Abstract
Stably retaining the water-soluble molybdate within electrodes is of great significance in phosphate analysis. This study fabricated an [Omim]6Mo7O24 carbon paste electrode (OCPE), which was able to effectively retain molybdate in the aqueous solution. The electrochemistry of phosphate at the surface of the OCPE was studied using cyclic voltammetry (CV). The voltammograms showed three reversible redox peaks corresponding to the redox of Mo(VI/V) as well as several irreversible peaks involving the formation of hydrogen molybdenum bronze. The currents of all three reversible redox peaks linearly varied versus phosphate concentrations in 0.1 mol·L−1 H2SO4-KCl. After optimizing the mass ratio of the [Omim]6Mo7O24 and carbon powder, peak C6 shows a wide linear range (1.0 × 10–2–1.0 × 10–7 mol·L−1; R2 = 0.9970) with a sensitivity of 5.3 (± 0.1) μA/μM. The limit of detection (LOD = 3σ/s) was 8.5 × 10–8 mol·L−1. Interferences from common anions, silicate, and glyphosate on phosphate analysis were also investigated. The proposed sensing method was successfully applied to the determination of phosphate in tap water, whose results were verified with ion chromatography (IC).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Determination of inorganic phosphate is important for environmental and life science applications (Macintosh et al. 2018; Ellam and Chico 2012). Therefore, there is an ongoing interest in both environmental researchers and bioanalytical chemists to develop simple, sensitive, and cost-effective approaches for phosphate analysis. Various analytical techniques have been developed to analyze the concentration of phosphate in environmental, industrial, clinical, and biological samples including ion chromatography with capillary electrophoresis, (Ruiz-Calero and Galceran 2005; Chang et al. 2005) colorimetry, (Yan et al. 2019; Liu et al. 2013; He et al. 2013) optical fluorescence, (Nagaraj et al. 2021; Zhang et al. 2022) spectrophotometry, (Snigur et al. 2020; Clinton-Bailey et al. 2017) and electrochemistry (Forano et al. 2018). Electrochemical methods have several advantages over other techniques including good detection sensitivity, selectivity, high throughput, miniaturization, cost-effectiveness, adaptability, and suitability for field measurements. Other than potentiometric methods, the most popular electroanalytical method for the determination of phosphate involves the treatment of the sample with an acidic molybdate solution to convert the phosphate into the Keggin anions (PMo12O403−), with subsequent electrochemical reduction leading to mixed molybdenum oxidation state(Nagul et al. 2015). The use of direct electroreduction techniques can lead to portability and excellent sensitivity, which make them very attractive for on-site monitoring of phosphate.
In general, the source of Mo used for the electrochemical phosphate detection is water-soluble salts such as Na2MoO4 (VI) and (NH4)6Mo7O24(VI) even when the reaction must occur on the surface of an electrode rather than in solution. Hence, the preparation of electrodes requires one to stably retain the water-soluble molybdate in an aqueous solution. Multiple papers have reported on the preparation or modification of electrodes with molybdenum sources utilizing a range of different materials such as graphite inks, (Lu et al. 2021a) carbon black nanoparticles, (Kumar et al. 2021) poly (vinyl chloride), (Kolliopoulos et al. 2015; Talarico et al. 2015) poly(vinylidene fluoride-co-hexafluoropropylene, (Cinti et al. 2016) chitosan ionic–liquid hybrid matrix, (Berchmans et al. 2011) and zirconium dioxide/zinc oxide/multiple-wall carbon nanotubes (Lu et al. 2021b) to achieve superior analytical performance for the phosphate analysis. An electrochemical sensor was prepared via zirconium dioxide, zinc oxide, multiple-wall carbon nanotubes, ammonium molybdate tetrahydrate, and a screen-printed electrode. This sensor was used to explore the electro-redox reaction of phosphomolybdate complexes on the surface of the electrode, thus resulting in a lower detection limit (LOD = 2.0 × 10–8 mol L−1). (Lu et al. 2021b) However, most phosphomolybdate-based methods that use voltammetry are commonly accompanied by tedious fabrication with the electrode decorated via nanomaterials to increase sensitivity. Therefore, phosphate-measuring electrodes with a simple fabrication method are always desirable.
