Abstract
The pesticide residues have become one of the issues in the fields of environmental health and food safety. Herein, an effective method was successfully developed to rapidly determine imidacloprid in environmental water samples based on magnetic molecular imprinted polymers (MMIP) coupled with high-performance liquid chromatography. MMIP were creatively synthesized in one-pot synthesis step via the nucleation process of Fe3O4 magnetic nanoparticles and the polymerization process of molecular imprinted polymers simultaneously, using imidacloprid as template and dopamine as functional monomer. The parameters effected on the extraction efficiency, including pH value and the amount of the adsorbent, have been optimized. Under the optimal conditions, MMIP was used to extract imidacloprid in water samples with excellent recovery (88–97.3%) and precision (1.2–3.3%). Therefore, magnetic nanoparticles-based dispersion solid-phase extraction could be a good candidate for highly efficient extraction of imidacloprid without complicated operations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Imidacloprid is an efficient contact neonicotinoid insecticide. It causes a blockage of the nicotinergic neuronal pathway. Imidacloprid was the most widely used insecticide in the world to prevent, control, or eliminate pests on crops. Presently, the increase usage of imidacloprid is due to boosting the productivity highly in modern agriculture (Fujii et al. 2019; Tao et al. 2019). However, excessive and improper use of imidacloprid also could cause wide spread contamination in surrounding, even cause the food safety issues severely (Li et al. 2019). Owing to the high solubility of imidacloprid in natural water systems, imidacloprid may cause a huge danger to human health and environmental safety (Sultana et al. 2018; Si et al. 2018; Wang et al. 2015). In addition, imidacloprid may damage the DNA of human peripheral lymphocytes even at trace levels (Sultana et al. 2018). Therefore, the monitoring of pesticide residues is of great significance to satisfy the demand for human health and to protect the ecosystem.
Various analytical methods sprang up in the last decades for the determination of imidacloprid, including enzyme-linked immunosorbent assay (Yan et al. 2012), capillary electrophoresis (Sanchez-Hernandez et al. 2014), electrochemical sensor (Ben Brahim et al. 2016), high-performance liquid chromatography (HPLC) (Mandic et al. 2005), HPLC–mass spectrometry (HPLC–MS) (Haroune et al. 2015; Zhang et al. 2018), and gas chromatography (GC) (Chen et al. 2017). Instrument-based analysis methods mentioned above could not accurately determine the quantity of imidacloprid by poor purification or direct instrument detection, because the environment matrix is very complex (Liu et al. 2015). Hence, sample pretreatment technology in pesticide residue detection is particularly important.
Solid-phase extraction was first discovered in the early 1970s and developed rapidly as a high-efficiency sample pretreatment technology (Kollmann and Brennan 1979).This method is developed to supplement or replace liquid–liquid extraction, which is widely used for residue analysis of pesticides, veterinary drugs and other harmful substances in environmental protection and food security field (Reinholds et al. 2019; Wang et al. 2019a, b). Compared with the traditional method, this method has the advantages of good reproducibility, time saving, and less solvent consumption. Recently, the technology of magnetic nanoparticles-based dispersion solid-phase extraction has been developed rapidly. Magnetic solid-phase extraction (MSPE) has received much attention due to its merits of environment friendliness, rapid separation process, excellent adsorption efficiency, and easily automated assay (Capriotti et al. 2019).
Molecular imprinted polymers (MIP) have high selectivity and affinity for a specific analyte or a group of structure-related compounds by forming molecular recognition holes matching template molecules in shape, size, and functional groups (Mirzajani et al. 2017; Wang et al. 2013). Magnetic molecular imprinted polymers (MMIP) have the advantages of both magnetic responsive and the high affinity of MIP. MMIP adsorbent for imidacloprid was prepared for selective separation of imidacloprid from honey and vegetable samples (Kumar et al. 2018). A novel and simple imprinting route based on graphene was proposed to fabricate an electrochemical sensor for sensitive and selective determination of imidacloprid residue (Zhang et al. 2017). Under the condition of the additional magnetic field, analytes can be separated directly and selectively. MMIP showed very superior performance in the field of trace pollutant’s extraction and separation, and gradually became one of the most promising fields in sample pretreatment.
