Abstract
In the present work, zinc oxide nanoparticles (ZnO NPs) were synthesized by using different precursors and the influence of different counter ions (acetate, sulfate, and nitrate) on various physical, morphological, and optical properties was investigated. The in vitro cytotoxicity of sonochemically synthesized ZnO NPs were assessed on murine NIH 3T3 cell line for 24 h. The synthesized materials were systematically analyzed by advanced characterization tools, and the obtained data confirm the prominent role of the precursor ions determining the various properties of ZnO. Transmission electron microscopy and field emission scattering electron micrographs revealed the active role of anions in determining the both interior and exterior surface morphology of ZnO; powder X-ray diffraction analysis exhibits hexagonal wurtzite structure of ZnO. Room temperature photoluminescence spectra show the blue and green emission bands along with large crystalline defects in the ZnO; also, energy dispersive X-ray spectroscopy analysis confirms the formation of pure ZnO. The neutral red uptake and lactate dehydrogenase tests were used to determine the cytotoxicity of synthesized ZnO NPs. Data showed induction of cytotoxic effects such as damage of membrane integrity, reduction of cell viability on a dose-dependent basis. The main aim of the present paper is to study the influence of different precursor ions on various properties of ZnO NPs as well as the cytotoxic studies on NIH murine 3T3 cell lines. The result justifies that the precursor ion has a substantial impact on determining the property of the nanostructured material.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanoparticles emerged as advanced tunable multifunctional particles due to their remarkable and unique physical, morphological, and optical features (Ong et al. 2018). Zinc oxide (ZnO) nanostructures have gained significant attraction due to its morphology and size-dependent optical and photocatalytic properties. ZnO is a hexagonal wurtzite semiconductor (n-type) with a wide direct band gap of 3.37 eV and a high exciton binding energy (60 meV) at RT (Janaki et al. 2015). In particular, ZnO nanostructures comprising of numerous ZnO nanoparticles are found suitable for various photochemical and electrochemical applications, where high surface area, surface chemical group, and good crystal structure are the prime requisite (Mayekar et al. 2014; Pauzi et al. 2019). Several synthesis techniques have been developed for the preparation of the nanostructure of different shapes because surface size and structure have a direct correlation in determining the optical and structural properties (Ciciliati et al. 2015). Among all, the chemical and physical methods available for ZnO nanostructured materials synthesis, the most common are—wet chemical (Shimpi et al. 2016), sol–gel (Rochman and Akwalia 2017), microemulsion (Emsaki et al. 2018), thermal decomposition of organic precursor (Ansari et al. 2017), electrodeposition (Yang et al. 2015), microwave-assisted (Assi et al. 2015), chemical vapor deposition (Meléndrez et al. 2016), hydrothermal (Kumaresan et al. 2017), sputtering (Rashid et al. 2015), precipitation method (Devi and Velu 2016), and sonochemical method (Khan et al. 2016, 2018). Most of the above-mentioned techniques used templates, capping agents, different precursor, and reducing agents, and other mediators to govern the morphology of the material, so far these methods were not been used on a large scale widely, but chemical synthesis has been commonly used due to its ease and cost-effectiveness (Madhumitha et al. 2016). One must emphasize that the precursor ions have a significant impact in determining the properties of ZnO nanoparticles, and it is the most efficient method for the introduction of different anions to the growth medium which explores the new way to tailor the properties of ZnO under the equivalent reaction conditions. Abbasi et al. (2001) observed the role of anions on the low-temperature growth of ZnO nanostructures by using four precursor salts for ZnO synthesis by the aqueous chemical growth method (Abbasi et al. 2001). Kumar et al. (2015) observed the effect of precursor ions (acetate and nitrate) on the morphology and photocatalytic activity; the group synthesized ZnO using three different methods and further advised that the precursor ions have a considerable effect on the morphology of the nanomaterial (Kumar et al. 2015). Barreto et al. (2013) reports the microwave-assisted ZnO synthesis and studied the effects of precursor materials, irradiation time, temperature, washing agents and additives on the shape and size (Barreto et al. 2013). Similarly, Kaur and Kalia (2016) observed the role of precursor ions on antimicrobial activity (Kaur and Kalia 2016). To date, the most commonly used precursor salts for the preparation of ZnO are zinc sulfate, zinc acetate, zinc nitrate, and zinc chloride (Pudukudy and Yaakob 2014). All the types of approaches applied for the synthesis of ZnO using different precursors have been summarized in Table 1.
The murine fibroblast cell line NIH 3T3 has been isolated from mouse embryonic fibroblast, since then it has become a standard fibroblast cell line and is used in several studies. Numerous in vitro studies have been done to evaluate the toxicity of several nanoparticles using different assay; still the potential toxicity of nanoparticles has not been fully understood. Due to its remarkable physico-chemical properties, ZnO is used in advanced biological applications such as antibacterial/antimicrobial agents, drug delivery, and cancer therapy. The exact mechanisms of cytotoxicity from ZnO NPs are not clearly understood, but the generation of hydroxyl radicals, superoxide anion, and per hydroxyl radicals from the surface of ZnO is thought to be major components.