Recently, we reported on the synthesis of [Omim]6Mo7O24 and its application in potentiometric PF6− ion sensors. (Shi et al. 2021) Here, we fabricate [Omim]6Mo7O24-carbon paste electrodes (OCPEs) and apply them to the determination of phosphate in tap water.
Experimental
Materials
Chemicals
The 1-octyl-3-methylimidazolium chloride (≥ 97%), ammonium molybdate tetrahydrate (AR), silicone oil (10 mPa‧s, 25 °C), carbon powder (3000 mesh), sodium dihydrogen phosphate anhydrous (≥ 99%), and potassium chloride (≥ 99%) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. Potassium hydroxide (≥ 95%), sulfuric acid, and hydrochloric acid were obtained from Sinopharm Chemical Reagent Co., Ltd. All chemicals were used as received without additional purification. All solutions were prepared with water purified in a Milli-Q System (Millipore, Bedford, MA, USA).
The phosphate standard solution was prepared as follows: The KCl electrolyte (0.7455 g) was dissolved in 100 mL of 0.1 mol·L−1 sulfuric acid solution to obtain 0.1 mol·L−1 H2SO4-KCl mixed solution. We then weighed 0.1380 g sodium dihydrogen phosphate monohydrate and added it to 10.0 mL of the above-mixed solution. The solution was stirred and vibrated fully to dissolve it and obtain 0.10 mol·L−1 phosphate stock solution. We then used a pipette to transfer 1.0 mL of phosphate stock solution into another test tube and added 9.0 mL of 0.10 mol·L−1 H2SO4-KCl mixed solution to obtain 1.0 × 10–2 mol·L−1 phosphate stock solution. This process was repeated to obtain a series of 1.0 × 10–2–1.0 × 10–8 mol·L−1 phosphate standard solutions.
Apparatus
Voltammetric measurements were collected using a CHI800D electrochemical analyzer (Shanghai Chenhua Instrument Co., Ltd.) with an Ag/AgCl reference electrode (CHI111) and a Pt wire counter electrode (CHI115) connected to a laptop (Fig. 1). A digital magnetic stirrer (HS501, IKA, Guangzhou, China) and clinical centrifuge (L420-A, Cence, Hunan Changsha, China) were used for [Omim]6Mo7O24 synthesis. Metrohm 883 IC + ion chromatography verified the phosphate concentration in tap water.
Synthesis of [Omim]6Mo7O24
[Omim]6Mo7O24 was synthesized according to the literature Shi et al. (2021). Briefly, 20 mL of 50 mmol·L−1 1-octyl-3-methylimidazolium chloride solution was added dropwise into a 100-mL beaker pre-loaded with 20 mL of 50 mmol·L−1 ammonium molybdate solution. This was stirred at room temperature for 30 min. The resulting white suspension was centrifuged at 4000 r·min−1 for 20 min and washed with small amounts of water until no chloride impurities could be detected by the silver nitrate test. The final milky white solid was dried in vacuum at 60 °C for 4 h, followed by ball milling with an agate mortar by hand to obtain a homogeneous paste around micron-sizes.
Preparation of OCPE
We started with a beaker containing 255 mg of carbon powder and 50 mg of silicon oil and then, added different quantities of [Omim]6Mo7O24 with mass ratios of 0, 5%, 10%, 15%, and 20%. After mixing for 30 min at room temperature, the homogeneous slurry was distributed into an φ 4.0-mm plastic syringe with the syringe rod under hand-pressure. An extrinsic electrical junction was approved by enforcing a copper wire down the syringe, which was followed by polishing the CPE surface with 1000-, 4000-, and 8000-mesh SiC abrasive papers to obtain an OCPE. This was then left to dry overnight at room temperature.
Characteristics of OCPE
Scanning electron micrographs (SEMs) were collected with an S-4800II field emission scanning electron microscope (Hitachi, Japan) equipped with Noran EDS (Thermo Electron Corporation) to obtain morphological characteristics of OCPE. The XPS spectrum of the surface layer was obtained using ESCALAB250Xi model (Thermo Fisher Scientific, USA) to confirm the molybdenum species on the OCPE surface layer.