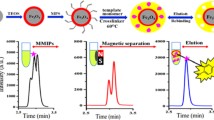
However, the preparation of MMIP normally contains several reaction steps, including the magnetic nanomaterial nucleation process and molecular imprinting polymerization process. The step-by-step preparation method of MMIP material undoubtedly brings the problems of the complexity of preparation, and of the uncontrollability of material performance. To this end, a novel MMIP was synthesized based on dopamine as functional monomer, and imidacloprid as template molecules in one reaction step in the presence of ferrous salt in an alkaline environment (Fig. 1). The obtained MMIP can adsorb imidacloprid with high efficiency and can be separated in the additional magnetic field easily.
Materials and methods
Reagents and apparatus
All chemicals were obtained from a commercial seller and used of analytical grade or better without further purification. Ferrous sulfateheptahydrate (FeSO4·7H2O), hydrazine hydrate (N2H4·H2O), dopamine, methanol, ethanol, dichloromethane, and tri-hydroxymethyl amino methane hydrochloride (Tris–HCl) were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Imidacloprid, triazophos, triazolone, methomyl, carbaryl, and chlorpyrifos were obtained from the department of agriculture environmental protection monitoring, China. The organic solvents used for chromatographic analysis were HPLC-grade water and methanol (Fisher Scientific Inc.).
Imidacloprid was diluted in pure methanol to obtain a stock solution at the concentration of 1000 µg/mL. The stock solution of imidaclopridwere stored in refrigerator at 4 °C. The working solutions were prepared daily by diluting with methanol: water (1:1). The stock was used to prepare spiking solutions for the method validation and for the recovery study, as well as for the calibration standards for analysis by HPLC–UV.
The morphology of the polymers structures was determined by a FEI Emission Scanning Electron Microscope (SEM, SU8200, Hitachi, Japan) with an acceleration voltage of 10 kV. Infrared absorption spectrum tests were conducted on a fourier-transform infrared spectroscopy (FTIR, Nicolet iS10 FTIR Spectrometer, Thermo Scientific, USA). HPLC analysis was performed on a Rigol L-3000 system. Separation of imidacloprid was achieved on a C-18 column (5 µm particle size, 250 × 4.6 mm) and the temperature was set at 40 ℃. A mixture of 70% solvent A (methanol of chromatographic purity) and 30% solvent B (ultrapure water) was used as the mobile phase. The flow rate of the mobile phase was set at 1 mL/min. The sample injection volume was 10 μL. The retention time of imidacloprid chromatography peak was at 2.84 min under the chromatography detection condition mentioned above.
Synthesis of magnetic molecular imprinted polymers
A novel MMIP was synthesized based on dopamine as functional monomer. The significant merit of the MMIP preparation methodology is processing the nucleation and polymerization procedures into one step into one pot. The synthesis processes of MMIP were declared briefly as follows.
0.2 mmol of FeSO4·7H2O was dissolved in 3 mL of ultra-pure water. The solution was transferred into a teflon beaker. 0.2 mL hydrazine hydrate was added. Then, 0.1 mmol of imidacloprid and 0.25 mmol of Tris–HCl was dissolved in 25 mL of ultra-pure water. Adjusted the sollution to pH = 8.5 before transferred the mixture into the teflon beaker contained ferrous salt. Lastly, 0.3 mmol of dopamine was added into the reactor. Heated the reactor gradually to 150 ℃ and maintained heating state for 5 h.
The reactor was then cooled to ambient temperature in 2 h. The product was separated by a magnet, and the supernatant was decanted. The products were washed with ultra-pure water and methanol for several times. The MMIP was obtained after the freeze drying. The MMIP material was kept into the referigrator at − 20 ℃ before usage. Because the stability and reusability of magnetic material was excellent under the storage conditions of drying and low temperature.
Extraction procedure
50 mg MMIP adsorbent was incubated into the 25 mL of imidacloprid solution at the concentration of 800 μg/L (pH = 6.5). After that, the adsorbent in the suspension was separated by a magnet and dried slightly by nitrogen flow. Eluent of the imidacloprid from the MMIP was accomplished by washing the adsorbent with 500 μL of organic solvents by fierce vortex for 1 min in 3 times. Finally, 100 μL of the eluent was drawn out and directly injected into the HPLC for analysis. The procedure of the extraction of imidacloprid by MMIP is illustrated in Fig. 2.