The present study is aimed to evaluate the cytotoxic effect of ZnO NPs prepared by the sonochemical method using different precursors also studying the impact of precursor ions on various properties.
Materials and analysis
Chemical and reagents
MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide], was purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and antibiotics were purchased from Gibco, USA.
Experimental methods
Here, we have reported the synthesis of ZnO by a modified sonochemical and chemical precipitation technique in the present work. The synthesis of ZnO was carried out by three different precursors of Zn to investigate the suitable precursor for the preparation of ZnO NPs and the effect of the different precursors on various physical, morphological, and optical properties.
Chemicals and synthesis method
The synthesis strategy affects the shape and size of the NPs which governs the various features of NPs. The sonochemical method emerges as a reliable and sustainable method for the synthesis of the novel material with exceptional properties and is based on the rapid development and explosion of the bubbles in an aqueous medium due to acoustic cavitation at ultrasonic frequency (20–40 kHz). The technique has numerous advantages over the other synthesis techniques in terms of simple, low cost, zero waste generation, less reaction time and provides access to control the size of synthesized nanomaterial.
Synthesis of ZnO NPs
Zinc acetate dihydrate Zn (CH3COO)2·2H2O (ZA), zinc nitrate hexahydrate (Zn (NO3)2·6H2O) (ZN), zinc sulfate heptahydrate (ZnSO4·7H2O) (ZS), sodium hydroxide (NaOH) pellets procured from Sigma-Aldrich, Germany. Samples were marked as ZnO-1, ZnO-2, and ZnO-3 for ZA, ZS, and ZN, respectively. The base concentration of precursor salts and reducing agents were kept fixed and the same reaction condition was provided for the synthesis and the method was almost similar for all the precursors used. The ZnO synthesis was carried out at RT under optimized conditions.
-
Zinc acetate precursor (ZnO-1): Firstly dissolution of Zn (CH3COO)2·2H2O (0.5 M) was done in deionized water (100 ml) under continuous stirring. Separately, 0.5 M NaOH was prepared and stirs continuously to get dissolved completely; sodium hydroxide aqueous solution was added dropwise to the zinc acetate (ZA) solution along with rigorous stirring on a magnetic stirrer until the formation of white precipitates. The round bottom flask was allowed continuing stir for 3 h under at 800 rpm. Once the reaction was complete the mixture was kept inside an ultrasonicator (Sonar 40 KHz) for 1 h at 37 °C. After the completion of the reaction, the mixture was allowed to cool at RT, followed by centrifugation at 7000 rpm for 5 min to obtain the precipitate, while the supernatant was discarded. The obtained residue was washed 2–3 times with distilled water and ethanol to eliminate any impurities present on the surface of nanoparticles. Finally, the synthesized precipitates were dried at 100 °C in an oven so that the precipitates get completely reduced into ZnO NPs. Lastly; the obtained powder was ground in a mortar and pestle to get homogenous ZnO powder.
-
Reaction scheme of formation of ZnO by acetate precursor:
$${\text{Zn}}\left( {{\text{CH}}_{3} {\text{COO}}} \right)_{2} \cdot 2{\text{H}}_{2} {\text{O}} + 2{\text{NaOH}} \to {\text{ Zn}}\left( {{\text{OH}}} \right)_{2} + 2{\text{CH}}_{3} {\text{COONa}}$$$${\text{Zn}}\left( {{\text{OH}}} \right)_{2} \mathop{\longrightarrow}\limits^{{{\text{Drying}}\,{\text{at}}\,{100}^{ \circ } {\text{C}}}}{\text{ZnO}} + {\text{H}}_{2} {\text{O}}$$ -
Sulfate Precursor (ZnO-2): The same experimental procedure was followed for ZnO-2 as well, replacing the precursor.
-
Reaction scheme of sulfate precursor:
$${\text{ZnSO}}_{{4}} + {\text{2NaOH}} \to {\text{Zn}}\left( {{\text{OH}}} \right)_{{2}} + {\text{2NaSO}}_{{4}}$$$${\text{Zn}}\left( {{\text{OH}}} \right)_{2} \mathop{\longrightarrow}\limits^{{{\text{Drying}}\,{\text{at}}\,{100}^{ \circ } {\text{C}}}}{\text{ZnO}} + {\text{H}}_{2} {\text{O}}$$ -
Nitrate Precursor (ZnO-3): The same experimental procedure was followed using the nitrate precursor. Figure 1 depicts the schematic diagram of the reaction process of the synthesis of ZnO.