Experimental conditions
The OCPE working electrode (WE), platinum wire counter electrode (CE), and Ag/AgCl/KCl (3 mol∙L−1) reference electrode (RE) were connected to the electrochemical workstation. CV was initially performed in 10 mL of supporting electrolyte solution consisting of 0.1 mol·L−1 H2SO4-KCl (volume ratio of 1:1) mixed solution under an operating potential range of − 0.4 – 1.2 V vs. SCE.
Sample measurements
The samples were laboratory tap water from Yangzhou University, China. First, 10 mL of tap water samples were collected, and 1000 µL of this sample was added to the supporting electrolyte (0.1 mol·L−1 H2SO4-KCl mixed solution (pH = 1.0)); CV was performed under 20 °C. The current changes at redox peaks were recorded as measurements in the absence or presence of phosphate. The concentrations of phosphate were determined via the standard addition method, and the accuracy was assessed via recovery testing with OCPEs.
Results and discussion
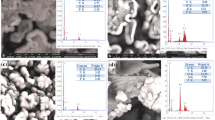
SEM–EDS result of OCPE
Figure 2 shows an [Omim]6Mo7O24-carbon paste electrode (OCPE) with Scanning Electron Microscope-Energy Dispersive Spectroscopy (SEM–EDS) acquired at 20.0 kV. We confirm that the OCPE morphology shows flaky clusters on the surface; the [Omim]6Mo7O24 is well dispersed on the CPE surface.
The XPS spectrum of the OCPE surface layer was carried out after multiple potentiodynamic cycling from − 0.4 to 1.2 V in an electrolyte containing phosphate (Fig. 3). The XPS spectrum exhibited a doublet in the Mo 3d electron-binding energy region. Peak i and Peak ii corresponding to Mo 3d appeared as a doublet Mo 3d5/2 at 234.93 eV and Mo 3d3/2 at 231.85 eV before reacting with phosphate. According to the literature, the binding energy of the peaks (232.5 eV and 235.6 eV) corresponds to the Mo6+ state.(Figueredo et al. 2021; Arvas et al. 2019) The corresponding peaks after CV in the presence of H2PO4− also exhibited two resolved peaks at 231.99 eV and 235.15 eV (peaks i' and ii'). The two peaks showed that binding energy 0.65 eV and 0.67 eV were lower than those of Peak i and Peak ii, respectively, which were assigned to Mo 3d5/2 and Mo 3d3/2 of Mo(V) states in agreement with the data reported in the literature (Sian and Reddy 2004; Maria et al. 2012). There was no indication of Mo (III) and Mo(IV) in the layer deposited on the OCPE surface (appearing at Mo4+(233.09 eV) and Mo5+(234.9 eV) (Figueredo et al. 2021). These results suggest that the parent Mo(VI) surface species convert into Mo(V) upon electroreduction.
Cyclic voltammograms of OCPE
To characterize the electrochemical properties of OCPE for phosphate analysis, an OCPE was subjected to CV in the 0.1 mol·L−1 H2SO4-KCl mixed solution in the absence or presence of phosphate from − 0.4 to 1.2 V and − 0.4 to 0.35 V (Fig. 4). The results are compared with pure GCEs in acidified molybdate (VI) (0.1 mol∙L−1 H2SO4 + 1.0 mmol∙L−1 MoO42−) solutions (Fig. 4A). The CV had a very large current response from electroactive polyoxomolybdate substrates due to the enhanced conductivity in the developed OCPE; this material was bulk modified via the uniform dispersion of OCPE in bulky carbons. No clear redox peaks were observed with [NH4]6Mo7O24-CPE due to its pure conductivity (data not shown), while a series of fine redox peaks were found with OCPE.