Results and discussion
Characterization of MMIP
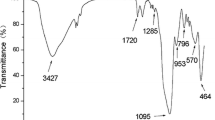
Magnetic non-molecular imprinted polymers (MNIP) and MMIP, were characterized by FTIR and SEM. The FTIR spectra of pure Fe3O4 (a), MNIP (b) and MMIP (c) shown the distinct differences each other (Fig. 3A). The band at 627 cm−1 of curve b and c, may be attributed to the Fe–O–Fe vibration of pure magnetite Fe3O4. The bands at 810 cm−1 were possibly related to C–C groups with skeletal vibration, and the bands at 1493 cm−1 were possibly related to C=C groups with skeletal vibration. The bands at 2463 cm−1 could indicate the unsaturated C bonds on the benzene ring stretching. Compared with the FTIR transmission bands of Fe3O4, MNIP and MMIP, it was obviously seen that dopamine was well-combined with the Fe3O4 microspheres forming the composites in MMIP.
The morphology of the obtained Fe3O4, MNIP and MMIP composites were identified by SEM (Fig. 3B–D). The SEM image of pure Fe3O4 particles in Fig. 3B. The SEM image of MNIP in Fig. 3C shown a high crystallinity of Fe3O4, which is in a large crystal size at about 20 μm with the structure of trigonal prism. There was more poly-dopamine enriched on the surface of MMIP than that of MNIP (Fig. 3D). Meanwhile, there are cavities in the poly-dopamine surface so that MMIP can adsorb imidacloprid specifically.
Adsorption efficiency of MMIP
To evaluate the adsorption efficiency of the different magnetic adsorbents toward the target analysts, the adsorption ability of Fe3O4, MNIP and MMIP to imidacloprid were compared, respectively (Fig. 4a). The MMIP exhibited a higher affinity to imidacloprid because of the more imidacloprid adsorbed onto the MMIP.
The cavities formed on the surface of MMIP can adsorb imidacloprid specifically because of the imitative structure of imidacloprid stemmed from the polymerization of dopamine. Meanwhile, the common properties of magnetic materials make the enrichment of imidacloprid easy from the water sample with a magnet (Fig. 4b).
The effect of the amount of MMIP on the adsorption of imidacloprid was optimized. For this, amounts of 1, 2, 4, 8, and 16 mg of MMIP were added into a standard solution of 10 mL containing 5 µg imidacloprid. Figure 5a shows that the adsorption quantity increased rapidly when the adsorption dosage increased from 1 to 4 mg. The adsorption quantity was increased slowly when absorbent dosage over 5 mg and gradually reached equilibrium. This result can be explained by the sufficient exposure of the adsorbent’s adsorption sites in the solution when adsorbent dosage was low. Thus, the adsorption could reach saturation in a short time, and the adsorbent got a higher adsorption capacity. However, with the increasing adsorbent dosage, the number of unoccupied sorption sites on the adsorbent surface was excessive, but the fixed amount of the imidacloprid molecules in the solution (Wang et al. 2014).
Effect of adsorbent dosage on imidacloprid extraction by MMIP (a); the detectable chromatographic peak area of imidacloprid in eluent after extraction treatment by MMIP (b); effect of pH on imidacloprid adsorption using the MMIP as absorbent (c); the recognizing selectivity of MMIP to Imidacloprid against the analogs (d)
Figure 5b shows peak areas abtained from chromatograms of imidacloprid solution treated according to the procedures desrcibred in “Extraction procedure” section. The value of the correlation coefficient (R2) of the fitting line was 0.985, which indicate that the proposed method could be used to extract trace pesticide satisfied.
The effect of solution pH on the adsorption of MMIP to imidacloprid was investigated. The pH value of a solution can control an electrostatic interaction between the adsorbate and the adsorbent surface by inducing the charge distribution (Limchoowong et al. 2017). However, there were no significant differences in the adsorption efficiency in a pH range of 6.0–8.0, whereas there was just a little increase on adsorption quantity when pH is 6.5 (Fig. 5c).
The recognizing selectivity of MMIP was investigated by recoveries rate studies in distilled water between the target and analogs of the target, as shown in Fig. 5d. From the results, we found that the recoveries of the pesticides triazophos, triazolone, methomyl, carbaryl, and chlorpyrifos were lower than that of imidacloprid. It is indicated that the prepared MMIP extracts imidacloprid with a high affinity.
The mechanism of polymerization is shown in Fig. 6. The catechol groups of dopamine were firstly oxidized to the quinones thus formed dopaminequinone. Then dopaminequinone was transformed into leukodopaminechrome through nucleophilic reaction process. Leukodopaminechrome transferred into indolequinone from the further oxidation reaction. Polydopamine was formed under the inter molecules cross-linking reaction of indolequinone (Pan et al. 2009).