-
Reaction scheme of nitrate precursor:
$${\text{Zn }}\left( {{\text{NO}}_{{3}} } \right)_{{2}} \cdot {\text{6H}}_{{2}} {\text{O }} + {\text{2NaOH}} \to {\text{Zn}}\left( {{\text{OH}}} \right)_{{2}} + {\text{2NaNO}}_{{3}} + {\text{6H}}_{{2}} {\text{O}}$$$${\text{Zn}}\left( {{\text{OH}}} \right)_{2} \mathop{\longrightarrow}\limits^{{{\text{Drying}}\,{\text{at}}\,{100}^{ \circ } {\text{C}}}}{\text{ZnO}} + {\text{H}}_{2} {\text{O}}$$
Characterization of synthesized ZnO NPs
The morphological features of the synthesized ZnO NPs were done by transmission electron microscopes (TECNAI 200 kV TEM-Fei, Electron Optics) and field emission-scanning electron microscopy (FE-SEM) (ZEISS, Model-SIGMA 5.05) performing at 20 kV (accelerating voltage). While the elemental analysis was done by the attached EDS analyzer with FE-SEM (EDS Model: INCA Oxford) instrument. The phase identification and crystallinity of the ZnO NPs were done by PXRD, (Bruker X-ray Diffractometer) graphite filtered CuK radiation (λ = 1.54 Å) at 40 kV. The scanning rate was 3/min (from 2θ = 20°–80°) used to record diffraction patterns. The UV-DRS study was done by UV–Vis spectrometer (Shimadzu) in the range of 200–800 nm to obtain optical absorption. The measurement was done by Perkin Elmer USA (Model-Lambda 950) in diffuse reflectance mode using BaSO4 as the DRS standard. Fourier Transmission Infrared spectroscopy was recorded on Perkin Elmer-Spectrum 6500, in the range of 4000–400 cm−1 with the resolution of 4 cm−1. The sample was prepared by using the solid KBr pellet technique. Photoluminescence spectra of ZnO were analyzed at RT at 315 nm excitation wavelengths using the Jobin Yvon instrument-JY Fluorolog-3-11 Japan and the emission spectrum was scanned over a wavelength range of 300–600 nm. Brunauer–Emmett–Teller (BET) was used to measure specific surface area, by using N at 77 K, and the Barrett–Joyner–Halenda (BJH) method was used to analyze the size and volume of the pore. The measurement was carried out by using NOVA-1000 version 3.70 using N as an adsorbent.
Culture of cells and exposure of ZnO NPs
The Swiss mouse embryonic fibroblast (NIH 3T3) cell line (SKU-93061524-1VL) were procured from Sigma-Aldrich Chemie GmbH Eschenstrasse 5D-82024 TAUFKIRCHEN. NIH 3T3 cells were sub-cultured in DMEM + 2 mM Glutamine + 10% Calf Serum (CS) at a 5% CO2 incubator at 37 °C. The cells at 80% confluence were sub-cultured into 96-well plates cytotoxicity test. NIH 3T3 cell lines were grown 24 h before exposure to ZnO NPs. The stock solution of ZnO NPs was made in DDW and diluted according to the treatment concentrations (0–50 µg/ml). Control cells were not exposed to ZnO NPs and were considered as controls.
NRU test
The lysosomal activity was measured by the NRU test (Neutral Red Cell Cytotoxicity Assay Kit 5/19 (Catalog # K447-1000). NRU solution (100 µl) was mixed with each well in a final concentration of 0.5 mg/ml and further left for incubation for 3 h. The absorbance accumulated NRU was determined at 540 nm using a micro plate reader (BioTek Instruments, Winooski, VT, the USA equipped with Gen5 software, version 1.09).
Release of lactate dehydrogenase (LDH) test
LDH test, used as an indicator of cell membrane injury, was measured by LDH assay kit (Cytotoxicity Detection Kit, Roche Diagnostics, Milan, Italy) on the culture medium of cells exposed to ZnO NPs (0, 1, 4, 10, 20 and 50 µg/ml) for 24 h. Unexposed cells were used as a negative control. This colorimetric assay is based on the cleavage of a tetrazolium salt when LDH activity is present in the cell culture supernatant. The absorbance was measured at 490 nm using a spectrophotometric microtiter plate reader (BioTek Instruments, Winooski, VT, the USA equipped with Gen5 software, version 1.09).
Statistical analysis
Data were statistically analyzed using a one-way ANOVA test with the least significant difference test. Values of *p < 0.05 were considered statistically significant.
Results and discussion
The as-synthesized ZnO nanomaterials obtained using three different precursors salts were systematically characterized, and the role of precursor ions on various properties was studied in detail.
Morphological analysis by FE-SEM
A typical FE-SEM image of ZnO NPs is depicted in Fig. 2. It was observed that the precursor ion such as acetate, sulfate, and nitrate generally leads to the formation of irregular shapes in the form of mesoporous structures. The BJH analysis supports the synthesized material is mesoporous. It was also observed that the agglomeration of the inside particle leads to the formation of highly condensed porous nanospheres for all the precursor ions.