Cyclic voltammetry (CV) comparative results of OCPE in a solution of 0.1 mol·L−1 of KCl + H2SO4 containing 0.010 mM phosphate (solid line) and pure carbon paste electrode (CPE) (dash line) in 1.0 mmol·L−1 [NH4]6Mo7O24 + 0.1 mol·L−1 of KCl-H2SO4 solution at potential ranges of − 0.4 to 1.2 V A, voltammogram of OCPE at potential ranges of − 0.2 to 0.36 V B. Scan rates of both CV measurements are 100 mV/s
It is obvious that the substrate current response was much larger with the OCPE than pure CPE (Fig. 4A). While there was a thick background response with OCPE due to the accessible capacitance of the [Omim]6Mo7O24, the resolving power from the peak current was seen by deducting background response and was markedly improved (Liu et al. 2005). The three typical couple peaks C2/A2, C3/A3, and C6/A6 in both potential ranges − 0.4 to 1.2 V and − 0.2 to 0.36 V (Fig. 4B) show in accordance with literature with reasonable small peak shifts (Ilangovan and Pillai 1997). It is reasonable by the literature that the three typical couple peaks C2/A2, C3/A3, and C6/A6 in both potential ranges − 0.4–1.2 V and − 0.2–0.36 V show small peak shifts (Ilangovan and Pillai 1997).
Comparing Fig. 4A and B, three additional irreversible peaks of A1, A5, and C4 emerge on the OCPE. Note that Fig. 4A voltammograms were obtained under a potential range from − 0.4 to 1.2 V. Peak C4 in Fig. 4A is presumably associated with an Mo(VI) reduction according to the following hydrogen molybdenum bronze reactions represented by the general formula HxMoO3 where 0 < x ≤ 2 in acid solution. According to literature, Peak C3 is generated from MoO42− + 4H+ + 2e → (H2MoO3)ads + H2O (Galicia et al. 2011). Then, the anodic peaks of A1, A5, and A7 in Fig. 4A must correspond to the sequence of oxidation of the hydrogen molybdenum bronze (H2MoO3)ads. This suggests that material dissolved in the solution returns to MoO42− according to following reaction (MoO3 + H2O → MoO42− + 2H+) along with A1 electrochemical oxidation (Galicia et al. 2011).
Figure 5A shows the plots of peak currents of C2/A2, C3/A3, and C6/A6 versus scan rates. Figure 5B clearly shows that the currents of C2/A2 and C3/A3 were proportional to the scan rate, thus implying that the redox processes are surface-controlled. The C6/A6(C) (Fig. 5C) currents were proportional to the square root of the scan rate, which indicates that the redox process is diffusion controlled.
Optimization of [Omim]6Mo7O24 percentage to carbon powder
To achieve the best detection sensitivity, the effect of [Omim]6Mo7O24 percentage on the CV currents was investigated in the [Omim]6Mo7O24 weight percent range of 0 to 20% in a supporting electrolyte solution containing a certain content of H2PO4− from − 0.4 to 1.2 V at a scan rate of 100 mV·s−1 (data not shown). The results showed that CPE with 0% [Omim]6Mo7O24 does not show any specific redox peaks. However, there were new and obvious redox peaks of kegging ions with 5–20% [Omim]6Mo7O24 after multiple CV sweeps. The current was highest when the mass ratio of [Omim]6Mo7O24 was 15%. This mass ratio was selected in all subsequent experiments for the phosphate analysis.
Analytical characteristics
The analytical features of OCPEs were evaluated by CV voltammograms using 1.0 × 10–2–1.0 × 10–8 mol∙L−1 standard solutions of phosphate (Fig. 6). According to Randles–Sevcik equation Ip = kc = 2.69 × 105n3/2AD1/2υ1/2c, Ip is the peak current (A), n is the number of electron transfers, A is the electrode area (cm2), D is the diffusion coefficient (cm2·s−1), υ is the scanning speed (V∙s−1), and c is the concentration (mol∙L−1). Under optimized experimental conditions, a linear sensing range was obtained between 1.0 × 10–2 and 1.0 × 10–7 mol·L−1described by the following equation: Ip = 5.2657c + 147.212 (R2 = 0.9970); here, Ip is the current intensity (μA), and c is the H2PO4− concentration (mol∙L−1), sensitivity is 5.3 (± 0.1) μA/μM. The limit of detection (LOD = 3σ/s, σ is the slope of linear equation, s is the standard deviation of the detection values for n = 6 blank samples) was calculated to be 8.5 × 10–8 mol·L−1.
