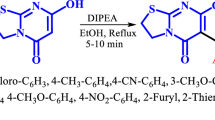
Abstract
An efficient multicomponent reaction was developed to synthesis of a novel series of (±)-7-methyl-4-aryl-4,6-dihydropyrido[4,3-d]pyrimidine-2,5(1H,3H)-dione/thione/imine from bio-based 4-hydroxy-6-methylpyridine-2-ones, aromatic aldehydes and urea/thiourea/guanidine in the presence of ZnCl2.2H2O in ethanol at 70 °C. Mild conditions as well as the operational simplicity, easy work up, environmentally friendly are the most advantages of this multicomponent for synthesis of potentially bioactive new products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
2-oxo/thioxo/imino-1,2,3,4-tetrahydropyrimidines (THPMs; Fig. 1) are a bunch of key heterocycle compounds introduced by an Italian chemist, Pietro Biginelli, during a one-pot reaction. THPM is obtained from the reaction of three components urea (thiourea), an aldehyde, and a 1,3-dicarbonyl compound (Kappe 2000). The change in three types of starting material provides a variety of THPOs that can show a wide range of biological activities, including antiviral I (Chitra et al. 2010; Kim et al. 2012) anticancer II (Mayer et al. 1999; Milović et al. 2022b), antibacterial III (Yadlapalli et al. 2012; Milović et al. 2022a) antifungal IV (Rajanarendar et al. 2010), anticancer (Ismaili et al. 2008; Janković et al. 2019) anti-inflammatory activity V (Gijsen et al. 2012), antioxidant agents VI (Ismaili et al. 2008) and Calcium channel inhibition VII (Ismaili et al. 2008). Due to these features, very quickly studies were conducted on THPMs, and almost all of the main chemistry publications contained articles about of the Biginelli reaction (Dallinger et al. 2004; Kolosov et al. 2009). Years after the discovery of THPMs, researchers still emphasize the introduction of new synthetic methods, the production of new THPM-based compounds, and their application in the industry, particularly new drug applications (Ling et al. 2021).
Although, so far, various methods for synthesizing THPMs have been based on using strong Lowry-Bronsted acids such as as H2SO4 (Folkers and Johnson 1933b), HCl (Folkers and Johnson 1933a), Lowry-Bronsted bases such as t-BuOK (Shen et al. 2010), Lewis acids such as InCl3 (Ranu et al. 2000; Nebo et al. 2019), Lewis bases such as (Debache et al. 2008), metal complex (Jankovic et al. 2015) and other types of conditions such as and zeolite (Rani et al. 2001) metal triflates (Su et al. 2005), such as ultrasonic in the presence of dendrimer-attached phosphotungstic acid nanoparticles immobilized on nanosilica (Safaei-Ghomi et al. 2018), low melting acidic (Gore et al. 2011) methods and ionic liquid (Valizadeh and Shockravi 2009) media have been presented in articles, the report of the new methods with mild conditions in terms of operation simplicity, economic viability, and greater selectivity for the preparation of potentially bioactive THPMs is still noticeable (Fig. 2).
From the point of view of the discovery of new drugs, bio-compound screening programs can be very effective for using natural compounds as starting materials in the preparation of new drugs from the category of known drug compounds (Newman and Cragg 2007). One class of these natural compounds are 4-hydroxy-2-pyridones (HPOs) alkaloids (Jessen and Gademann 2010). HPO derivatives are six-membered heterocycles that are mainly produced by fungi, and cause pathogenicity or incapacitate insects and actually regulate insect behavior (Molnár et al. 2010). Most of them were isolated from fungi (Hubka et al. 2015) and some from plants (Nebo et al. 2019). These compounds have many biological activities such as antifungal (Breinholt et al. 1997) (A), antibiotic (Singh et al. 2012) (B), insecticidal (Wachira et al. 2014) (C), cytotoxic (Bergmann et al. 2007), neurotoxic (Ferraz et al. 1999), anti-proliferative (Ding et al. 2014), antibacterial (Alfatafta et al. 1994), and anti-oxidant (D) (Kamali et al. 2020).
In continuation of our previous research (Kamali and Keramat Pirolghor 2022), we wish to present an efficient one-pot multicomponent synthesis of a new series of THPMs using HPOs and aromatic aldehydes and urea/thiourea/guanidine in the presence of ZnCl2.2H2O as the catalyst (Fig. 3).
Results and discussion
According to the main goal of this research, the synthesis of 4-hydroxy-2-pyridones-based THPM, we first treated 1-ethyl-4-hydroxy-6-methylpyridin-2-one (1a), benzaldehyde (2a) and urea (3a) in the presence of HCl as the catalyst in ethanol under reflux conditions (Scheme 1 and Table 1; entry 1). As a result, it was afforded 4a (65% yield). For synthesizing of THMP (5a), this reaction was done in the presence of p-TSA instead of HCl (Scheme 1 and Table 1; Entry 2). In this reaction, the product 5a was not obtained, too. We repeated this reaction in the presence of a basic catalyst (Et3N; Table 1, Entry 3), and also with p-chlorobenzaldehyde (2b; Table 1, Entry 4). But 5a or 5b were not observed. Therefore, it was tested this reaction in the presence of SnCl2.2H2O (based on our previous work(Kamali and Keramat Pirolghor 2022)) as Lewis acid catalysts (Table 2; Entry 5). As a result, SnCl2.2H2O unlike to HCl or p-TSA (Brønsted-Lowry acids) and TEA (base catalyst) produced 5a as a main product. This behavior is probably due to the effect of tin chloride in the accumulation of substrates, and iminium intermediate, which provides a better catalytic role, and also causes dehydration in the last step of the reaction (Scheme 2) (Kamali and Keramat Pirolghor 2022). So we turned to some other metal chlorides (Table 2; Entry 1–3) to optimize the reaction conditions, and also performed a reaction without of any catalyst for a more investigation (Table 1; Entry 6). The SnCl2.2H2O gave better yield product (63%) than the other metal chloride salts (Table 2; Entry 1–3). But, because of more eco-friendly, ZnCl2.2H2O with slightly lower product yield (59%) than SnCl2.2H2O was selected as main catalyst for this reaction. For optimization of other reaction condition, the reaction was performed in some other solvents (Table 2; Entry 4–7), different amounts of catalyst (Table 1; Entry 4 and Table 3; Entry 1–3), different temperatures and times (Table 3; Entry 4–9). Consequently, the best amount of catalyst, solvent type, temperature and time of reaction for synthesis of 5a with 83% yield were 20 mol%, ethanol, 70 °C and 3 h, respectively. This reaction was economic and environmental friendly.
