McMenamin J, Gunson RN, Murcia PR (2020) Epidemiology of seasonal coronaviruses: establishing the context for the emergence of coronavirus disease 2019. J Infect Dis 222:17–25
Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al-Jabir A, Iosifidis C, Agha M, Agha R (2020) The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg 78:185–193
Nie J, Li Q, Wu J, Zhao C, Hao H, Liu H, Zhang L, Nie L, Qin H, Wang M (2020) Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg Microb Infect 9:680–686
Ning B, Yu T, Zhang S, Huang Z, Tian D, Lin Z, Niu A, Golden N, Hensley K, Threeton B, Lyon CJ (2021) A smartphone-read ultrasensitive and quantitative saliva test for COVID-19. Sci Adv 7(2):eabe3703
Nobel YR, Phipps M, Zucker J, Lebwohl B, Wang TC, Sobieszczyk ME, Freedberg DE (2020) Gastrointestinal symptoms and coronavirus disease 2019: a case-control study from the United States. Gastroenterology 159:373–375
Nooraei S, Bahrulolum H, Hoseini ZS, Katalani C, Hajizade A, Easton AJ, Ahmadian G (2021) Virus-like particles: preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J Nanobiotechnol 19:1–27
Ozer EA, Simons LM, Adewumi OM, Fowotade AA, Omoruyi EC, Adeniji JA, Dean TJ, Taiwo BO, Hultquist JF, Lorenzo-Redondo R (2021) High prevalence of SARS-CoV-2 B. 1.1. 7 (UK variant) and the novel B. 1.5. 2.5 lineage in Oyo State, Nigeria. Medrxiv 4(09):212552065
J. Papenburg, M.P. Cheng, R. Corsini, C. Caya, E. Mendoza, K. Manguiat, L.R. Lindsay, H. Wood, M.A. Drebot, A. Dibernardo, (2021) Evaluation of a commercial culture-free neutralization antibody detection kit for severe acute respiratory syndrome-related coronavirus-2 and comparison with an antireceptor-binding domain enzyme-linked immunosorbent assay, open forum infectious diseases, Oxford University Press US. ofab220.
Park G-S, Ku K, Baek S-H, Kim S-J, Kim SI, Kim B-T, Maeng J-S (2020) Development of reverse transcription loop-mediated isothermal amplification assays targeting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). J Mol Diagn 22:729–735
Park WB, Hwang YH, Cheong HJ (2023a) COVID-19 vaccination in Korea. Infect Chemother 55:135
Park E, Choi SY, Kim J, Hildebrandt N, Lee JS, Nam JM (2023b) Nanotechnologies for the diagnosis and treatment of SARS-CoV-2 and its variants. Small Methods. https://doi.org/10.1002/smtd.202300034
Poggiali E, Dacrema A, Bastoni D, Tinelli V, Demichele E, Mateo Ramos P, Marcianò T, Silva M, Vercelli A, Magnacavallo A (2020) Can lung US help critical care clinicians in the early diagnosis of novel coronavirus (COVID-19) pneumonia? Radiology 295:E6–E6
Popowski K, Lutz H, Hu S, George A, Dinh P-U, Cheng K (2020) Exosome therapeutics for lung regenerative medicine. J Extracell Veh 9:1785161
Pouresmaieli M, Ekrami E, Akbari A, Noorbakhsh N, Moghadam NB, Mamoudifard M (2021) A comprehensive review on efficient approaches for combating coronaviruses. Biomed Pharmacother 144:112353
Qiu G, Gai Z, Tao Y, Schmitt J, Kullak-Ublick GA, Wang J (2020) Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano 14:5268–5277
Rahayu T, Pertiwi KR, Kushartanti W, Arovah NI (2023) Physical activity and post-COVID-19 syndrome in older adults: a systematic review, international journal of kinesiology and sports. Science 11:42–52
Rani PR, Imran M, Lakshmi JV, Jolly B, Jain A, Surekha A, Senthivel V, Chandrasekhar P, Divakar MK, Srinivasulu D (2021) Symptomatic reinfection of SARS-CoV-2 with spike protein variant N440K associated with immune escape. J Med Virol 93:4163
Rödel J, Egerer R, Suleyman A, Sommer-Schmid B, Baier M, Henke A, Edel B, Löffler B (2020) Use of the variplex™ SARS-CoV-2 RT-LAMP as a rapid molecular assay to complement RT-PCR for COVID-19 diagnosis. J Clin Virol 132:104616
Sahu RK, Salem-Bekhit MM, Bhattacharjee B, Almoshari Y, Ikbal AMA, Alshamrani M, Bharali A, Salawi A, Widyowati R, Alshammari A (2021) Mucormycosis in Indian COVID-19 patients: insight into its patho-genesis, clinical manifestation, and management strategies. Antibiotics 10:1079