Morphological analysis by TEM
The internal morphology of the NPs was further analyzed by TEM as shown in Fig. 3. The obtained particulates were dispersed in a suitable solvent and then deposited on the carbon-coated grid for TEM studies. The TEM micrographs of ZnO reveal that the particles spherical in shape aggregate together to form a particle of size less than 50 nm. It was confirmed by the micrograph that the ZnO exhibits precursor ions dependent morphology. The average particle size obtained was 29.3 nm, 43.8 nm, and 20.3 nm for ZnO-1, ZnO-2, and ZnO-3, respectively (Fig. 3).
ZnO synthesized using acetate precursor shows highly agglomerated nanostructure, whereas elongated and round particle aggregates to form chain-like morphology were observed for sulfate ions. In the case of nitrate precursors, homogeneously arranged spherical NPs were formed with a minimum average particle size as compared to the other precursor. The porous sphere-like texture appears in ZnO-3 due to the internal agglomeration of the particle ranging from 6 to 20 nm (Fig. 3c). A significant variation was observed in the surface morphology of the particle w.r.t the precursor ion due to the adsorption of few faces of ZnO lattice causing the hindrance of the growth of nanoparticles results in the difference in size and shape of ZnO NPs. The differences in the shape of the nanoparticles are attributed to the fact that sulfate anions adsorb strongly on the surface as compared to nitrate and acetate ions which exhibit weak surface interactions. When the surface adsorption increases, the growth process decreases resulting in smaller particle sizes. The size of ZnO NPs decreases in the following order for different precursors: NO3− > CH3COO− > SO4−. It is very important to note that the precursor salt's structural chemistry also influences the morphology of the resultant product, i.e., ZnO.
Powder X-ray diffraction (PXRD)
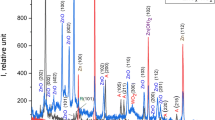
The comparative XRD diffractogram of ZnO synthesized using three precursors is presented Fig. 4. A sharp diffraction peak specifies that the particles are crystalline in nature (Pillai et al. 2020). The characteristic diffraction peaks of ZnO appeared at 31.7°, 34.3°, 36.2°, 47.4°, 56.5°, and 62.8° by relieving their reflecting planes at (100), (002), (101), (102), (110), and (103), respectively, for ZnO-3. The position of these peaks was perfectly matched with the hexagonal wurtzite phase of zinc oxide (JCPDS data: ICSD card no. 16-973) (Moazzen et al. 2012).
The pure wurtzite diffraction pattern was observed in the ZnO-3. The calculation of the mean particle size was calculated from Full-width half-maximum, using the XRD values by Scherer’s formula:
where D = diameter of average crystal size, λ = wavelength of Angstrom (1.54), β = width at half-maximum and θ = Bragg diffraction angle.
The average crystalline size was calculated by using the averages of all the diffraction peaks. The average crystal size of ZnO-1 is 80.8 nm, ZnO-2 is 51.9 nm and ZnO-3 is 17.7 nm. The difference in average crystalline size was attributed to the fact that NO32− is the non-coordinative ligand so that the ZnO wurtzite chooses to grow in the c-axis orientation, i.e., 0001 planes. As the crystallite size decreases, the average surface area increases which are confirmed by BET analysis. The smallest crystal size was observed for ZnO-3 with nitrate precursor ions. The comparative data of the diffraction peak with the standard (JCPDS) were also summarized in Tables 2 and 3 illustrate the detailed XRD analysis.
Identification of functional groups by FT-IR:
FT-IR helped to identify the associated functional groups and bond structure of as-synthesized ZnO nanomaterial (Rammah et al. 2020). Figure 5 shows typical FT-IR spectra of ZnO (1, 2, and 3).
The strong absorption band situated between 430and 458 cm−1 is due to the characteristic fingerprint bands due to the Zn–O stretching vibration mode of the ZnO lattice. The wavenumbers would shift when acetate groups form a complex with a metal such as zinc. A broad band at 3400–3900 cm−1 is attributed to the surface absorbance of water and hydroxyl groups at the surface of particles, whereas band in the range of 1570–1640 cm−1 is attributed to the strong asymmetric mode of vibration of C=O, which is due to the absorption of atmospheric carbon in all the samples. The symmetric stretching occurs from 1380 to 1520 cm−1 due to the presence of the C-O group. The IR peak confirms the stretching at 460, 448, and 431 cm−1 attributed to the ZnO stretching for acetate, sulfate and nitrate precursors, respectively. The vibrational pattern is different for the ZnO-1, 2, and 3, clearly due to the influence of precursor ions. The different precursor ion shifts the vibrational frequency of Zn–O in the fingerprinting region as seen in the figure. Table 4 shows the comparative data of the FT-IR spectra of the precursors used for the preparation of ZnO with the corresponding bonds, their characteristic bands, and intensity.