To further investigations and extend the library synthesis of THPM, some aromatic aldehydes (2a–2h) with electron withdrawing or donating groups, two 2-pyridones (1a, 1b) and urea/thiourea/guanidine (3a–3b) were treated to give the THPMs (5a–5l) in good to excellent yields (Scheme 1 and Table 4). It was seen that aldehydes with donor groups, didn’t afford the THPMs and the bis-product such as 6 (Table 4, Entry 15, in the presence of urea) were produced. This is probably due to that the reaction goes forward from other pathway (in compare to proposed mechanism; Scheme 2) (Kamali et al. 2020) in which first one pyridone molecule interact with an aldehyde, and the knoevenagel reaction occurs, and then the second pyridone molecule be added to knoevenagel intermediate and produces product 4. It should also be mentioned that the aldehydes having the strong acceptor groups such as NO2, the better reacts to urea/thiourea/guanidine, and as a result, THPM are obtained with more yield (Table 4, 5c, 5i and 5l). Also, the reaction with this method, in the presence of aliphatic aldehydes (formaldehyde, acetaldehyde and butyraldehyde) instead of aromatic aldehydes did not produce THPM. At last, generally, the yield of THPMs were lower using pyridone 1b than 1a (Table 4, 5h–5l), this is probably due to the more aromatic property and less acidic character of alpha hydrogen of carbonyl of 1b which itself is a result of the tautomerization of the carbonyl group and NH (Hejazi et al. 2016) (2-Hydroxypyridine/2-Pyridone; Scheme 3).
Experimental section
The 4-hydroxy-6-methylpyridine-2-ones were synthesized according to known method (Kraus et al. 2016). The other starting materials were purchased from Merck and Fluka chemical companies. Melting points were determined with a Branstead Electrothermal model 9200 apparatus and are uncorrected. IR spectra were recorded on a Perkin Elmer RX1 Fourier transform infrared spectrometer. The 1H and 13C NMR spectra were recorded in DMSO-d6 on Bruker Avance 300 MHz spectrometers. Elemental analyses were carried out by a Perkin Elmer 2400 series II CHN/O analyzer.
Synthesis of 5a as a general procedure
The 1-ethyl-4-hydroxy-6-methylpyridine-2-one (1 mmol), the benzaldehyde (1 mmol) and the urea (1 mmol) were added to a solution of ZnCl2.2H2O (20 mol%) in absolute ethanol (1 mL), and the mixture was stirred at 70 ℃ for 3 h. Then the reaction mixture was poured in ice water (5 mL) and the precipitated was collected by filtration, washed with distilled water (5 mL). The resulting product was recrystallized from DMF/H2O (1 mL; V/V = 2:1) to give the pure 5a as White solid in 81% yield; m.p.: 188–190 °C. Compounds 4a–4b, 5b–5l and 6 were obtained in the same way. Only in the cases of 4a and 4b products, p-TSA (50 mol%) was used instead ZnCl2.2H2O and for isolation of them, after washing with water, the precipitate was added to ethanol 95% (5 mL), stirred for 0.5 h, and filtered. The filtrate solution was evaporated, and the solid recrystallized from ethanol 95%/water (4 mL; v/v = 3:1). The physical properties of products are in below, and the 1H NMR, 13C NMR, and IR spectra attached in the Supporting Information.
(±)-1-((1-ethyl-4-hydroxy-6-methyl-2-oxo-1,2-dihydropyridin-3-yl)(phenyl)methyl)urea (4a) White solid in 83% yield; m.p.: 228–230 °C; IR (KBr) υ: 3336 (NH), 3204 (NH), 2983 (CH), 2038 (CH), 1647 (C=O cm−1, 1H NMR (300 MHz, DMSO-d6) δ: 10.53 (s, 1H, OH of pyridone), 7.38–7.14 (m, 5H, ArH), 7.11 (d, J = 7.2 Hz, 1H, NH of urea), 6.24 (d, J = 7.2 Hz, 1H, CH residue of CHO), 5.87 (s, 1H, CH of pyridone), 5.81 (s, 2H, NH2 of urea), 3.88 (2dq, J = 13.6, 6.8 Hz, 2H, CH2 of pyridone), 2.29 (s, 3H, CH3 of pyridone), 1.10 (t, J = 6.9 Hz, 3H, CH3 pyridone) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 162.7 (=C–OH of pyrdone), 160.8 (C=O of urea), 158.1 (N–C=O of pyridone), 145.2 (=C–N of pyridone), 144.7 (C of ArH), 127.5 (CH of ArH), 126.1 (CH of ArH), 125.8 (CH of ArH), 109.1 (=CH of pyridone), 99.5 (=C of pyridone), 46.9 (CH residue of CHO), 19.5 (CH2 of pyridone), 13.8 (CH3 of pyridone) ppm. Anal. Calcd for C16H19N3O3 (301.14): C, 63.77; H, 6.36; N, 13.94. Found: C, 63.64; H, 6.36; N, 14.08.