Salahandish R, Hyun JE, Haghayegh F, Tabrizi HO, Moossavi S, Khetani S, Ayala-Charca G, Berenger BM, Niu YD, Ghafar-Zadeh E, Nezhad AS (2023) CoVSense: ultrasensitive nucleocapsid antigen immunosensor for rapid clinical detection of wildtype and variant SARS-CoV-2. Adv Sci. https://doi.org/10.1002/advs.202206615
Salzberger B, Buder F, Lampl B, Ehrenstein B, Hitzenbichler F, Holzmann T, Schmidt B, Hanses F (2021) Epidemiology of SARS-CoV-2. Infection 49:233–239
C. Samal, K. Jakimowicz, K. Dasgupta, A. Vashishtha, A. Natarajan, H. Nazir, A.S. Varma, T. Dahake, A.A. Pandey, I. Singh, (2021) Vaccination Worldwide: Strategies, Distribution and Challenges, arXiv preprint arXiv:2107.14139
Sarma P, Shekhar N, Prajapat M, Avti P, Kaur H, Kumar S, Singh S, Kumar H, Prakash A, Dhibar DP (2021) In-silico homology assisted identification of inhibitor of RNA binding against 2019-nCoV N-protein (N terminal domain). J Biomol Struct Dyn 39:2724–2732
Schultz JS, Mace KE, Tan KR (2019) Return to travel in the coronavirus disease, pandemic recovery period and implications for imported malaria: reinforcing prevention early diagnosis, and appropriate treatment of malaria. Clin Infect Dis 76:1161–1163
Sebbar E-H, Choukri M (2023) Interleukin 6: A biomarker for COVID-19 progression. Mater Today: Proc 72:3351–3355
Seo G, Lee G, Kim MJ, Baek S-H, Choi M, Ku KB, Lee C-S, Jun S, Park D, Kim HG (2020) Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano 14:5135–5142
Serrano MM, Rodríguez DN, Palop NT, Arenas RO, Córdoba MM, Mochón MDO, Cardona CG (2020) Comparison of commercial lateral flow immunoassays and ELISA for SARS-CoV-2 antibody detection. J Clin Virol 129:104529
Shafaati M, Saidijam M, Soleimani M, Hazrati F, Mirzaei R, Amirheidari B, Tanzadehpanah H, Karampoor S, Kazemi S, Yavari B (2022) A brief review on DNA vaccines in the era of COVID-19. Futur Virol 17:49–66
Shan B, Broza YY, Li W, Wang Y, Wu S, Liu Z, Wang J, Gui S, Wang L, Zhang Z (2020) Multiplexed nanomaterial-based sensor array for detection of COVID-19 in exhaled breath. ACS Nano 14:12125–12132
Shen Z, Xiao Y, Kang L, Ma W, Shi L, Zhang L, Zhou Z, Yang J, Zhong J, Yang D (2020) Genomic diversity of severe acute respiratory syndrome–coronavirus 2 in patients with coronavirus disease 2019. Clin Infect Dis 71:713–720
Shinn J, Kwon N, Lee SA, Lee Y (2022) Smart pH-responsive nanomedicines for disease therapy. J Pharm Investig 52:427–441
Simon DS, Yew C-W, Kumar VS (2023) Multiplexed reverse transcription loop-mediated isothermal amplification coupled with a nucleic acid-based lateral flow dipstick as a rapid diagnostic method to detect SARS-CoV-2. Microorganisms 11:1233
Singh D, Yi SV (2021) On the origin and evolution of SARS-CoV-2. Exp Mol Med 53:537–547
Singh R, Kang A, Luo X, Jeyanathan M, Gillgrass A, Afkhami S, Xing Z (2021) COVID-19: Current knowledge in clinical features, immunological responses, and vaccine development. FASEB J 35:e21409
Škapars R, Gašenko E, Broza YY, Sīviņš A, Poļaka I, Bogdanova I, Pčolkins A, Veliks V, Folkmanis V, Lesčinska A (2023) Breath volatile organic compounds in surveillance of gastric cancer patients following radical surgical management. Diagnostics 13:1670
Smyrlaki I, Ekman M, Lentini A, Rufino de Sousa N, Papanicolaou N, Vondracek M, Aarum J, Safari H, Muradrasoli S, Rothfuchs AG (2020) Massive and rapid COVID-19 testing is feasible by extraction-free SARS-CoV-2 RT-PCR. Nat Commun 11:4812
Song J, El-Tholoth M, Li Y, Graham-Wooten J, Liang Y, Li J, Li W, Weiss SR, Collman RG, Bau HH (2021) Single-and two-stage, closed-tube, point-of-care, molecular detection of SARS-CoV-2. Anal Chem 93:13063–13071
Stefańska K, Józkowiak M, Angelova Volponi A, Shibli JA, Golkar-Narenji A, Antosik P, Bukowska D, Piotrowska-Kempisty H, Mozdziak P, Dzięgiel P (2023) The role of exosomes in human carcinogenesis and cancer therapy-recent findings from molecular and clinical research. Cells 12:356