Band gap measurements
The DRS study helped to calculate the energy bandgap (EBG) (Eg in eV) as a function of reflectance percent. Optical bandgap (OBG) spectra and DRS of the synthesized ZnO NPs are exhibited in Fig. 6a, b. The calculation of EBG was done by Kubelka–Munk function F(R) transformation of measured % reflection as exhibited in the equation.
where K = transformed reflectance as per the Kubelka–Munk, R = reflection percent (R %).
The energy bandgap (EBG) of 3.23 eV was inferred from the graph which satisfies the EBG of bulk ZnO (3.32 Ev), whereas the other two precursor ions ZnO-1 (5.3 eV) and ZnO-2 (5.0 eV) shows the large shift in OBG which does not correspond to the bulk ZnO. It is a known fact that the optical band gap decreases when particle size increases, ZnO-3 has the lowest particle size whereas ZnO-2 has the highest as confirmation was done by TEM and XRD results. The large shifting in the bandgap of the other two precursors is due to the emergence of quantum confinement effect, synthesis technique, and morphology of the material that influences the band gap values significantly. Thus, based on the OBG studies, it was found that the ZnO-3 exhibits the EBG of 3.23 eV near the visible region. It is also reported that there is no change in the crystalline structure and symmetry of the material with the different methods of synthesis, also supported by the XRD data.
Based on the above observation, it was concluded that the precursor ions and size of the particles have a large impact on the optical property of ZnO. DRS results were also supported by the PL data as bandgap may be due to the surface-oriented defects as seen in PL spectra of ZnO-3 which will be discussed in the later section.
Elemental analysis of ZnO NPs by EDX
The elemental composition and atomic percent of the particles were confirmed by EDX. ZnO-3 shows the nanoparticles without any impurity and in good atomic percent. The EDX spectrum of all three ZnO nanoparticles is shown in Fig. 7.
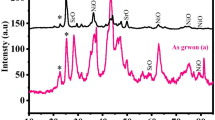
Photoluminescence spectroscopy (PL)
The PL spectra of the synthesized ZnO NPs were recorded at 315 nm excitonic wavelength and at RT to determine the crystalline defects and optical property (Fig. 8). Excitonic near band edge (NBE) emission and deep-level emission (DLE) are the two components of ZnO PL spectra in the visible region (Do et al. 2017). NBE is associated with the UV emission due to excitonic-related recombination (excitonic–excitonic collision near the bandgap of ZnO), while DLE is associated with the defect in the interstitial structure of ZnO in the visible region (VR) (Dash et al. 2019). The precursors showed almost similar bands with different intensities due to the presence of crystalline defects, different sizes, and morphology indicating the role of anions on the optical property. The PL spectra show a narrow emission peak at 364 nm and 378 nm covering the UV region of PL spectra. Sharp and narrow emission peaks were observed at 451 nm due to the transition between the defect level and the valence band (VB). The DLE was observed at 468 nm in the visible region of PL spectra is attributed to the presence of singlet ionized oxygen vacancies. Blue band emission peak at 422 nm, 438 nm, 451 nm, 468 nm, 481 nm, 510 nm has a higher intensity in comparison to the other peaks observes in the VR was observed in both acetate and nitrate precursor. This is ascribed to the various intrinsic and extrinsic defects inbuilt in the native structure of ZnO including oxygen and Zn vacancy present in the crystal lattice of ZnO (Bekkari et al. 2017). By comparing the PL results, it was observed that ZnO nanostructures synthesized using nitrate precursors have higher DLE as compared to the other used precursors, while ZnO nanostructures synthesized using sulfate precursors have the lowest DLE. Thus, anion significantly affects the intensity of DLE (Amalraj and Pius 2015; Gnanamoorthy et al. 2021; Khan et al. 2020).
Surface area measurement by BET
The average surface area (ASA) and surface texture of the synthesized samples were calculated using BET/BJH analyses. The highest surface area was obtained for the nitrate precursor which is 46.80 m2/g. The difference in the ASA and pore size is clear evidence of the role of different precursor ions, as nitrate precursors have the maximum surface area whereas acetate has the minimum due to the role of anions. The specific surface area was calculated by applying the BET equation from the BET plot. The BET equation is
where ν = adsorbed gas, νm = monolayer of the adsorbed gas, P0 and P = saturation and adsorbed pressure of adsorbate, and C = BET constant.
The graph is extrapolated between 1/W [(P/P0) – 1] and (P/P0), and it should produce a straight line in the relative pressure range 0.05–0.3. The summarized form of BET surface area and BJH pore diameter of ZnO NPs is shown in Table 5.