(±)-1-((4-chlorophenyl)(1-ethyl-4-hydroxy-6-methyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)urea (4b) White solid in 87% yield; m.p.: 199–200 °C; IR (KBr) υ: 3345 (NH), 3196 (NH), 2989 (CH), 1647 (C=O) cm−1, 1H NMR (300 MHz, DMSO-d6) δ: 10.62 (s, 1H, OH of pyridone), 7.23–7.27 (m, 4H, CH of ArH), 7.13 (d, J = 7.2 Hz, 1H, NH of urea), 6.21 (d, J = 7.2 Hz, 1H, residue CH of CHO), 5.87 (s, 1H, CH of pyridone), 5.84 (s, 2H, NH2 of urea), 3.88 (2dq, J = 13.6, 7.0 Hz, 2H, CH2 of pyridone), 2.29 (s, 3H, CH3 of pyridone), 1.10 (t, J = 7.0 Hz, 3H, CH3 of pyridone) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 162.7 (=C–OH of pyridone), 161.0 (C=O of urea), 158.1 (C=O of pyridone), 145.5 (=C–N of pyridone), 143.8 (C of ArH), 130.2 (C of ArH), 127.6 (CH of ArH), 127.5 (CH of ArH), 108.7 (=CH of pyridone), 99.5 (=C of pyridone), 46.6 (CH residue of CHO), 35.8 (CH2 of pyridone), 19.6 (CH3 of pyridone), 13.9 (CH3 of pyridone) ppm. Anal. Calcd for C16H18ClN3O3 (335.10): C, 57.23; H, 5.40; N, 12.51. Found: C, 57.09; H, 5.31; N, 12.83.
(±)-6-ethyl-7-methyl-4-phenyl-4,6-dihydropyrido[4,3-d]pyrimidine-2,5(1H,3H)-dione (5a) White solid in 81% yield; m.p.: 188–190 °C; IR (KBr) υ:3341 (NH), 3196 (NH), 2956 (CH), 1661 (C=O) cm−1, 1H NMR (300 MHz, DMSO-d6) δ: 10.33 (s, 1H, NH of pyrimidine), 7.57 (d, J = 7.6 Hz, 1H, NH of pyrimidine), 7.14–7.18 (m, 5H, CH of ArH), 6.39 (d, J = 7.6 Hz, 1H, CH of pyrimidine), 5.69 (s, H, CH of pyridone), 3.83–3.97 (m, 2H, CH2 of pyridone), 2.29 (s, 3H, CH3 of pyridone), 1.03 (t, J = 9.2 Hz, 3H, CH3 of pyridone) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 162.6 (C=O pyridone), 160.2 (C=O of pyrimidine), 154.2 (N–C= of pyridone), 147.3 (C of ArH), 144.8 (=C–NH of pyridone), 127.6 (CH of ArH), 126.0 (CH of ArH), 124.3 (CH of ArH), 106.6 (=CH of pyridone), 98.0 (=C of pyridone), 48.6 (CH of pyrimidine), 34.0 (CH of pyridone), 17.6 (CH3 of pyridone), 11.2 (CH3 of pyridone). Anal. Calcd for C16H17N3O2 (283.13): C, 67.83; H, 6.05; N, 14.83. Found: C, 67.71; H, 5.94; N, 14.94.
(±)-4-(4-chlorophenyl)-6-ethyl-7-methyl-4,6-dihydropyrido[4,3-d]pyrimidine-2,5(1H,3H)-dione (5b) White solid in 86% yield; m.p.: 180–182 °C; IR (KBr) υ: 3346 (NH), 3207 (NH), 2969 (CH), 2942 (CH), 1658 (C=O) cm−1. 1H NMR (300 MHz, DMSO-d6) δ: 10.08 (s, 1H, NH of pyrimidine), 7.58 (d, J = 7.7 Hz, 1H, NH of pyrimidine), 7.25 (d, 2H, J = 7.9 Hz, CH of ArH), 7.18 (d, 2H, J = 7.9 Hz, CH of ArH), 6.32 (d, J = 7.7 Hz, 1H, CH of pyrimidine), 5.72 (s, H, CH of pyridone), 3.85–39.98 (m, 2H, CH2 of pyridone), 2.24 (s, 3H, CH3 of pyridone), 1.03 (t, J = 9.2 Hz, 3H, CH3 of pyridone) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 162.8 (C=O of pyrimidine), 161.0 (C=O pyridone), 155.0 (N–C of pyridone), 145.6 (C of ArH), 142.3 (C of ArH), 130.7 (=C–N of pyridone), 129.9 (CH of ArH), 127.8 (CH of ArH), 106.4 (=CH of pyridone), 98.3 (=C of pyridone), 48.6 (CH of pyrimidine), 34.0 (CH of pyridone), 19.2 (CH3 of pyridone), 14.1 (CH3 of pyridone) ppm. Anal. Calcd for C16H16ClN3O2 (317.09): C, 60.48; H, 5.08; N, 13.22. Found: C, 60.39; H, 4.96; N, 13.34.
(±)-6-ethyl-7-methyl-4-(4-nitrophenyl)-4,6-dihydropyrido[4,3-d]pyrimidine-2,5(1H,3H)-dione (5c) Yellow solid in 92% yield; m.p.: 183–185 °C; IR (KBr) υ: 3304 (NH), 3190 (NH), 2992 (CH), 2943 (CH), 1642 (C=O cm−1, 1H NMR (300 MHz, DMSO-d6) δ: 10.68 (s, 1H, NH of pyrimidine), 8.08 (d, J = 8.0 Hz, 2H, CH of ArH), 7.69 (d, J = 7.6 Hz, 1H, NH of pyrimidine), 7.47 (d, J = 8.0 Hz, 2H, CH of ArH), 6.35 (d, J = 7.6 Hz, 1H, CH of pyrimidine), 5.86 (s, H, CH of pyridone), 3.90 (m, 2H, CH2 of pyridone), 2.30 (s, 3H, CH3 of pyridone), 1.12 (t, J = 9.2 Hz, 3H, CH3 of pyridone) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 162.4 (C=O pyridone), 161.3 (C=O of pyrimidine), 153.6 (N–C= of pyridone), 145.7 (C of ArH), 137.5 (C of ArH), 127.0 (=C–NH of pyridone), 126.9 (CH of ArH), 122.9 (CH of ArH), 108.2 (=CH of pyridone), 99.3 (=C of pyridone), 47.6 (CH of pyrimidine), 35.7 (CH of pyridone), 19.6 (CH3 of pyridone), 13.8 (CH3 of pyridone) ppm. Anal. Calcd for C16H16N4O4 (328.12): C, 58.53; H, 4.91; N, 17.06. Found: C, 58.40; H, 4.81; N, 17.21.