Subramaniyan B, Gurung S, Bodas M, Moore AR, Larabee JL, Reuter D, Georgescu C, Wren JD, Myers DA, Papin JF (2023) The isolation and in vitro differentiation of primary fetal baboon tracheal epithelial cells for the study of SARS-CoV-2 host-virus interactions. Viruses 15:862
Suresh S, Chattamvelli R (2022) Simple techniques to predict the onset of pandemics. Int J Comput Appl 184:22–25
Suthar MS, Zimmerman MG, Kauffman RC, Mantus G, Linderman SL, Hudson WH, Vanderheiden A, Nyhoff L, Davis CW, Adekunle O (2020) Rapid generation of neutralizing antibody responses in COVID-19 patients. Cell Rep Med 1:100040
Szabó GT, Mahiny AJ, Vlatkovic I (2022) COVID-19 mRNA vaccines: platforms and current developments. Mol Ther 30:1850–1868
Truong PL, Yin Y, Lee D, Ko SH (2023) Advancement in COVID-19 detection using nanomaterial-based biosensors. InExploration 3(1):20210232
A.R. Uğur, M. Özdemir, (2023) Emerging technologies for COVID-19 diagnosis prevention and management, Omics Approaches and Technologies in COVID-19, 389–404.
Vasireddy D, Vanaparthy R, Mohan G, Malayala SV, Atluri P (2021) Review of COVID-19 variants and COVID-19 vaccine efficacy: What the clinician should know? J Clin Med Res 13:317
Voloch CM, da Silva Francisco Jr R, de Almeida LG, Cardoso CC, Brustolini OJ, Gerber AL, Guimarães APdC, Mariani D, da Costa RM, Ferreira Jr OC (2021) Genomic characterization of a novel SARS-CoV-2 lineage from Rio de Janeiro Brazil. J Virol 95:e00119-00121
Walach H, Klement RJ, Aukema W (2021) The safety of covid-19 vaccinations—we should rethink the policy. Vaccines 9:693
Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W (2020a) Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 323:1843–1844
Wang H, Wu X, Zhang X, Hou X, Liang T, Wang D, Teng F, Dai J, Duan H, Guo S (2020b) SARS-CoV-2 proteome microarray for mapping COVID-19 antibody interactions at amino acid resolution. ACS Cent Sci 6:2238–2249
Wang H, Li X, Li T, Zhang S, Wang L, Wu X, Liu J (2020c) The genetic sequence, origin, and diagnosis of SARS-CoV-2. Eur J Clin Microbiol Infect Dis 39:1629–1635
Wang X, Zhong M, Liu Y, Ma P, Dang L, Meng Q, Wan W, Ma X, Liu J, Yang G (2020d) Rapid and sensitive detection of COVID-19 using CRISPR/Cas12a-based detection with naked eye readout, CRISPR/Cas12a-NER. Sci Bull 65:1436
Wang C, Li W, Drabek D, Okba NM, van Haperen R, Osterhaus AD, van Kuppeveld FJ, Haagmans BL, Grosveld F, Bosch B-J (2020e) A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun 11:2251
Wang Z, Liu Y, Wei L, Ji JS, Liu Y, Liu R, Zha Y, Chang X, Zhang L, Liu Q (2022a) What are the risk factors of hospital length of stay in the novel coronavirus pneumonia (COVID-19) patients? A survival analysis in Southwest China. PLoS ONE 17:e0261216
Wang J, Drelich AJ, Hopkins CM, Mecozzi S, Li L, Kwon G, Hong S (2022b) Gold nanoparticles in virus detection: recent advances and potential considerations for SARS-CoV-2 testing development. Wiley Interdiscip Rev: Nanomed Nanobiotechnol 14:e1754
Wang Z, Popowski KD, Zhu D, de Juan Abad BL, Wang X, Liu M, Lutz H, De Naeyer N, DeMarco CT, Denny TN (2022c) Exosomes decorated with a recombinant SARS-CoV-2 receptor-binding domain as an inhalable COVID-19 vaccine. Nat Biomed Eng 6:791–805
Whitman JD, Hiatt J, Mowery CT, Shy BR, Yu R, Yamamoto TN, Rathore U, Goldgof GM, Whitty C, Woo JM (2020) Evaluation of SARS-CoV-2 serology assays reveals a range of test performance. Nat Biotechnol 38:1174–1183
Wong HYF, Lam HYS, Fong AH-T, Leung ST, Chin TW-Y, Lo CSY, Lui MM-S, Lee JCY, Chiu KW-H, Chung TW-H (2020) Frequency and distribution of chest radiographic findings in patients positive for COVID-19. Radiology 296:E72–E78
World Health Organization, Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: interim guidance, 2 March 2020, World Health Organization, 2020.