Cytotoxicity of ZnO NPs
Cytotoxicity of ZnO NPs in NIH 3T3 cell line murine was determined by using NRU and LDH tests. The cytotoxicity data were represented in Fig. 9a, b. ZnO NP-induced cytotoxicity on NIH 3T3 cell line based on dose-dependent and higher cytotoxicity was recorded at 50 µg/ml ZnO NPs (Fig. 9a–c). The correlation between LDH release and percentage of viable NIH 3T3 cells after 24 h exposure to ZnO NPs is presented in Fig. 9c.
There are not many in vitro studies on cytotoxic effects of synthesized ZnO NPs in NIH 3T3 cells. We determined cytotoxicity all together by duplicate test endpoints that confirmed cell viability, leakage of LDH as a biomarker of membrane injury, and morphological alteration of the cell layer. The results obtained in this work contribute to increase the knowledge on the cytotoxic potential of ZnO NPs in general but a further investigation is needed to understand the actual mechanism of underlying cytotoxic effects observed.
Conclusion
ZnO NPs were successfully synthesized by utilizing three different precursor salts of Zinc. Based on the analysis results, it was observed that out of the three precursors used for the ZnO synthesis; nitrate has been found be most suitable. The FE-SEM and TEM images revealed that synthesized nanoparticles exhibit different morphologies clearly due to the impact of precursor ions and the optical bandgap of ZnO-3 observed Ebg value at 3.23 eV was in close resemblance to bulk ZnO (3.37). The XRD data reveal the formation of pure hexagonal wurtzite crystal and the EDX analysis confirms the formation of pure ZnO nanostructures. Based on the study conducted, it was proved that by changing the precursor salts; a remarkable effect on the shape, size, optical and physical properties of the ZnO was observed even under a similar experimental condition. It was suggested that the cytotoxic activity of sonochemically synthesized ZnO NPs were highly dependent on concentration. Thus, it was concluded that the precursor salt plays an important role in the synthesis of ZnO and also affects its various physical and chemical properties and ZnO NPs induced toxicity on NIH-3T3 cells.
Change history
29 December 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11696-021-02025-8
References
Abbasi MA, Khan Y, Hussain S, Nur O, Willander M (2001) Anions effect on the low-temperature growth of ZnO nanostructures. Vacuum 86(12):1998–2001. https://doi.org/10.1016/j.vacuum.2012.05.020
Adam RE, Pozina G, Willander M, Nur O (2018) Synthesis of ZnO nanoparticles by co-precipitation method for solar driven photodegradation of Congo red dye at different pH. Photonics Nanostruct Fundam Appl 32:11–18
Agarwal S, Rai P, Gatell EN, Llobet E, Güell F, Kumar M, Awasthi K (2019) Gas sensing properties of ZnO nanostructures (flowers/rods) synthesized by hydrothermal method. Sens Actuators B Chem 292:24–31
Amalraj A, Pius A (2015) Photocatalytic degradation of monocrotophos and chlorpyrifos in aqueous solution using TiO2 under UV radiation. J Water Process Eng 7:94–101. https://doi.org/10.1016/j.jwpe.2015.06.002
Ansari SA, Ansari SG, Foaud H, Cho MH (2017) Facile and sustainable synthesis of carbon-doped ZnO nanostructures towards the superior visible light photocatalytic performance. New J Chem 41(17):9314–9320
Assi N, Mohammadi A, Sadr Manuchehri Q, Walker RB (2015) Synthesis and characterization of ZnO nanoparticle synthesized by a microwave-assisted combustion method and catalytic activity for the removal of ortho-nitrophenol. Desalin Water Treat 54(7):1939–1948
Barreto GP, Morales GR, Quintanilla ML (2013) Microwave-assisted synthesis of ZnO nanoparticles: effect of precursor reagents, temperature, irradiation time, and additives on nano-ZnO morphology development. J Mater 2(13):478681. https://doi.org/10.1155/2013/47868
Bayrami A, Parvinroo S, Habibi-Yangjeh A, Rahim Pouran S (2018) Bio-extract-mediated ZnO nanoparticles: microwave-assisted synthesis, characterization and antidiabetic activity evaluation. Artif Cells Nanomed Biotechnol 46(4):730–739
Bekkari R, Boyer D, Mahiou R, Jaber B (2017) Influence of the sol gel synthesis parameters on the photoluminescence properties of ZnO nanoparticles. Mater Sci Semicond Process 71:181–187