(±)-4-(2-chlorophenyl)-6-ethyl-7-methyl-4,6-dihydropyrido[4,3-d]pyrimidine-2,5(1H,3H)-dione (5d) White solid in 78% yield; m.p.: 184–185 °C; IR (KBr) υ: 336 (NH), 3067 (CH), 3158 (NH), 2988 (CH), 1646 (C=O), cm−1, 1H NMR (300 MHz, DMSO-d6) δ: 10.39 (s, 1H, NH of pyrimidine), 7.67 (d, J = 7.5 Hz, 1H, NH of pyrimidine), 7.08–7.29 (m, 4H, CH of ArH), 6.28 (d, J = 7.5 Hz, 1H, CH of pyrimidine) 5.76 (s, 1H, CH of pyridone), 3.93 (dq, J = 13.1, 7.0 Hz, 1H, CH2 of pyridone), 3.74 (dq, J = 13.1, 7.0 Hz, 1H, CH2 of pyridone), 2.26 (s, 3H, CH3 of pyridone), 1.10 (t, J = 7.0 Hz, 3H, CH3 of pyridone) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 162.7 (C=O of pyridone), 162.5 (C=O of pyrimidine), 161.6 (N–C= of pyridone), 157.8 (C of ArH), 156.3 (=C–N of pyridone), 145.3 (C of ArH), 141.3 (CH of ArH), 131.7 (CH of ArH), 128.6 (CH of ArH), 125.5 (CH of ArH), 107.2 (=CH of pyridone), 99.5 (=C of pyridone), 47.1 (CH of pyrimidine), 35.8 (CH3 of pyridone), 19.6 (CH3 of pyridone),14.0 (CH3 of pyridone) ppm. Anal. Calcd for C16H16ClN3O2 (317.09): C, 60.48; H, 5.08; N, 13.22. Found: C, 60.32; H, 4.95; N, 13.41.
(±)-4-(4-chlorophenyl)-6-ethyl-7-methyl-2-thioxo-2,3,4,6-tetrahydropyrido[4,3-d]pyrimidin-5(1H)-one (5e) White solid in 65% yield; m.p.: 187–189 °C; IR (KBr) υ: 3294 (NH), 3161 (CH), 2983 (CH), 1640 (C=O), 1090 (C=S) cm−1. 1H NMR (300 MHz, DMSO-d6) δ: 10.61 (s, 1H, NH of pyrimidine), 7.22 (d, J = 6.9 Hz, 2H, CH of ArH), 7.13 (d, J = 6.0 Hz, 1H, NH of pyrimidine), 6.94 (d, J = 6.9 Hz, 2H, CH of ArH), 6.10 (d, J = 6.0 Hz, 1H, CH of pyrimidine), 5.91 (s, 1H, CH of pyridone), 3.91 (m, 2H, CH2 of pyridone), 2.31 (s, 3H, CH3 of pyridone), 1.11 (t, J = 6.0 Hz, 3H, CH3 of pyridone) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 182.5 (C=S of pyrimidine), 162.2 (C=O of pyridone), 161.2 (=C–NH of pyridone), 145.5 (=C of pyridone), 143.4 (C of ArH), 128.5 (C of ArH), 127.6 (CH of ArH), 126.1 (CH of ArH), 107.93 (=C of pyridone), 99.6 (CH of pyridone), 52.3 (CH of pyrimidine), 35.4 (CH2 of pyridone), 19.5 (CH3 of pyridone), 13.7 (CH3 of pyridone) ppm. Anal. Calcd for C16H16ClN3OS (333.07): C, 57.57; H, 4.83; N, 12.59. Found: C, 57.39; H, 4.60; N, 12.73.
(±)-4-(2-chlorophenyl)-6-ethyl-2-imino-7-methyl-2,3,4,6-tetrahydropyrido[4,3-d]pyrimidin-5(1H)-one (5f) White solid in 78% yield; m.p.: 231–233 °C; IR (KBr) υ: 3324 (NH), 3140 (CH), 2978 (CH), 1640 (C=O). cm−1 .1H NMR (300 MHz, DMSO-d6) δ: 11.53 (s, 1H, NH of pyrimidine), 7.37 (s, 1H, = NH of pyrimidine), 7.13–7.26 (m, 4H, CH of ArH), 6.98 (d, J = 6.9 Hz, 1H, NH of pyrimidone), 6.23 (d, J = 6.9 Hz, 1H, CH of pyrimidone), 5.96 (s, 1H, CH of pyridone), 3.91 (m, 2H, CH2 of pyridone), 2.33 (s, 3H, CH3 of pyridone), 1.09 (t, J = 7.2 Hz, 3H, CH3 of pyridone) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 164.4 (C=O of pyridone), 162.3 (=C–NH of pyridone), 164.0 (C=NH of pyrimidine), 144.9 (=C–N of pyridone), 138.7 (C of ArH), 130.7 (C of ArH), 130.0 (CH of ArH), 129.5 (CH of ArH), 127.2 (C of ArH), 126.2 (CH of ArH), 108.8 (=C of pyridone), 99.8 (CH of pyridone), 46.2 (CH of pyrimidine), 34.5 (CH2 of pyridone), 19.3 (CH3 of pyridone), 13.6 (CH3 of pyridone) ppm. Anal. Calcd for C16H17ClN4O (316.11): C, 60.66; H, 5.41; N, 17.69. Found: C, 60.41, H, 5.32, N, 17.83.