Wu J-L, Tseng W-P, Lin C-H, Lee T-F, Chung M-Y, Huang C-H, Chen S-Y, Hsueh P-R, Chen S-C (2020) Four point-of-care lateral flow immunoassays for diagnosis of COVID-19 and for assessing dynamics of antibody responses to SARS-CoV-2. J Infect 81:435–442
Wu L, Wu L-P, Wu J, Sun J, He Z, Rodriguez-Rodriguez C, Saatchi K, Dailey LA, Hafeli UO, Cun D (2021) Poly (lactide-co-glycolide) nanoparticles mediate sustained gene silencing and improved biocompatibility of siRNA delivery systems in mouse lungs after pulmonary administration. ACS Appl Mater Interfaces 13:3722–3737
Xia S, Chen X (2020) Single-copy sensitive, field-deployable, and simultaneous dual-gene detection of SARS-CoV-2 RNA via modified RT–RPA. Cell Discov 6:37
Xia J, Tong J, Liu M, Shen Y, Guo D (2020) Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol 92:589–594
Xiao F, Sun J, Xu Y, Li F, Huang X, Li H, Zhao J, Huang J, Zhao J (2020) Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerg Infect Dis 26:1920
Xiao H, Hu J, Huang C, Feng W, Liu Y, Kumblathan T, Tao J, Xu J, Le XC, Zhang H (2023) CRISPR techniques and potential for the detection and discrimination of SARS-CoV-2 variants of concern. TrAC Trends Anal Chem 2:117000
Yadav SK, Yadav RD, Tabassum H, Arya M (2023) Recent developments in nanotechnology-based biosensors for the diagnosis of coronavirus. Plasmonics 18(3):955–969
Yang Z, Ma Y, Zhao H, Yuan Y, Kim BY (2020) Nanotechnology platforms for cancer immunotherapy. Wiley Interdiscip Rev: Nanomed Nanobiotechnol 12:e1590
Ye G, Liu B, Li F (2022) Cryo-EM structure of a SARS-CoV-2 omicron spike protein ectodomain. Nat Commun 13:1214
Yu L, Wu S, Hao X, Dong X, Mao L, Pelechano V, Chen W-H, Yin X (2020) Rapid detection of COVID-19 coronavirus using a reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) diagnostic platform. Clin Chem 66:975–977
Yüce M, Filiztekin E, Özkaya KG (2021) COVID-19 diagnosis-a review of current methods. Biosens Bioelectron 172:112752
Żak MM, Stock A, Stadlbauer D, Zhang W, Cummings K, Marsiglia W, Zargarov A, Amanat F, Tamayo M, Cordon-Cardo C (2021) Development and characterization of a quantitative ELISA to detect anti-SARS-CoV-2 spike antibodies. Heliyon 7:e08444
Zhang Q, Honko A, Zhou J, Gong H, Downs SN, Vasquez JH, Fang RH, Gao W, Griffiths A, Zhang L (2020) Cellular nanosponges inhibit SARS-CoV-2 infectivity. Nano Lett 20:5570–5574
Zhang W, Davis BD, Chen SS, Martinez JMS, Plummer JT, Vail E (2021) Emergence of a novel SARS-CoV-2 variant in Southern California. JAMA 325:1324–1326
Zhao Z, Cui H, Song W, Ru X, Zhou W, Yu X (2020) A simple magnetic nanoparticles-based viral RNA extraction method for efficient detection of SARS-CoV-2. BioRxiv. https://doi.org/10.1101/2020.02.22.961268
Zhu X, Wang X, Han L, Chen T, Wang L, Li H, Li S, He L, Fu X, Chen S (2020) Multiplex reverse transcription loop-mediated isothermal amplification combined with nanoparticle-based lateral flow biosensor for the diagnosis of COVID-19. Biosens Bioelectron 166:112437
Ziegler CG, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, Cao Y, Yousif AS, Bals J, Hauser BM (2020) SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 181:1016–1035
Author information
Authors and Affiliations
Contributions
BB and SS did conceptualization; BB, DR, SC, AN and ABA done writing—original draft preparation; SS, RKS, SC and JK contributed to writing—review and editing; all authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors that there is no conflict of interest.
Human and animal participants
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhattacharjee, B., Rynjah, D., Ahmed, A.B. et al. Overview of diagnostic tools and nano-based therapy of SARS-CoV-2 infection. Chem. Pap. 78, 2123–2154 (2024). https://doi.org/10.1007/s11696-023-03271-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-03271-8