Abstract
This study reports the simultaneous voltammetric determination of Cd and Pb by square wave voltammetry (SWV) using carbon paste electrode modified with bismuth film. For the ex situ film formation, the influence of Bi concentration, time and deposition potential was evaluated. Regarding the SWV parameters, frequency, amplitude, deposition time and deposition potential were evaluated. The ideal conditions of film formation were achieved with 1 mg/L Bi applying –0.6 V during 300 s (0.01 M acetate buffer, pH 4.6). The highest current values of Cd and Pb were obtained in 0.01 M acetate buffer (pH 4.6) with deposition potential of –1.1 V, time deposition of 180 s, frequency of 40 Hz, amplitude of 40 mV and potential step of 0.005 V. The detection limits calculated for Cd and Pb were 0.5 and 0.3 µg/L, respectively, and the method was successfully applied to the analysis of commercial potable water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Metallic compounds are known as potentially highly toxic metals, they can cause serious health problems representing a great threat to living organisms due to their non-biodegradability and persistence in the environment [1–3]. The presence of metals in the environment comes mainly from anthropogenic sources such as industrial effluents, crops and mining, and this contamination is highly harmful, being able to compromise the quality of several products as well as the aquatic environment [1–3]. Among these metals, Cd and Pb deserve special attention, since they do not have any known biological functions and can be harmful when present in organisms (even at low concentrations) [4, 5]. Cadmium intoxication occurs mainly through the respiratory, oral or parenteral route affecting the liver, kidney, lung, bones, central nervous system and reproductive organs [3, 5]. The main routes of Pb contamination are respiratory, oral and cutaneous, causing anemia, neurological dysfunction, renal weakening and nerves paralysis [4]. According to Brazilian legislation, the maximum concentrations allowed for Cd and Pb in drinking water are 5 and 10 μg/L, respectively [6]. The Environmental Protection Agency also recommends maximum Cd concentration in water for human consumption of 5 μg/L [7]. Therefore, the development of a simple, sensitive and low-cost method for Cd and Pb determination in potable water is important. Spectrometric methods are commonly used for this purpose [8]. However, they demand sophisticated expensive instrumentation and long-time analysis. Among the electroanalytical methods, voltammetry has been increasingly used for metals determination, since it is highly sensitive, low-cost, allowing the direct analysis of liquid samples and simultaneous and/or sequential determination of several compounds [9, 10]. Among the electrodes used in voltammetry, the mercury and solid electrodes have the highest application [11]. However, due to Hg toxicity and the consequent concern about the environment quality, the Hg electrode is falling into disuse [12]. On the other hand, solid electrodes have a high cost and often low reproducibility [13], which makes their use more difficult. In view of these problems, the application of carbon paste electrodes (CPE) modified or not has grown [10, 13, 14]. One type of CPE modification that improves the sensitivity and reproducibility of the voltammetric methods is the metal film formation by electrodeposition on the CPE surface [15]. Since 2000, the bismuth film electrode (BiFE) has been widely applied not only due to similar characteristics to the Hg electrode such as high H+ reduction potential and amalgam formation with metals (such as Cd and Pb) [16, 17], but also because BiFE deserves special attention related to the decrease in interference caused by the reduction of dissolved O2 [18]. In addition, BiFE can be easily prepared (ex or in situ), it is considered eco-friendly and is obtained in a low-cost way generating sensitive methods, well-defined and symmetrical peaks with excellent resolution [18]. Despite BiFE could be obtained on the solid surface such as glassy carbon [19], its obtaining on CPE surface can present some advantages such as low background current, easy surface renovation and high reproducibility [13].
Here, we describe for the first time the construction of a bismuth film sensor deposited on a carbon paste composed of the carbon black nanomaterial (BiFE-CPE). This carbonaceous material has high electronic transfer capacity due to its high surface area and low cost compared to other carbonaceous materials such as graphene [20], multiwalled carbon nanotubes [21–23] and graphite [24–26]. In this context, this study aims to develop a voltammetric method for the simultaneous determination of Cd and Pb using a chemically modified electrode based on carbon black and bismuth film.