(±)-4-(4-chlorophenyl)-6-ethyl-2-imino-7-methyl-2,3,4,6-tetrahydropyrido[4,3-d]pyrimidin-5(1H)-one (5g) White solid in 87% yield; m.p.: 209–210 °C;; IR (KBr) υ: 3305 (NH), 3194 (NH), 3082 (CH), 2922 (CH), 1646 (C=O). cm−1 .1H NMR (300 MHz, DMSO-d6) δ: 11.97 (s, 1H, NH of pyrimidine), 7.78 (s, 1H, NH of pyrimidine), 7.26 (d, J = 7.25, 2H, CH of ArH), 7.24 (d, 1H, J = 6.96 Hz, = NH of pyrimidine), 6.94 (d, J = 6.96 Hz, 2H, CH of ArH), 6.13 (d, 1H, J = 6.96 Hz, CH of pyrimidine), 5.96 (s, 1H, CH of pyridone), 3.95 (m, 2H, CH2 of pyridone), 2.37 (s, 3H, CH3 of pyridone), 1.13 (t, J = 7.1 Hz, 3H, CH3 of pyridone) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 164.8 (C=O of pyridone), 164.4 (=C–NH of pyridone), 159.2 (NH =C of pyrimidine), 145.6 (=C–N of pyridone), 138.0 (C of ArH), 129.8 (C of ArH), 128.1 (CH of ArH), 127.7 (CH of ArH), 109.8 (=C of pyridone), 103.3 (=C of pyridone), 45.2 (CH of pyrimidine), 35.0 (CH2 of pyridone), 19.2 (CH3 of pyridone), 13.6 (CH3 of pyridone) ppm. Anal. Calcd for C16H17ClN4O (316.11): C, 60.66; H, 5.41; N, 17.69. Found: C, 60.41; H, 5.32; N, 17.83.
(±)-7-methyl-4-phenyl-4,6-dihydropyrido[4,3-d]pyrimidine-2,5(1H,3H)-dione (5h) White solid in 61% yield; m.p.: 275–277 °C; IR (KBr) υ: 3376 (NH), 3286 (NH), 3058 (CH), 1616 (C=O) cm−1. 1H NMR (300 MHz, DMSO-d6) δ: 11.19 (s, 1H, NH of pyridone), 10.48 (s, 1H, NH of pyrimidine), 7.93 (d, J = 8.6 Hz, 1H, NH of pyrimidine), 7.17–7.29 (m, 5H, CHs of ArH), 6.19 (d, J = 8.6 Hz, 1H, CH of primidine), 5.72 (s, 1H, CH of pyridine), 2.05 (s, 3H, CH3 of pyridone) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 163.6 (C=O of pyridone), 162.1 (=C–NH of pyridone), 156.8 (O=C of pyrimidine), 145.0 (=C–NH of pyridone), 144.4 (C of ArH), 128.1 (CH of ArH), 127.7 (CH of ArH), 126.1 (CH of ArH), 107.9 (=C of pyridone), 96.1 (=C of pyridone), 50.0 (CH of pyrimidine), 18.3 (CH3 of pyridone) ppm. Anal. Calcd for C14H13N3O2 (255.10): C, 65.87; H, 5.13; N, 16.46. Found: C, 65.71; H, 4.98; N, 16.57.
(±)-7-methyl-4-(4-nitrophenyl)-4,6-dihydropyrido[4,3-d]pyrimidine-2,5(1H,3H)-dione (5i) Yellow solid in 85% yield; m.p.: 209–211 °C; IR (KBr) υ: 3359 (NH), 3263 (NH), 3048 (CH), 1616 (C=O) cm−1. 1H NMR (300 MHz, DMSO-d6) δ: 11.13 (s, 1H, NH of pyridone), 10.70 (s, 1H, NH of pyrimidine), 8.12 (d, J = 8.0 Hz, 2H, CH of ArH), 7.66 (d, J = 7.7 Hz, 1H, NH of pyrimidine), 7.49 (d, J = 8.0 Hz, 2H, CH of ArH), 6.30 (d, J = 7.7 Hz, 1H, CH of pyrimidine), 5.74 (s, 1H, CH of pyridone), 2.07 (s, 3H, CH3 of pyridone) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 164.1 (C=O of pyridone), 162.0 (=C–NH of pyridone), 156.9 (O=C of pyrimidine), 152.0 (C of ArH), 151.8 (C of ArH), 147.0 (=C of pyridone), 129.5 (CH of ArH), 123.2 (CH of ArH), 108.7 (=C of pyridone), 106.6 (=C of pyridone), 49.9 (CH of pyrimidine), 18.2 (CH3 of pyridone) ppm. Anal. Calcd for C14H12N4O4 (300.09): C, 56.00; H, 4.03; N, 18.66. Found: C, 55.87; H, 3.91; C, 18.81.
(±)-4-(4-chlorophenyl)-7-methyl-4,6-dihydropyrido[4,3-d]pyrimidine-2,5(1H,3H)-dione (5j) White solid in 68% yield; m.p.: 204–206 °C; IR (KBr) υ: 3359 (NH), 3267 (NH), 2924 (CH), 1620 (C=O) cm−1. 1H NMR (300 MHz, DMSO-d6) δ: 11.08 (s, 1H, NH of pyridone), 10.72 (s, 1H, NH of pyrimidine), 7.55 (d, J = 7.6 Hz, 1H, NH of pyrimidine), 7.25 (d, br, 4H, CHs of ArH), 6.16 (d, J = 7.6 Hz, 1H, CH of pyrimidine), 5.77 (s, 1H, CH of pyridone), 2.06 (s, 3H, CH3 of pyridone) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 163.7 (C=O of pyridone), 163.0 (=C–NH of pyridone), 156.8 (O=C of pyrimidine), 145.7 (=C–NH of pyridone), 143.4 (C of ArH), 132.4 (C of ArH), 129.9 (CH of ArH), 127.7 (CH of ArH), 113.4 (=C of pyridone), 107.4 (=C of pyridone), 49.9 (CH of pyrimidine), 18.3 (CH3 of pyridone) ppm. Anal. Calcd for: C14H12ClN3O2 (289.06): C, 58.04; H, 4.18; N, 14.50. Found: C, 58.17; H, 4.01; N, 14.69.