The high adsorption capacity and extraction efficiency of MMIP for imidacloprid could result in the miscellaneous adsorption mechanism synergistically. In the case of imidacloprid as template, polymerization of dopamine performed molecular imprinting program, so polymer has specific adsorption for imidacloprid. Meanwhile, there were plenty of negatively charged surfaces of the magnetic adsorbent due to the existence of numerous phenolic groups of polydopamine. These negatively charged surfaces could provide adsorption sites for electrostatic interaction or p–p stacking interaction with imidacloprid.
Analytical performance
To evaluate the applicability and accuracy of the propose analysis method based on imidacloprid extraction by MMIP and determination with HPLC, the adsorption and extraction of imidaclopridat trace levels were performed with the addition/recovery tests of imidacloprid in water samples. Table 1 shows that the determined imidacloprid concentrations were in a good agreement with the spiked concentrations values with the recoveries ranged from 88 to 97.4%, and the relative standard deviation (RSD) ranged from 1.2 to 3.3%. These results demonstrated the reliability of the method for the accurate determination of imidacloprid in real water samples.
A comparative study was also evaluated between our proposed method and other reported methods for analysing imidacloprid, and the results are presented in Table 2. As can be seen, the LOD of the proposed method of this work (1 µg/L) for the imidacloprid were better than that of ion mobility spectrometry and comparable with the HPLC–MS in terms of LOD (Aria et al. 2019). The imidacloprid chromatogram is shown in Fig. 7. The recovery of imidacloprid extracted by proposed adsorbent was comparable with the recoveries by MIP made from monomer of methacrylic acid, acrylic acid, or p-vinylbenzoic acid (Kumar et al. 2018; Zhang et al. 2017; Jovanov et al. 2014). Moreover, the extraction procedure using MMIP will take place in a short time to adsorb the target. In addition, solid-phase was separated easily. Therefore, the proposed method is obviously featured with good effective, fast adsorption dynamics and easy-to-operate resulting from magnetism in the preconcentration and determination of imidacloprid.
Conclusions
The poly-dopamine functionalized magnetic particles were facilely prepared in one pot and used as magnetic dispersive solid-phase extraction adsorbents to simplify the isolation of imidacloprid from the water sample. The synthesized sorbent was characterized by SEM and FTIR. The prepared adsorbent MMIP exhibited good adsorption performance and selectivity for the imidacloprid. The adsorption saturation time of MMIP to imidacloprid was less than 30 min. The highly monodisperse and magnetically separable sorbent was applied for the isolation of imidacloprid with high efficiency due to the molecular imprinted polymer cavities on the surface of megnetic core. The adsorbed imidacloprid by MMIP was eluted using methonal followed by HPLC–UV analysis. The suggested method offered advantages such as simplicity, good enhancement factor in a short analysis time. As an outcome, the proposed method can be recommended as a proper alternative for the preconcentration imidacloprid in environmental matrix.
References
Aria MM, Sorribes-Soriano A, Jafari MT, Nourbakhsh F, Esteve-Turrillas FA, Armenta S, Herrero-Martinez JM, de la Guardia M (2019) Uptake and translocation monitoring of imidacloprid to chili and tomato plants by molecularly imprinting extraction—ion mobility spectrometry. Microchem J 144:195–202. https://doi.org/10.1016/j.microc.2018.09.007
Ben Brahim M, Ammar HB, Abdelhedi R, Samet Y (2016) Electrochemical behavior and analytical detection of Imidacloprid insecticide on a BDD electrode using square-wave voltammetric method. Chin Chem Lett 27(5):666–672. https://doi.org/10.1016/j.cclet.2015.12.032
Capriotti AL, Cavaliere C, La Barbera G, Montone CM, Piovesana S, Lagana A (2019) Recent applications of magnetic solid-phase extraction for sample preparation. Chromatographia 82(8):1251–1274. https://doi.org/10.1007/s10337-019-03721-0