EXPERIMENTAL
Chemicals and instrumentation. All reagents used in this work were of analytical grade. Ultrapure water was purified by a Direct-Q UV3® purification system (resistivity of 18.2 MΩ cm, Millipore, USA). 1000 mg/L standard solutions of Pb (Merck) and Cd (Assurance) were used, and a working standard solution was prepared daily (1.0 mg/L Cd and 1.5 mg/L Pb) with ultrapure water. Bismuth solution (50 mg/L) was prepared by dissolving Bi(NO3)3·5H2O (Synth) in 0.04 M HNO3 (Merck). The electrolyte (0.01 M acetate buffer, pH 4.6) was made by mixing appropriate quantities of acetic acid (Synth) and sodium acetate (Merck). Carbon black (VXC72R) was kindly supplied by Cabot Corporation. The mineral oil was commercially acquired (Synth). All materials used in this work were previously cleaned by immersion in 10% (v/v) HNO3 (Merck) solution for 24 h and rinsed with ultrapure water.
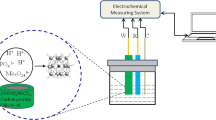
The electrochemical measurements were performed using Palmsens EmStat2 (Palm Instruments) connected to a computer controlled by PSTrace 4.6. All electrochemical experiments were carried out in a three-electrode configuration employing a modified electrode based on carbon black and bismuth film as the working electrode, platinum wire as the counter electrode and Ag/AgCl (3.0 M KCl) as the reference electrode. All the experiments were performed in a 30 mL glass cell at room temperature and without removing oxygen. A Hanna pH-meter (model pH21, Brazil) was used to check the solution pH.
Preparation of the working electrode. The preparation of carbon paste was performed by mixing carbon black and mineral oil in the ratio of 80 : 20 (w/w). The mixture was manually homogenized for 40 min. Next, the paste was inserted into a cylindrical plastic tube (syringe: o.d. 8 mm, i.d. 6 mm) equipped with a copper rod to provide the external electric contact. The paste was subsequently pressed with the copper rod, and the surface was polished by rubbing it using a clean sheet of paper. The influence of Bi concentration (0.2 to 10 mg/L), time (60 to 420 s) and potential deposition (–0.6 to –1.2 V) on the film formation was evaluated. BiFE on CPE was deposited ex situ (–0.6 V during 300 s) in 0.01 M acetate buffer (pH 4.6) containing 1 mg/L Bi.
Optimization of voltammetric parameters and sample analysis. The analytical procedure for the simultaneous determination of Pb and Cd was performed by square wave voltammetry (SWV). After Bi film formation on CPE, the electrode was carefully rinsed with ultrapure water and introduced into the electrochemical cell. The ideal conditions of frequency (10 to 100 Hz), amplitude (10 to 70 mV), deposition time (120 to 540 s) and deposition potential (–1.4 to –1 V) were obtained systematically in 0.01 M acetate buffer (pH 4.6). For the sample analysis, the electrochemical cell contained 3 mL of 0.01 M acetate buffer (pH 4.6) and 27 mL of sample. The deposition potential applied was –1.1 V during 180 s. After that, the voltammograms were recorded from –1.1 to 0 V by SWV (frequency of 40 Hz, amplitude of 40 mV, Estep of 0.005 V). The quantification of Cd and Pb was achieved by standard addition of 5 to 15 and 7.5 to 22.5 µg/L, respectively. All voltammetric measurements were performed at 22(±2)°C, and the samples were analyzed in replicate (n = 3). The BiFE-CPE was applied to Pb and Cd determination in ten water samples (with and without gas) purchased in a supermarket in Rio Grande City (Rio Grande do Sul, Brazil).