Benhebal H et al (2013) Photocatalytic degradation of phenol and benzoic acid using zinc oxide powders prepared by the sol–gel process. Alex Eng J 52:517–523
Ciciliati MA, Silva MF, Fernandes DM, de Melo MA, Hechenleitner AAW, Pineda EA (2015) Fe-doped ZnO nanoparticles: synthesis by a modified sol–gel method and characterization. Mater Lett 159:84–86
Dash P, Manna A, Mishra NC, Varma S (2019) Synthesis and characterization of aligned ZnO nanorods for visible light photocatalysis. Phys E 107:38–46
Devi PG, Velu AS (2016) Synthesis, structural and optical properties of pure ZnO and Co doped ZnO nanoparticles prepared by the co-precipitation method. J Theor Appl Phys 10(3):233–240
Do TAT, Giang HT, Ngan PQ, Thai GH, Dai Lam T (2017) Correlation between photoluminescence spectra with gas sensing and photocatalytic activities in hierarchical ZnO nanostructures. RSC Adv 7(16):9826–9832
Emsaki M, Hassanzadeh-Tabrizi SA, Saffar-Teluri A (2018) Microemulsion synthesis of ZnO–ZnWO4 nanoparticles for superior photodegradation of organic dyes in water. J Mater Sci Mater Electron 29(3):2384–2391
Gnanamoorthy G, Yadav VK, Ali D, Narayanan V, Katubi MS, K., & Alarifi, S. (2021) Trigger action of copper aminophosphate (X-CuAP) nanoparticles for enhanced electrochemical, photocatalyst and biological properties. Opt Mater 117:111113. https://doi.org/10.1016/j.optmat.2021.111113
Goswami M, Adhikary NC, Bhattacharjee S (2018) Effect of annealing temperatures on the structural and optical properties of zinc oxide nanoparticles prepared by chemical precipitation method. Optik 158:1006–1015
Isai KA, Shrivastava VS (2019) Photocatalytic degradation of methylene blue using ZnO and 2% Fe–ZnO semiconductor nanomaterials synthesized by sol–gel method: a comparative study. SN Appl Sci 1(10):1–11
Janaki AC, Sailatha E, Gunasekaran S (2015) Synthesis, characteristics, and antimicrobial activity of ZnO nanoparticles. Spectrochim Acta Part A Mol Biomol Spectrosc 144:17–22
Kaur M, Kalia A (2016) Role of salt precursors for the synthesis of zinc oxide nanoparticles and in imparting variable antimicrobial activity. J Appl Nat Sci 8(2):1039–1048
Khan SH, Pathak B, Fulekar MH (2016) Development of zinc oxide nanoparticle by sonochemical method and study of their physical and optical properties. In: AIP conference proceedings, vol 1724, no 1. AIP Publishing LLC, p 020108
Khan SH, Pathak B, Fulekar MH (2018) Synthesis, characterization and photocatalytic degradation of chlorpyrifos by novel Fe:ZnO nanocomposite material. Nanotechnol Environ Eng 3(1):1–14
Khan SH, Pathak B, Fulekar MH (2020) A study on the influence of metal (Fe, Bi, and Ag) doping on structural, optical, and antimicrobial activity of ZnO nanostructures. Adv Compos Hybrid Mater 3(4):551–569
Khoshhesab ZM, Sarfaraz M, Houshyar Z (2012) Influence of urea on precipitation of zinc oxide nanostructures through chemical precipitation in ammonium hydrogen carbonate solution. Synth React Inorg Met Org Nano Met Chem 42:1363–1368
Kołodziejczak-radzimska A, Jesionowski T (2014) Zinc oxide—from synthesis to application: a review. Materials. https://doi.org/10.3390/ma7042833
Kumar N, Mittal H, Reddy L, Nair P, Ngilaa JC, Parashara V (2015) Morphogenesis of ZnO nanostructures: the role of acetate (COOH) and nitrate (NO3) ligand donors from zinc salt precursors in synthesis and morphology dependent photocatalytic properties. RSC Adv 5:38801. https://doi.org/10.1039/c5ra04162g
Kumaresan N, Ramamurthi K, Babu RR, Sethuraman K, Babu SM (2017) Hydrothermally grown ZnO nanoparticles for effective photocatalytic activity. Appl Surf Sci 418:138–146
Lanje AS et al (2013) Low-temperature dielectric studies of zinc oxide (ZnO) nanoparticles prepared by precipitation method. Adv Powder Technol 24(1):331–335
Li R, Wang J, He Y, Dong F, Bian L, Li B (2019) Mechanochemical synthesis of defective molybdenum trioxide, titanium dioxide, and zinc oxide at room temperature. ACS Sustain Chem Eng 7(14):11985–11989
Madhumitha G, Elango G, Roopan SM (2016) Biotechnological aspects of ZnO nanoparticles: overview on synthesis and its applications. Appl Microbiol Biotechnol 100(2):571–581
Mao Y, Li Y, Zou Y, Shen X, Zhu L, Liao G (2019) Solvothermal synthesis and photocatalytic properties of ZnO micro/nanostructures. Ceram Int 45(2):1724–1729