Chen J, Zhang WT, Shu Y, Ma XH, Cao XY (2017) Detection of organophosphorus pesticide residues in leaf lettuce and cucumber through molecularly imprinted solid-phase extraction coupled to gas chromatography. Food Anal Methods 10(10):3452–3461. https://doi.org/10.1007/s12161-017-0875-5
Fujii T, Sanada-Morimura S, Oe T, Ide M, Thanh DV, Chien HV, Tuong PV, Loc PM, Cuong LQ, Liu ZW, Zhu ZR, Li JH, Wu G, Huang SH, Estoy GF, Sonoda S, Matsumura M (2019) Long-term field insecticide susceptibility data and laboratory experiments reveal evidence for cross resistance to other neonicotinoids in the imidacloprid-resistant brown planthopper Nilaparvata lugens. Pest Manag Sci. https://doi.org/10.1002/ps.5533
Haroune L, Cassoulet R, Lafontaine MP, Belisle M, Garant D, Pelletier F, Cabana H, Bellenger JP (2015) Liquid chromatography–tandem mass spectrometry determination for multiclass pesticides from insect samples by microwave-assisted solvent extraction followed by a salt-out effect and micro-dispersion purification. Anal Chim Acta 891:160–170. https://doi.org/10.1016/j.aca.2015.07.031
Jovanov P, Guzsvany V, Franko M, Lazic S, Sakac M, Milovanovic I, Nedeljkovic N (2014) Development of multiresidue DLLME and QuEChERS based LC–MS/MS method for determination of selected neonicotinoid insecticides in honey liqueur. Food Res Int 55:11–19. https://doi.org/10.1016/j.foodres.2013.10.031
Kollmann G, Brennan J (1979) Radioimmunoassay of carcinoembryonic antigen (CEA) without extraction and dialysis using solid-phase antibody. J Immunol Methods 29(4):387–394. https://doi.org/10.1016/0022-1759(79)90009-7
Kumar N, Narayanan N, Gupta S (2018) Application of magnetic molecularly imprinted polymers for extraction of imidacloprid from eggplant and honey. Food Chem 255:81–88. https://doi.org/10.1016/j.foodchem.2018.02.061
Leiva JA, Nkedi-Kizza P, Borejsza-Wysocki WS, Bauder VS, Morgan KT (2016) Imidacloprid extraction from citrus leaves and analysis by liquid chromatography–mass spectrometry (HPLC–MS/MS). Bull Environ Contam Toxicol 96(5):671–677. https://doi.org/10.1007/s00128-016-1769-8
Li HX, Jin R, Kong DS, Zhao X, Liu FM, Yan X, Lin YH, Lu GY (2019) Switchable fluorescence immunoassay using gold nanoclusters anchored cobalt oxyhydroxide composite for sensitive detection of imidacloprid. Sens Actuators B Chem 283:207–214. https://doi.org/10.1016/j.snb.2018.12.026
Limchoowong N, Sricharoen P, Areerob Y, Nuengmatcha P, Sripakdee T, Techawongtien S, Chanthai S (2017) Preconcentration and trace determination of copper(II) in Thai food recipes using Fe3O4@Chi-GQDs nanocomposites as a new magnetic adsorbent. Food Chem 230:388–397. https://doi.org/10.1016/j.foodchem.2017.03.066
Liu ZP, Liu JF, Wang K, Li WH, Shelver WL, Li QX, Li J, Xu T (2015) Selection of phage-displayed peptides for the detection of imidacloprid in water and soil. Anal Biochem 485:28–33. https://doi.org/10.1016/j.ab.2015.05.014
Mandic AI, Lazic SD, Okresz SN, Gaal FF (2005) Determination of the insecticide imidacloprid in potato (Solanum. tuberosum L.) and onion (Allium cepa) by high-performance liquid chromatography with diode-array detection. J Anal Chem 60(12):1134–1138. https://doi.org/10.1007/s10809-005-0256-x
Mirzajani R, Ramezani Z, Kardani F (2017) Selective determination of thidiazuron herbicide in fruit and vegetable samples using molecularly imprinted polymer fiber solid phase microextraction with ion mobility spectrometry detection (MIPF-SPME-IMS). Microchem J 130:93–101. https://doi.org/10.1016/j.microc.2016.08.009
Pan F, Jia H, Qiao S, Jiang Z, Wang J, Wang B, Zhong Y (2009) Bioinspired fabrication of high performance composite membranes with ultrathin defect-free skin layer. J Membr Sci 341:279–285. https://doi.org/10.1016/j.memsci.2009.06.020
Reinholds I, Jansons M, Pugajeva I, Bartkevics V (2019) Recent applications of carbonaceous nanosorbents in solid phase extraction for the determination of pesticides in food samples. Crit Rev Anal Chem 49(5):439–458. https://doi.org/10.1080/10408347.2018.1542586