RESULTS AND DISCUSSION
Optimization of film formation parameters. Bismuth concentration. The effect of Bi concentration in the range of 0.2 to 10 mg/L on the film formation was investigated using fixed Cd and Pb concentrations (100 µg/L), and their current intensities were evaluated. As it can be observed in Fig. 1, the highest oxidation peak intensities for Cd and Pb were determined using 1 mg/L Bi. At Bi concentrations higher than 1 mg/L, a decrease in the current values of Cd and Pb was observed, making evident the dependence between the analyte peak intensity and the film thickness, since the deposition time and Bi concentration can influence the process [27]. Additionally, a thick layer of bismuth can compromise the electrical properties of the electrode surface, resulting in mass transfer limitations of the metal ions diffusing out of the film during the stripping step [28, 29]. Therefore, the optimum concentration of Bi is 1 mg/L which was chosen for the following experiments.
The relation between Bi concentration used in the film formation at CPE and Cd (100 µg/L) and Pb (100 µg/L) current intensities by square wave voltammetry. Supporting electrolyte: 0.01 M acetate buffer solution (pH 4.6); Estart: –1.1 V; Eend: 0 V; Estep: 0.005 V; deposition E: –1.2 V; deposition time: 60 s; frequency: 30 Hz; amplitude: 0.04 V.
Influence of the deposition potential and deposition time on the film formation. The deposition potential for the film formation was evaluated from –1.2 to –0.4 V (Fig. 2). It was verified that the highest current peak for Cd (25 µg/L) and Pb (50 µg/L) was obtained when the applied deposition potential was –0.6 V. Applying –0.4 V, the current intensity for Cd and Pb decreased, and it can occur due to the proximity of their oxidation potentials. On the other hand, applying potentials more negative than –0.8 V, the current intensity of target analytes also decreases, probably because this region does not favor the reduction of Cd and Pb. Additionally, it is known that hydrogen is formed on the electrode surface when negative potentials are applied, and it can damage the metallic alloys deposited on the electrode surface affecting the electrode sensitivity [30, 31]. Hence, a deposition potential of -0.6 V was adopted. The deposition time evaluated for the film formation was from 60 to 420 s (not shown here), and Pb current intensity was not altered with time variation, while for Cd, when deposition time greater than 300 s was applied, the signals became almost constant, inferring the complete accumulation of Cd on the sensor surface [21]. Therefore, the deposition time chosen for the film formation was 300 s.
The relation between deposition potential used in the Bi film formation at CPE and Cd (25 µg/L) and Pb (50 µg/L) current intensities by square wave voltammetry. Supporting electrolyte: 0.01 M acetate buffer solution (pH 4.6); Estart: –1.1 V; Eend: 0 V; Estep: 0.005 V; Bi concentration: 1 mg/L; deposition time: 60 s; frequency: 30 Hz; amplitude: 0.04 V.
To confirm the formation of bismuth film on the carbon paste electrode surface, the sweep from 0 to 0.2 V was performed. As can be seen in Fig. 3, it is possible to observe oxidation of Bi at 0.08 V, which was in agreement with studies published in the literature [24, 25].
Optimization of parameters for Cd and Pb determination by square wave voltammetry. Influence of the deposition potential and deposition time. The deposition potentials evaluated for voltammetric determination of Cd and Pb were between –1.0 and –1.4 V. As it can be seen in Fig. 4, among the deposition potentials evaluated, –1.1 V provided the highest current intensity for Cd and Pb. It is possible to observe that for potentials more negative and more positive than ‒1.1 V the current response was lower for both compounds. Therefore, –1.1 V was chosen as the ideal potential. Generally, the analyte concentration is proportional to the deposition time used. Thus, according to the analyte concentration, the deposition time can be altered to obtain the most sensitive response. The deposition time was evaluated from 120 to 540 s, and the results are shown in Fig. 5. As expected, when using longer deposition time, the current intensity obtained for Cd and Pb was higher. However, the peak resolution was reduced at deposition time greater than 180 s (not shown here). It should be noted that under these conditions (180 s), the quantification limits calculated for Cd and Pb were below those established by Brazilian law. Thus, the deposition time of 180 s was adopted for the studies shown below.