(±)-4-(4-bromophenyl)-7-methyl-4,6-dihydropyrido[4,3-d]pyrimidine-2,5(1H,3H)-dione (5k) White solid in 84% yield; m.p.: 208–210 °C; IR (KBr) υ: 3356 (NH), 3110 (CH), 3077 (CH), 2932 (CH), 1619 (C=O) cm−1. 1H NMR (300 MHz, DMSO-d6) δ: 11.13 (s, 1H, NH of pyridone), 10.69 (s, 1H, NH of pyrimidine), 7.54 (d, J = 7.58 Hz, 1H, NH of pyrimidine), 7.36 (d, J = 7.52 Hz, 2H, CH of ArH), 7.21 (d, J = 7.52 Hz, 2H, CH of ArH), 6.15 (d, J = 7.58 Hz, 1H, CH of pyrimidine), 5.73 (s, 1H, CH of pyridone), 2.05 (s, 3H, CH3 of pyridone) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 164.0 (C=O of pyridone), 162.5 (=C–NH of pyridone), 157.0 (C=O of pyrimidine), 144.9 (=C–NH of pyridone), 143.7 (C of ArH), 130.5 (CH of ArH), 128.1 (CH of ArH), 122.4 (C of ArH), 112.7 (=C of pyridone), 107.9 (=C of pyridone), 47.8 (CH of pyrimidine), 19.6 (CH3 of pyridone)Anal. Calcd for: C14H12BrN3O2 (333.01): C, 50.32; H, 3.62; N, 12.57. Found: C, 50.17; H, 3.62; N, 12.73.
(±)-2-imino-7-methyl-4-(4-nitrophenyl)-2,3,4,6-tetrahydropyrido[4,3-d]pyrimidin-5(1H)-one (5l) yellow solid in 90% yield; m.p.: 256–258 °C; IR (KBr) υ: 3328 (NH), 3312 (NH), 2952 (CH), 3077 (CH), 2932 (CH), 1657 (C=O) cm−1. 1H NMR (300 MHz, DMSO-d6) δ: 11.21 (s, 1H, NH of pyridone), 10.86 (s, 1H, NH of pyrimidone), 8.37 (d, J = 7.5 Hz, 1H, CH of ArH), 7.58 (d, J = 7.1 Hz, 1H, NH of pyrimidine), 7.53 (s, 1H, NH of pyrimidine), 7.30 (d, J = 7.5 Hz, 2H, CH of ArH), 6.46 (d, J = 7.1 Hz, 1H, CH of pyrimidine), 5.97 (s, 1H, CH of pyridone), 2.21 (s, 3H, CH3 of pyridone) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 164.1 (C=O of pyridone), 162.0 (=C–NH of pyridone), 157.0 (NH=C of pyrimidine), 152.0 (=C–NH of pyridone), 151.8 (C of ArH), 146.0 (C of ArH), 129.5 (CH of ArH), 123.2 (CH of ArH), 114.1 (=C of pyridone), 106.6 (=C of pyridone), 48.4 (CH of pyrimidine), 19.6 (CH3 of pyridone). Anal. Calcd for: C14H13N5O3 (299.10): C, 56.18; H, 4.38; N, 23.40. Found: C, 56.01; H, 4.29, N, 23.57.
2,8-diethyl-10-(4-methoxyphenyl)-3,7-dimethyl-8,10-dihydro-1H-pyrano[3,2-c:5,6-c']dipyridine-1,9(2H)-dione (6) White solid in 56% yield; m.p.: 228–229 °C; IR (KBr) υ: 3076 (CH), 2975 (CH), 1638 (C=O) cm−1. 1H NMR (300 MHz, DMSO-d6) δ: 6.75–6.84 (m, 4H, CH of ArH), 6.05 (s, 2H, CH of pyridone), 5.89 (s, 1H, CH residue of CHO), 3.91–3.98 (m, 2H, CH2 of pyridone), 3.68 (s, 3H, CH3 of aldehyede), 2.36 (s, 6H, CH3 of pyridone), 1.12 (t, J = 6.2 Hz, 6H, CH3 of pyridone) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 168.9 ((=C of pyridone), 155.2 (C=O of pyridone), 154.2 (C-N of ArH),145.7 (=C of pyridone), 137.0 (C of ArH), 130.7 (CH of ArH), 114.1 (CH of ArH), 105.2 (=C of pyridone), 97.6 (=C of pyridone), 54.8 (O-CH3), 50.0 (CH of pyran), 48.7 (CH2 of pyridone), 20.3 (CH3 of pyridone), 13.7 (CH3 of pyridone).Anal. Calcd for: C24H26N2O4 (406.19): C, 70.92; H, 6.45; N, 6.89. Found: C, 70.73; H, 6.21; N, 6.61.
Conclusion
The multicomponent synthesis of a novel potentially bioactive series of dihydropyrimidin-2(1H)-ones/thione/imine based on 4-hydroxy-2-pyridone alkaloids has been provided by a rapid, straight, and efficient method using ZnCl2.2H2O as inexpensive catalyst. The advantages of this reaction consist generality and simplicity operational, short reaction time, simple workup, and high to excellent yields and eco-compatibility.
Availability of data and materials
The data in this manuscript have not been published elsewhere, nor are they under consideration by another publisher. The corresponding author declares that all the data and materials are available with in the article.