Sanchez-Hernandez L, Hernandez-Dominguez D, Bernal J, Neususs C, Martin MT, Bernal JL (2014) Capillary electrophoresis–mass spectrometry as a new approach to analyze neonicotinoid insecticides. J Chromatogr A 1359:317–324. https://doi.org/10.1016/j.chroma.2014.07.028
Si FF, Zou RB, Jiao SS, Qiao XS, Guo YR, Zhu GNA (2018) Inner filter effect-based homogeneous immunoassay for rapid detection of imidacloprid residue in environmental and food samples. Ecotoxicol Environ Saf 148:862–868. https://doi.org/10.1016/j.ecoenv.2017.11.062
Sultana T, Murray C, Kleywegt S, Metcalfe CD (2018) Neonicotinoid pesticides in drinking water in agricultural regions of southern Ontario, Canada. Chemosphere 202:506–513. https://doi.org/10.1016/j.chemosphere.2018.02.108
Tang JS, Zhang M, Cheng GH, Lu YT (2009) Development and application of molecularly imprinted polymer as solid phase extraction of imidacloprid in environmental samples. J Liq Chromatogr Relat Technol 32(1):59–71. https://doi.org/10.1080/10826070802548622
Tao Y, Phung D, Dong FS, Xu J, Liu XG, Wu XH, Liu QY, He M, Pan XL, Li RN, Zheng YQ (2019) Urinary monitoring of neonicotinoid imidacloprid exposure to pesticide applicators. Sci Total Environ 669:721–728. https://doi.org/10.1016/j.scitotenv.2019.03.040
Wang X, Mu ZD, Liu R, Pu YP, Yin LH (2013) Molecular imprinted photonic crystal hydrogels for the rapid and label-free detection of imidacloprid. Food Chem 141(4):3947–3953. https://doi.org/10.1016/j.foodchem.2013.06.024
Wang Y, Xie J, Wu YC, Hu XY (2014) A magnetic metal-organic framework as a new sorbent for solid-phase extraction of copper(II), and its determination by electrothermal AAS. Microchim Acta 181(9–10):949–956. https://doi.org/10.1007/s00604-014-1183-z
Wang L, Liu TZ, Liu F, Zhang JJ, Wu YH, Sun HW (2015) Occurrence and profile characteristics of the pesticide imidacloprid, preservative parabens, and their metabolites in human urine from rural and urban China. Environ Sci Technol 49(24):14633–14640. https://doi.org/10.1021/acs.est.5b04037
Wang X, Jia RB, Song Y, Wang MQ, Zhao QH, Sun SH (2019a) Determination of pesticides and their degradation products in water samples by solid-phase extraction coupled with liquid chromatography–mass spectrometry. Microchem J 149:104013. https://doi.org/10.1016/j.microc.2019.104013
Wang Z, Wang XY, Tian H, Wei QH, Liu BS, Bao GM, Liao ML, Peng JL, Huang XQ, Wang LQ (2019b) High through-put determination of 28 veterinary antibiotic residues in swine wastewater by one-step dispersive solid phase extraction sample cleanup coupled with ultra-performance liquid chromatography–tandem mass spectrometry. Chemosphere 230:337–346. https://doi.org/10.1016/j.chemosphere.2019.05.047
Yan X, Shi HY, Wang MH (2012) Development of an enzyme-linked immunosorbent assay for the simultaneous determination of parathion and imidacloprid. Anal Methods 4(12):4053–4057. https://doi.org/10.1039/c2ay25760b
Zhang M, Zhao HT, Xie TJ, Yang X, Dong AJ, Zhang H, Wang J, Wang ZY (2017) Molecularly imprinted polymer on graphene surface for selective and sensitive electrochemical sensing imidacloprid. Sens Actuators B Chem 252:991–1002. https://doi.org/10.1016/j.snb.2017.04.159
Zhang Q, Wang XM, Li Z, Jin HB, Lu ZB, Yu C, Huang YF, Zhao MR (2018) Simultaneous determination of nine neonicotinoids in human urine using isotope-dilution ultra-performance liquid chromatography tandem mass spectrometry. Environ Pollut 240:647–652. https://doi.org/10.1016/j.envpol.2018.04.144
Acknowledgements
This work was supported by the Collaborative Innovation Project of Anhui Provincial Department of Education (Grant Number GXXT-2019-034), the Science Research Project of Anhui Provincial Education Department (Grant Number KJ2019A0758 and KJ2019JD08).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cui, Z., Xiang, L. & Tang, J. Facile one-pot synthesis of magnetic molecular imprinting polymers as a novel adsorbent for the enrichment of imidacloprid based on a magnetic dispersive micro-solid-phase extraction in water samples. Chem. Pap. 75, 3787–3795 (2021). https://doi.org/10.1007/s11696-021-01622-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-021-01622-x