The relation between the deposition potential applied in BiFE-CPE and current responses of Cd (25 µg/L) and Pb (50 µg/L) by square wave voltammetry. Supporting electrolyte: 0.01 M acetate buffer solution (pH 4.6); Estart: –1.1 V; Eend: 0 V; Estep: 0.005 V; deposition time: 60 s; frequency: 30 Hz; amplitude: 0.04 V.
The relation between the deposition time applied in BiFE-CPE and current responses of Cd (0.5 µg/L) and Pb (3 µg/L) by square wave voltammetry. Supporting electrolyte: 0.01 M acetate buffer solution (pH 4.6); Estart: –1.1 V; Eend: 0 V; Estep: 0.005 V; deposition potential: –1.1 V; frequency: 30 Hz; amplitude: 0.04 V.
Influence of the frequency and amplitude. The relationships between Cd and Pb oxidation peak intensities and the evaluated frequencies are represented in Fig. 6. It can be observed that as the frequencies increase, the current intensities increase as well. However, when frequencies higher than 40 Hz were applied, there was a loss in the peak resolution (not shown here). The amplitude influence was also evaluated. As it can be observed in Fig. 7, Cd and Pb current intensities increase gradually with the increase in the amplitude until reaching a maximum at 40 mV. Therefore, the frequency of 40 Hz and the amplitude of 40 mV were chosen as ideal for this method.
The relation between the frequency applied in BiFE-CPE and current responses of Cd (25 µg/L) and Pb (50 µg/L) by square wave voltammetry. Supporting electrolyte: 0.01 M acetate buffer solution (pH 4.6); Estart: ‒1.1 V; Eend: 0 V; Estep: 0.005 V; deposition potential: ‒1.1 V; deposition time: 180 s; amplitude: 0.04 V.
The relation between the amplitude used in BiFE-CPE and current responses of Cd (25 µg/L) and Pb (50 µg/L) by square wave voltammetry. Supporting electrolyte: 0.01 M acetate buffer solution (pH 4.6); Estart: ‒1.1V; Eend: 0 V; Estep: 0.005 V; deposition potential: ‒1.1 V; deposition time: 180 s; frequency: 40 Hz.
Method validation and sample analysis. Calibration curves were obtained by standard addition in the concentration range of 5 to 15 μg/L for Cd and 7.5 to 22.5 μg/L for Pb, and the correlation coefficients obtained were 0.994 and 0.998, respectively. The detection limits (LOD) calculated as 3s/S (where s is the standard deviation of electrolyte, and S is the angular coefficient of calibration curve) for Cd and Pb were 0.5 and 0.3 µg/L, respectively. The quantification limits (10 s/S) calculated for Cd and Pb were 1.6 and 1.0 µg/L. In order to determine the method accuracy, mineral water sample (A1) was fortified at two concentration levels (5 and 10 μg/L for Cd, 7.5 and 15 μg/L for Pb), and the recoveries obtained were between 70 and 96% with the relative standard deviation (RSD) values smaller than 8% (Table 1). Therefore, this method can be considered accurate. Figure 8 depicts the voltammogram obtained from the fortified sample (5 μg/L Cd and 7.5 μg/L Pb) using the proposed electrode (BiFE-CPE) and the optimized SWV method.
Voltammograms of natural water sample fortified with 5 µg/L Cd and 7.5 µg/L Pb obtained using BiFE-CPE by square wave voltammetry. Voltammetric conditions: 27 mL of sample, 3 mL of 0.01 M acetate buffer solution (pH 4.6); Estart: –1.1 V; Eend: 0 V; Estep: 0.005 V; deposition potential: –1.1 V; deposition time: 180 s; amplitude: 0.04 V; frequency: 40 Hz. (1) Sample fortified with Cd (5 µg/L) and Pb (7.5 µg/L) and (2–4) standard addition of Cd (5 to 15 µg/L) and Pb (7.5 to 22.5 µg/L).
This chemically modified electrode was applied to determine Cd and Pb in ten water samples available in the supermarket of Rio Grande City (Brazil). However, the compounds were not detected in the analyzed samples. Therefore, these samples are considered suitable for the human consumption.