Mayekar J, Dhar V, Radha S (2014) Role of salt precursor in the synthesis of zinc oxide nanoparticles. Int J Res Eng Technol 3(3):43–45
Meléndrez MF, Solis-Pomar F, Gutierrez-Lazos CD, Flores P, Jaramillo AF, Fundora A, Pérez-Tijerina E (2016) A new synthesis route of ZnO nanonails via microwave plasma-assisted chemical vapor deposition. Ceram Int42(1):1160–1168
Moazzen MAM, Borghei SM, Taleshi F (2012) Synthesis and characterization of nano-sized hexagonal and spherical nanoparticles of zinc oxide. JNS 2:295–300
Ong CB, Ng LY, Mohammad AW (2018) A review of ZnO nanoparticles as solar photocatalysts: synthesis, mechanisms, and applications. Renew Sustain Energy Rev 81:536–551
Ortega PP, Silva CC, Ramirez MA, Biasotto G, Foschini CR, Simoes AZ (2021) Multifunctional environmental applications of ZnO nanostructures synthesized by the microwave-assisted hydrothermal technique. Appl Surf Sci 542:148723
Pauzi N, Zain NM, Yusof NAA (2019) Microwave-assisted synthesis for environmentally ZnO nanoparticle synthesis. In: Proceedings of the 10th national technical seminar on underwater system technology 2018. Springer, Singapore, pp 541–546
Pillai AM, Sivasankarapillai VS, Rahdar A, Joseph J, Sadeghfar F, Rajesh K, Kyzas GZ (2020) Green synthesis and characterization of zinc oxide nanoparticles with antibacterial and antifungal activity. J Mol Struct 1211:128107
Pudukudy M, Yaakob Z (2014) Simple chemical synthesis of novel ZnO nanostructures: role of counter ions. Solid State Sci 30:78–88
Rammah YS, El-Agawany FI, Mahmoud KA, El-Mallawany R, Ilik E, Kilic G (2020) FTIR, UV–Vis–NIR spectroscopy, and gamma rays shielding competence of novel ZnO-doped vanadium borophosphate glasses. J Mater Sci Mater Electron 31(12):9099–9113
Rashid J, Barakat MA, Salah N, Habib SS (2015) ZnO-nanoparticles thin films synthesized by RF sputtering for photocatalytic degradation of 2-chlorophenol in synthetic wastewater. J Ind Eng Chem 23:134–139
Rochman NT, Akwalia PR (2017) Fabrication and characterization of zinc oxide (ZnO) nanoparticle by sol-gel method. In: Journal of physics: conference series, vol 853, no. 1. IOP Publishing, p 012041
Samanta PK, Patra SK, Ghosh A, Roy Chaudhuri P (2009) Visible emission from ZnO nanorods synthesized by a simple wet chemical method. Int J NanoSci Nanotechnol 1(1–2):81–90
Shimpi NG, Jain S, Karmakar N, Shah A, Kothari DC, Mishra S (2016) Synthesis of ZnO nanopencils using wet chemical method and its investigation as LPG sensor. Appl Surf Sci 390:17–24
Taghavi Fardood S, Ramazani A, Moradnia F, Afshari Z, Ganjkhanlu S, Yekke Zare F (2019) Green synthesis of ZnO nanoparticles via sol–gel method and investigation of its application in solvent-free synthesis of 12-aryl-tetrahydrobenzo [α] xanthene-11-one derivatives under microwave irradiation. Chem Methodol 3(6):632–642
Weintraub B, Deng Y, Wang ZL (2007) Position-controlled seedless growth of ZnO nanorod arrays on a polymer substrate via wet chemical synthesis. J Phys Chem C 111:28
Wojnarowicz J, Chudoba T, Gierlotka S, Lojkowski W (2018) Effect of microwave radiation power on the size of aggregates of ZnO NPs prepared using microwave solvothermal synthesis. Nanomaterials 8(5):343
Yang J, Wang Y, Kong J, Jia H, Wang Z (2015) Synthesis of ZnO nanosheets via electrodeposition method and their optical properties, growth mechanism. Opt Mater 46:179–185
Zare M, Namratha K, Byrappa K, Surendra DM, Yallappa S, Hungund B (2018) Surfactant assisted solvothermal synthesis of ZnO nanoparticles and study of their antimicrobial and antioxidant properties. J Mater Sci Technol 34(6):1035–1043
Zhang J et al (2010) Ionic liquid-controlled synthesis of ZnO microspheres. J Mater Chem 20:9798–9804
Zhang X, Han Y, Liu W, Pan N, Li D, Chai J (2021) A novel synthesis of hexagonal cylinder-like ZnO with an excellent performance by a surfactant-free microemulsion-hydrothermal method. J Ind Eng Chem 97:326–336
Acknowledgements
This work was funded by Researchers Supporting Project No. (RSP-2021/165), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, S.H., Yadav, V.K., Ali, D. et al. Influence of precursor ions on the structural morphological and optical properties of ZnO nanostructure and cytotoxicity on murine NIH 3T3 cells. Chem. Pap. 76, 477–489 (2022). https://doi.org/10.1007/s11696-021-01877-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-021-01877-4