References
Alfatafta AA, Gloer JB, Scott JA, Malloch D (1994) Apiosporamide, a new antifungal agent from the coprophilous fungus apiospora montagnei. J Nat Prod 57:1696–1702. https://doi.org/10.1021/np50114a012
Bergmann S, Schümann J, Scherlach K et al (2007) Genomics-driven discovery of PKS-NRPS hybrid metabolites from Aspergillus nidulans. Nat Chem Biol 3:213–217. https://doi.org/10.1038/nchembio869
Breinholt J, Ludvigsen S, Rassing BR et al (1997) Oxysporidinone: a novel, antifungal N-methyl-4-hydroxy-2-pyridone from Fusarium oxysporum. J Nat Prod 60:33–35. https://doi.org/10.1021/np9605596
Chitra S, Devanathan D, Pandiarajan K (2010) Synthesis and in vitro microbiological evaluation of novel 4-aryl-5-isopropoxycarbonyl-6-methyl-3,4-dihydropyrimidinones. Eur J Med Chem 45:367–371. https://doi.org/10.1016/j.ejmech.2009.09.018
Dallinger D, Stadler A, Kappe CO (2004) Solid- and solution-phase synthesis of bioactive dihydropyrimidines. Chem Inform. https://doi.org/10.1002/chin.200452250
Debache A, Amimour M, Belfaitah A et al (2008) A one-pot Biginelli synthesis of 3,4-dihydropyrimidin-2-(1H)-ones/thiones catalyzed by triphenylphosphine as Lewis base. Tetrahedron Lett 49:6119–6121. https://doi.org/10.1016/j.tetlet.2008.08.016
Ding F, Leow ML, Ma J et al (2014) Collective synthesis of 4-hydroxy-2-pyridone alkaloids and their antiproliferation activities. Chem Asian J 9:2548–2554. https://doi.org/10.1002/asia.201402466
Ferraz AC, Angelucci MEM, Da Costa ML et al (1999) Pharmacological evaluation of ricinine, a central nervous system stimulant isolated from ricinus communis. Pharmacol Biochem Behav 63:367–375. https://doi.org/10.1016/S0091-3057(99)00007-6
Folkers K, Johnson TB (1933a) Researches on pyrimidines. CXXXIII. Some reactions and derivatives of 2-keto-4-phenyl-5-carbethoxy-6-methyl-1,2,3,4-tetrahydropyrimidine. J Am Chem Soc 55:2886–2893. https://doi.org/10.1021/ja01334a043
Folkers K, Johnson TB (1933b) Researches on pyrimidines. CXXXVI. The mechanism of formation of tetrahydropyrimidines by the Biginelli reaction 1. J Am Chem Soc 55:3784–3791. https://doi.org/10.1021/ja01336a054
Gijsen HJM, Berthelot D, De Cleyn MAJ et al (2012) Tricyclic 3,4-dihydropyrimidine-2-thione derivatives as potent TRPA1 antagonists. Bioorg Med Chem Lett 22:797–800. https://doi.org/10.1016/j.bmcl.2011.12.068
Gore S, Baskaran S, Koenig B (2011) Efficient synthesis of 3,4-dihydropyrimidin-2-ones in low melting tartaric acid–urea mixtures. Green Chem 13:1009. https://doi.org/10.1039/c1gc00009h
Hejazi S, Osman O, Alyoubi A et al (2016) The thermodynamic and kinetic properties of 2-hydroxypyridine/2-pyridone tautomerization: a theoretical and computational revisit. Int J Mol Sci 17:1893. https://doi.org/10.3390/ijms17111893
Hubka V, Nováková A, Kolařík M et al (2015) Revision of Aspergillus section Flavipedes: seven new species and proposal of section Jani sect. nov. Mycologia 107:169–208. https://doi.org/10.3852/14-059
Ismaili L, Nadaradjane A, Nicod L et al (2008) Synthesis and antioxidant activity evaluation of new hexahydropyrimido[5,4-c]quinoline-2,5-diones and 2-thioxohexahydropyrimido[5,4-c]quinoline-5-ones obtained by Biginelli reaction in two steps. Eur J Med Chem 43:1270–1275. https://doi.org/10.1016/j.ejmech.2007.07.012
Jankovic N, Bugarcic Z, Markovic S (2015) Double catalytic effect of (PhNH3)2CuCl4 in a novel, highly efficient synthesis of 2-oxo and thioxo-1,2,3,4-tetra-hydopyrimidines. J Serbian Chem Soc 80:595–604. https://doi.org/10.2298/JSC141028011J
Janković N, Trifunović Ristovski J, Vraneš M et al (2019) Discovery of the Biginelli hybrids as novel caspase-9 activators in apoptotic machines: lipophilicity, molecular docking study, influence on angiogenesis gene and miR-21 expression levels. Bioorg Chem 86:569–582. https://doi.org/10.1016/j.bioorg.2019.02.026
Jessen HJ, Gademann K (2010) 4-Hydroxy-2-pyridone alkaloids: structures and synthetic approaches. Nat Prod Rep 27:1168. https://doi.org/10.1039/b911516c
Kamali M, Keramat Pirolghor F (2022) One-pot three-component synthesis of novel chromeno[3,2- c ]pyridine-1,9(2 H )-diones by using SnCl 2 ⋅2H 2 O as catalyst. J Heterocycl Chem 59:655–663. https://doi.org/10.1002/jhet.4404
Kamali M, Shahi S, Mashhadi Akbar Bujar M (2020) Temperature-dependent green synthesis of new series of Mannich bases from 4-hydroxy-pyridine-2-one and their antioxidant activity evaluation. ChemistrySelect 5:1709–1712. https://doi.org/10.1002/slct.201904615
Kappe CO (2000) Biologically active dihydropyrimidones of the Biginelli-type—a literature survey. Eur J Med Chem 35:1043–1052. https://doi.org/10.1016/S0223-5234(00)01189-2
Kim J, Park C, Ok T et al (2012) Discovery of 3,4-dihydropyrimidin-2(1H)-ones with inhibitory activity against HIV-1 replication. Bioorg Med Chem Lett 22:2119–2124. https://doi.org/10.1016/j.bmcl.2011.12.090