The analytical conditions, linear range, sensitivity, and LOD of OCPE were compared with previously reported phosphate sensing data (Table 1). The results are comparable with or prior to existing methods (Lu et al. 2021a, b; Shi et al. 2021; Figueredo et al. 2021; Arvas et al. 2019; Sian and Reddy 2004). The resulting LOD value of the proposed method allowed us to select tap water samples to quantitate phosphate concentrations.
Interferences
Interferences from common anions of SO42−, Cl−, HCO3−, and NO3− were tested, and it was found that they did not interfere with the phosphate quantification. Moreover, the interferences of silicate and glyphosate on the OCPE were also investigated. Silicate and glyphosate are commonly found in surface waters, and the former is the main interfering substance for the electrochemical determination of phosphate ions (Udnan et al. 2005), while the latter is the most widely used herbicide. To study the effect of silicate and glyphosate on OCPE, the concentration of both interfering substances was configured to be 10 times of that phosphate standard solutions, then record the CV current (Fig. 7). The mixed standard solutions of phosphate with sodium silicate have signals that decrease nearly linearly with all phosphate standard solutions (RSD = 2.27%). These data explain the higher rate constant for the phosphomolybdate complex [PMo12O40]3− relative to the silicatemolybdate complex [SiMo12O40]4− formation at a chosen pH (pH = 1.0) (Quintana et al. 2004). Therefore, the OCPEs have anti-silicate performance when silicate concentration is tenfold larger than phosphate at the chosen pH = 1.0. Glyphosate did not interfere with OCPE signals under the above experimental conditions. However, it is not difficult to speculate that organic phosphorus pesticides (OPPs) will have a significant impact on the phosphate determination in the samples with micro-organisms (lake or pond water) which can secret organic phosphorus hydrolases (OPHs) and convert OPPs to inorganic phosphate.
Determination of phosphate in tap water
The analytical features above were optimized in standard solutions. Then, the suitability of the proposed OCPE system in tap water samples was exploited. Water samples were analyzed using both the proposed and the reference IC method (Table 2). The phosphate concentration was determined to be 0.29 ± 0.02 μmol·L−1 in the tap water sample. This agreed well with that measured by the IC method. To evaluate the accuracy of this sensor, the tap water samples were spiked with a phosphate standard of 10 μmol·L−1 leading to satisfactory recovery values comparable to the reference IC method.
The repeatability of the OCPE was evaluated with three sets of H2PO4− detection. The relative standard deviation (RSD) curve slope was 3.5%. The reproducibility of the OCPEs tested with three electrodes and a set of H2PO4− additions obtained from the RSD calibration plot was 4.1%. The storage stability of the electrode was evaluated after seven days of storage at room temperature leading to an RSD of 8.9%.
Conclusions
The [Omim]6Mo7O24-based OCPE can effectively retain molybdate in the acidic H2SO4-KCl electrolyte solution. It shows a five-order wide linear range with a high sensitivity for phosphate sensing with relatively simple and low-cost fabrication. The developed CV method with an OCPE could extend to the quantification of phosphate in various samples including environmental and bio-samples.