Some works describing modified electrodes for Cd and Pb determination in water [17, 27, 32–34], including well [26] and tap [20, 21, 23, 25, 26, 35–37] water, by voltammetry are given in Table 2. Nanoparticles [17, 27, 32], biopolymer [33] and different carbon types [21, 23, 25, 26, 34–37] were used for the electrode construction. Despite the low detection limits obtained, most of these works involve several preparation steps and materials with high cost. Additionally, in most of the cases, the deposition time applied and the detection limits calculated were higher than those obtained using the method proposed here.
As a result, the combination of carbon paste electrode modified with bismuth film and SWV allows to monitor the water quality in relation to Cd and Pb contamination with accuracy, simplicity, high sensitivity and low cost.
CONCLUSIONS
In this work, we showed the development of chemically modified electrode based on carbon black and bismuth film applied to the simultaneous voltammetric determination of Cd and Pb. The proposed method was accurate (recovery values between 70 and 96% with RSD values below 8%), with low limits of detection and quantification for the analytes. Additionally, it is important to mention that the developed electrode is very cheap compared to solid electrode and not toxic (like the Hg electrode normally used for Cd and Pb determination). Therefore, the proposed chemically modified electrode can be used for determining Cd and Pb in water.
REFERENCES
Heavy Metals and Human Health, Environmental Health. http://cdn.intechopen.com/pdfs/27687/InTech-Heavy_metals_and_human_health.pdf. Accessed May 20, 2021.
Jaishankar, M., Tseten, T., Anbalagan, N., Mathew, B.B., and Beeregowda, K.N., Interdiscip. Toxicol., 2014, vol. 7, p. 60.
Castro-González, M.I. and Méndez-Armenta, M., Environ. Toxicol. Pharmacol., 2008, vol. 26, p. 263.
Agency for Toxic Substance and Disease Registry. Toxicological Profile for Lead, U.S. Department of Health and Humans Services, Public Health Service. https://stacks.cdc.gov/view/cdc/37676/cdc_37676_ DS1.pdf. Accessed May 20, 2021.
Agency for Toxic Substance and Disease Registry. Toxicological Profile for Cadmium, U.S. Department of Health and Humans Services, Public Health Service. https://www.atsdr.cdc.gov/toxprofiles/tp5.pdf. Accessed May 20, 2021.
Brazil, Conselho Nacional do Meio Ambiente, resolução no. 396/2008. http://www.mma.gov.br/port/conama/legiabre.cfm? codlegi= \562. Accessed May 20, 2021.
Environmental Protection Agency (EPA). https:// www.epa.gov/sites/production/files/2015-06/documents/ny_hh_303_w_03121998.pdf. Accessed May 20, 2021.
Rosini, F., Matos, W.O., Santos, M.C., and Nóbrega, J.A., Analytica, 2006, vol. 22, p.74.
Wang, Y., Xu, H., Zhang, J., and Li, G., Sensors, 2008, vol. 8, p. 2043.
Maciel, J.V., Durigon, A.M., Souza, M.M., Quadrado, R.F.N., Fajardo, A.R., and Dias, D., TrAC, Trends Environ. Anal., 2019, vol. 22, e00062.
Stozhko, N.Y., Malakhova, N.A., Fyodorov, M.V., and Brainina, K.Z., J. Solid State Electrochem., 2008, vol. 12, p. 1185.
Ouyang, R., Zhu, Z., Tatum, C.E., Chambers, J.Q., and Xue, Z.L., J. Electroanal. Chem., 2011, vol. 656, p. 78.
Uslu, B. and Ozkan, S.A., Anal. Lett., 2007, vol. 40, p. 817.
Heinemann, M.G., Gonçalves, B.L., Vicenti, J.R.M., and Dias, D., Anal. Sci., 2019, vol. 35, p. 1255.
Kalcher, K., Kauffmann, J.M., Wang, J., Svancara, I., Vytras, K., Neuhold, C., and Yang, Z., Electroanalysis, 1995, vol. 7, p. 5.
Wang, J., Lu, J., Hocevar, S.B., and Farias, P.A.M., Anal. Chem., 2000, vol. 72, p. 3218.