Kolosov MA, Orlov VD, Beloborodov DA, Dotsenko VV (2009) A chemical placebo: NaCl as an effective, cheapest, non-acidic and greener catalyst for Biginelli-type 3,4-dihydropyrimidin-2(1H)-ones (-thiones) synthesis. Mol Divers 13:5–25. https://doi.org/10.1007/s11030-008-9094-8
Kraus GA, Wanninayake UK, Bottoms J (2016) Triacetic acid lactone as a common intermediate for the synthesis of 4-hydroxy-2-pyridones and 4-amino-2-pyrones. Tetrahedron Lett 57:1293–1295. https://doi.org/10.1016/j.tetlet.2016.02.043
Ling Y, Hao Z-Y, Liang D et al (2021) The expanding role of pyridine and Dihydropyridine scaffolds in drug design. Drug Des Devel Ther 15:4289–4338. https://doi.org/10.2147/DDDT.S329547
Mayer TU, Kapoor TM, Haggarty SJ et al (1999) Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science (80-) 286:971–974. https://doi.org/10.1126/science.286.5441.971
Milović E, Janković N, Petronijević J et al (2022a) Synthesis, characterization, and biological evaluation of tetrahydropyrimidines: dual-activity and mechanism of action. Pharmaceutics 14:2254. https://doi.org/10.3390/pharmaceutics14102254
Milović E, Petronijević J, Joksimović N et al (2022b) Anticancer evaluation of the selected tetrahydropyrimidines: 3D-QSAR, cytotoxic activities, mechanism of action, DNA, and BSA interactions. J Mol Struct 1257:132621. https://doi.org/10.1016/j.molstruc.2022.132621
Molnár I, Gibson DM, Krasnoff SB (2010) Secondary metabolites from entomopathogenic Hypocrealean fungi. Nat Prod Rep 27:1241. https://doi.org/10.1039/c001459c
Nebo L, Varela RM, Fernandes JB, Palma M (2019) Microwave-assisted extraction of Ricinine from Ricinus communis leaves. Antioxidants 8:438. https://doi.org/10.3390/antiox8100438
Newman DJ, Cragg GM (2007) Natural products as sources of new drugs over the last 25 years. J Nat Prod 70:461–477. https://doi.org/10.1021/np068054v
Rajanarendar E, Reddy MN, Murthy KR et al (2010) Synthesis, antimicrobial, and mosquito larvicidal activity of 1-aryl-4-methyl-3,6-bis-(5-methylisoxazol-3-yl)-2-thioxo-2,3,6,10b-tetrahydro-1H-pyrimido[5,4-c]quinolin-5-ones. Bioorg Med Chem Lett 20:6052–6055. https://doi.org/10.1016/j.bmcl.2010.08.060
Rani VR, Srinivas N, Kishan MR et al (2001) Zeolite-catalyzed cyclocondensation reaction for the selective synthesis of 3,4-dihydropyrimidin-2(1H)-onesIICT Communication No. 4737. Green Chem 3:305–306. https://doi.org/10.1039/b107612b
Ranu BC, Hajra A, Jana U (2000) Indium(III) chloride-catalyzed one-pot synthesis of Dihydropyrimidinones by a three-component coupling of 1,3-dicarbonyl compounds, aldehydes, and urea: an improved procedure for the Biginelli reaction. J Org Chem 65:6270–6272. https://doi.org/10.1021/jo000711f
Safaei-Ghomi J, Tavazo M, Mahdavinia GH (2018) Ultrasound promoted one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones using dendrimer-attached phosphotungstic acid nanoparticles immobilized on nanosilica. Ultrason Sonochem 40:230–237. https://doi.org/10.1016/j.ultsonch.2017.07.015
Shen Z-L, Xu X-P, Ji S-J (2010) Brønsted base-catalyzed one-pot three-component Biginelli-type reaction: an efficient synthesis of 4,5,6-triaryl-3,4-dihydropyrimidin-2(1 H )-one and mechanistic study. J Org Chem 75:1162–1167. https://doi.org/10.1021/jo902394y
Singh SB, Liu W, Li X et al (2012) Antifungal spectrum, in vivo efficacy, and structure-activity relationship of Ilicicolin H. ACS Med Chem Lett 3:814–817. https://doi.org/10.1021/ml300173e
Su W, Li J, Zheng Z, Shen Y (2005) One-pot synthesis of dihydropyrimidiones catalyzed by strontium(II) triflate under solvent-free conditions. Tetrahedron Lett 46:6037–6040. https://doi.org/10.1016/j.tetlet.2005.07.021
Valizadeh H, Shockravi A (2009) Imidazolium-based phosphinite ionic liquid as reusable catalyst and solvent for one-pot synthesis of 3,4-dihydropyrimidin-2(1 H )- (thio)ones. Heteroat Chem 20:284–288. https://doi.org/10.1002/hc.20549
Wachira S, Omar S, Jacob J et al (2014) Toxicity of six plant extracts and two pyridone alkaloids from Ricinus communis against the malaria vector Anopheles gambiae. Parasit Vectors 7:312. https://doi.org/10.1186/1756-3305-7-312
Yadlapalli RK, Chourasia OP, Vemuri K et al (2012) Synthesis and in vitro anticancer and antitubercular activity of diarylpyrazole ligated dihydropyrimidines possessing lipophilic carbamoyl group. Bioorg Med Chem Lett 22:2708–2711. https://doi.org/10.1016/j.bmcl.2012.02.101
Acknowledgements
The authors are grateful for financial support provided by Kharazmi University.
Funding
This declaration is not applicable.
Author information
Authors and Affiliations
Contributions
MK designed, directed the project and wrote the article; SM performed the experiments and collected data.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This declaration is not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kamali, M., Mohammadzadeh, S. One-pot Biginelli synthesis of novel series of 2-oxo/thioxo/imino-1,2,3,4-tetrahydropyrimidine based on 4-hydroxy-2-pyridone. Chem. Pap. 77, 4943–4952 (2023). https://doi.org/10.1007/s11696-023-02832-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-02832-1