References
Arvas MB, Gorduk O, Gencten M, Sahin Y (2019) Preparation of a novel electrochemical sensor for phosphate detection based on a molybdenum blue modified poly(vinyl chloride) coated pencil graphite electrode. Anal Methods 11:3874. https://doi.org/10.1039/C9AY01275C
Berchmans S, Karthikeyan R, Gupta S, Poinern GEJ, Issa TB, Singh P (2011) Glassy carbon electrode modified with hybrid films containing inorganic molybdate anions trapped in organic matrices of chitosan and ionic liquid for the amperometric sensing of phosphate at neutral pH. Sens Actuat B 160:1224–1231. https://doi.org/10.1016/10.1016/j.snb.2011.09.052
Chang SY, Tseng W-L, Mallipattu S, Chang H-T (2005) Determination of small phosphorus-containing compounds by capillary electrophoresis. Talanta 66:411–421. https://doi.org/10.1016/j.talanta.2004.10.014
Cinti S, Talarico D, Palleschi G, Moscone D, Arduini F (2016) Novel reagentless paper-based screen-printed electrochemical sensor to detect phosphate. Anal Chim Acta 919:78–84. https://doi.org/10.1016/j.aca.2016.03.011
Clinton-Bailey GS, Grand MM, Beaton AD, Nightingale AM, Owsianka DR, Slavik GJ, Connelly DP, Cardwell CL, Mowlem MC (2017) A lab-on-chip analyzer for in situ measurement of soluble reactive phosphate: improved phosphate blue assay and application to fluvial monitoring. Environ Sci Technol 51:9989–9995. https://doi.org/10.1021/acs.est.7b01581
Ellam TJ, Chico TJA (2012) Phosphate: the new cholesterol? The role of the phosphate axis in non-uremic vascular disease. Atherosclerosis 220:310–318. https://doi.org/10.1016/j.atherosclerosis.2011.09.002
Figueredo F, Girolametti F, Aneggi E, Lekka M, Annibaldi A, Susmel S (2021) Plastic electrode decorated with polyhedral anion tetrabutylammonium octamolybdate [N(C4H9)4]4Mo8O26 for nM phosphate electrochemical detection. Anal Chim Acta 1161:338469. https://doi.org/10.1016/j.aca.2021.338469
Forano C, Farhat H, Mousty C (2018) Recent trends in electrochemical detection of phosphate in actual waters. Curr Opin Electrochem 11:55–61. https://doi.org/10.1016/j.coelec.2018.07.008
Galicia A, Zayas T, Salgado L (2011) Voltammetric study of the molybdate reduction and subsequent oxidation on glassy carbon electrode in acid media. ECS Trans 36(1):13–19. https://doi.org/10.1149/1.3660594
He G, Zhao L, Chen K, Liu Y, Zhu H (2013) Highly selective and sensitive gold nanoparticle-based colorimetric assay for PO43- in aqueous solution. Talanta 106:73–78. https://doi.org/10.1016/j.talanta.2012.11.064
Ilangovan G, Pillai KC (1997) Electrochemical and XPS characterization of glassy carbon electrode surface effects on the preparation of a monomeric molybdate(VI)-modified electrode. Langmuir 13(3):566–575. https://doi.org/10.1021/la960053n
Kolliopoulos AV, Kampouris DK, Banks CE (2015) Rapid and portable electrochemical quantification of phosphorus. Anal Chem 87:4269–4274. https://doi.org/10.1021/ac504602a
Kumar DR, Dhakal G, Nguyen VQ, Lee J, Lee YR, Shim J-J (2021) Ammonium heptamolybdate preloaded on flexible carbon-matrix film electrode for the electrochemical phosphate sensor in a river water sample. Microchem J 170:106639. https://doi.org/10.1016/j.microc.2021.106639
Liu H, He P, Li Z, Sun C, Shi L, Liu Y, Zhu G, Li J (2005) An ionic liquid-type carbon paste electrode and its polyoxometalate-modified properties. Electrochem Commun 7:1357–1363. https://doi.org/10.1016/j.elecom.2005.09.018
Liu W, Du Z, Qian Y, Li F (2013) A specific colorimetric probe for phosphate detection based on anti-aggregation of gold nanoparticles. Sensor Actuat B 176:927–931. https://doi.org/10.1016/j.snb.2012.10.074
Lu Y, Lan Q, Zhang C, Liu B, Wang X, Xu X, Liang X (2021a) Trace-level sensing of phosphate for natural soils by a nanoscreen-printed electrode. Environ Sci Technol 55(19):13093–13102. https://doi.org/10.1021/acs.est.1c05363