Yang, D., Wang, L., Chen, Z., Megharaj, M., and Naidu, R., Microchim. Acta, 2014, vol. 181, p. 1199.
Wang, J., Lu, J., Kirgöz, U.A., Hocevar, S.B., and Ogorevc, B., Anal. Chim. Acta, 2001, vol. 434, p. 29.
Zhao, G., Wang, H., Liu, G., and Wang, Z., Sens. Actuators, B, 2016, vol. 235, p. 67.
Cesarino, I., Caridade, C.G., Pauliukaite, R., Cavalheiro, E.T.G., and Brett, C.M.A., Electroanalysis, 2010, vol. 13, p. 1437.
Luo, J.H., Jiao, X.X., Li, N.B., and Luo, H.Q., J. Electroanal., 2013, vol. 689, p. 130.
Hai, T.L., Hung, L.C., Phuong, T.T.B., Ha, B.T.T., Nguyen, B.-S., Hai, T.D., and Nguyen, V.-H., Microchem. J., 2020, vol. 153, 104456.
Oularbi, L., Turmine, M., and Rhazi, M.E., Synth. Met., 2019, vol. 253, p. 1.
Flechsig, G-U., Kienbaum, M., and Gründler, P., Electrochem. Commun., 2005, vol. 7, p. 1091.
Hočevar, S.B., Švancara, I., Vytřas, K., and Ogorevc, B., Electrochim. Acta, 2005, vol. 51, p. 706.
Salih, F.E., Ouarzane, A., and Rhazi, M.E., Arab. J. Chem., 2017, vol. 10, p. 596.
Yang, D., Wang, L., Chen, Z., Megharaj, M., and Naidu, R., Electrochim. Acta, 2014, vol. 132, p. 223.
Arduini, F., Calvo, J.Q., Amine, A., Palleschi, G., and Moscone, D., TrAC, Trends Anal. Chem., 2010, vol. 29, p. 1295.
Baldrianova, L., Svancara, I., Vlcek, M., Economou, A., and Sotiropoulos, S., Electrochim, Acta, 2006, vol. 52, p. 481.
Liu, B.Z, Lu, L.Y., Wang, M., and Zi, Y.Q., Electroanalysis, 2008, vol. 20, p. 2363.
Krolicka, A., Bobrowski, A., and Kowal, A., Electroanalysis, 2006, vol. 18, p. 1649.
Zhu, L., Xu, L., Huang, B., Jia, N., Tan, L., and Yao, S., Electrochim. Acta, 2014, vol. 115, p. 471.
Fort, C.I., Cotet, L.C., Vulpoi, A., Turdean, G.L., Danciu, V., Baia, L., and Popescu, I.C., Sens. Actuators, B, 2015, vol. 220, p. 712.
Chajangali, M.A., Kouhestani, H., Masdarolomoor, F., and Daneshinejad, H., Sens. Actuators, B, 2015, vol. 216, p. 384.
Xiao, L., Xu, H., Zhao, S., Song, T., Wang, H., Li, S., Gan, W., and Yuan, Q., Electrochim. Acta, 2014, vol. 143, p. 143.
Dai, H., Wang, N., Wang, D., Ma, H., and Lin, M., Chem. Eng. J., 2016, vol. 299, p. 150.
Lee, S., Bong, S., Ha, J., Kwak, M., and Park, S.K., Sens. Actuators, B, 2015, vol. 215, p. 62.
ACKNOWLEDGMENTS
The authors are grateful to Cobat Corporation for carbon black and to CAB English Lessons for the English correction.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
There are no conflicts of interests to declare.
Rights and permissions
About this article
Cite this article
de Oliveira, C., Maciel, J.V., Christ-Ribeiro, A. et al. A Voltammetric Approach for the Simultaneous Determination of Cd and Pb in Water Applying Carbon Paste Electrode Modified with Bismuth Film. J Anal Chem 77, 369–375 (2022). https://doi.org/10.1134/S1061934822030078
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934822030078