Lu Y, Li X, Li D, Compton RG (2021b) Amperometric environmental phosphate sensors. ACS Sens 6:3284–3294. https://doi.org/10.1021/acssensors.1c01035
Macintosh KA, Mayer BK, McDowell RW, Powers SM, Baker LA, Boyer TH, Rittmann BE (2018) Managing diffuse phosphorus at the source versus at the sink. Environ Sci Technol 52:11995–12009. https://doi.org/10.1021/acs.est.8b01143
Maria B-B, Tomasz W, Teresa Ł, Józef C (2012) Catalytic effect of molybdenum oxo-species on reduction of allyl alcohol at a Pt electrode in strongly acidic solutions. Electrochim Acta 63:89–99. https://doi.org/10.1016/j.electacta.2011.12.081
Nagaraj K, Nityananda SA, Darshak RT (2021) Recent advances in the fluorescent and colorimetric detection of dihydrogen phosphate. Supramol Chem 33(8):408–441. https://doi.org/10.1080/10610278.2022.2035387
Nagul EA, McKelvie ID, Worsfold P, Kolev SD (2015) The molybdenum blue reaction for the determination of orthophosphate revisited: opening the black box. Anal Chim Acta 890:60. https://doi.org/10.1016/j.aca.2015.07.030
Quintana JC, Idrissi L, Palleschi G, Albertano P, Armine A, El Rhazi M, Moscone D (2004) Investigation of amperometric detection of phosphate: Application in seawater and cyanobacterial biofilm samples. Talanta 63:567–574. https://doi.org/10.1016/j.talanta.2003.11.040
Ruiz-Calero V, Galceran MT (2005) Ion chromatographic separations of phosphorus species: a review. Talanta 66:376–410. https://doi.org/10.1016/j.talanta.2005.01.027
Shi J, Guo Z, Tian S, Li M (2021), Determanation of PF6- in environmental water by [Omim]6Mo7O24 carbon paste ion selective electrode (in Chinese). PTCA (Part B: Chem Anal) 57:193. https://doi.org/10.11973/lhjy-hx202103001
Sian TS, Reddy GB (2004) Optical, structural and photoelectron spectroscopic studies on amorphous and crystalline molybdenum oxide thin films. Sol Energy Mater Sol Cells 82:375–386. https://doi.org/10.1016/j.solmat.2003.12.007
Snigur D, Chebotarev A, Bulat K, Duboviy V (2020) Fast room temperature cloud point extraction procedure for spectrophotometric determination of phosphate in water samples. Anal Biochem 597:113671. https://doi.org/10.1016/j.ab.2020.113671
Talarico D, Cinti S, Arduini F, Amine A, Moscone D, Palleschi G (2015) Phosphate detection through a cost-effective carbon black nanoparticle-modified screen-printed electrode embedded in a continuous flow system. Environ Sci Technol 49:7934–7939. https://doi.org/10.1021/acs.est.5b00218
Udnan Y, Mckelvie ID, Grace MR, Jakmunee J, Grudpan K (2005) Evaluation of on-line preconcentration and flow-injection amperometry for phosphate determination in fresh and marine waters. Talanta 66(2):461–466. https://doi.org/10.1016/j.talanta.2004.12.064
Yan JC, Ren J, Ren LL, Jian J, Yang Y, Yang SF, Ren TL (2019) Development of a portable setup using a miniaturized and high precision colorimeter for the estimation of phosphate in natural water. Anal Chim Acta 1058:70–79. https://doi.org/10.1016/j.aca.2019.01.030
Zhang Z, Tao H, Cao Q, Li L, Xu S, Li Y, Liu Y (2022) Ratiometric fluorescence sensor for sensitive detection of inorganic phosphate in environmental samples. Anal Bioanal Chem 414:3507–3515. https://doi.org/10.1007/S00216-022-03973-0
Acknowledgements
This work was supported by Science and Technology Program Nanjing Customs, China (No. 2021KJ21) and Postgraduate Research and Practice Innovation Program of Jiangsu Province (Yangzhou University).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, J., Fei, J., Zeng, C. et al. Cyclic voltammetric determination of phosphate in tap water via [Omim]6Mo7O24-carbon paste electrodes. Chem. Pap. 77, 5873–5880 (2023). https://doi.org/10.1007/s11696-023-02906-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-02906-0