Abstract
Carbon-supported ternary Pt70Sn10In20, Pt70Sn15In15 and Pt70Sn20In10/C electrocatalysts were synthesized by co-impregnation method for the electrooxidation of ethanol in membraneless fuel cells. The resulting catalysts were characterized by X-ray diffraction (XRD), transmission electron microscopy (TEM) and energy-dispersive X-ray spectroscopy (EDX) analyses compared to Pt50Sn50, Pt50In50 and Pt100/C catalysts produced in the same way. The results revealed that all catalysts showed a similar particle morphology and the lattice constant of Pt–Sn–In was between Pt–Sn and Pt–In. Cyclic voltammetry (CV) and chronoamperometry (CA) showed that the Pt–Sn–In catalyst has improved electrocatalytic activity for ethanol oxidation compared to Pt–Sn and Pt–In catalysts, which is in agreement with the results of the single cell test. The improvement in activity is due to a greatly increased electrochemically active surface and the promoting function of indium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In order to meet the ever increasing demand for electricity, it is necessary to recognize the significant change in fuel consumption without harming the environment. Therefore, fuel cells, especially membraneless ethanol fuel cells (MLEFC), are considered to be a source of clean energy that converts chemical energy directly into electricity through oxido-reduction reactions (Choban et al. 2004; Gowdhamamoorthi et al. 2014). MLEFCs have the most important properties associated with the use of portable electronic devices and have the ability to change batteries due to the provision of adequate current density, greater durability and stability (Ferrigno et al. 2002; Arun et al. 2015). Due to their easy handling and high availability, alcohols have so far been an important source of energy in fuel cells (Ramos et al. 2012). In alcohols, ethanol is often used as a renewable fuel in MLEFCs due to its high energy density (26.8 MJ kg–1), its low freezing point (114.1 °C) and its simple electro-oxidation (Ponmani et al. 2014). Moreover, the low toxicity, easy storage and easy transportation from one place to another make this colorless liquid an absolute fuel (Zhou et al. 2004). Although ethanol has the above advantages, its growth in MLEFC is hampered by the difficulty of breaking the C−C bond at low temperatures (Ponmani et al. 2016). So far, precious metal catalysts, particularly carbon-supported Pt catalysts, have been identified as the leading candidate for the electrooxidation of ethanol in MLEFCs, primarily because of their advantages such as specific functionality, greater stability and longer life (Jayasayee et al. 2012). However, the high cost, the lack of Pt and the toxicity of intermediates such as COads on Pt limit their practical application (Antolini 2009). Therefore, considerable efforts have been made to develop new low-cost Pt catalysts for ethanol oxidation in order to achieve effective catalytic performance in such systems (Bonesi et al. 2010). To solve this problem, new catalysts can be made by alloying inexpensive and readily available metals with significantly lower Pt mass (Cunha et al. 2011). For example, Pt–Sn alloy catalysts have unique CO-poisoning tolerance properties compared to other reported bimetallic Pt-M (M = Ru, Rh, Mo, Pd, Fe, Co, Ni) catalysts (Wang et al. 2006; Drzymała et al. 2020; Zeng et al. 2020). However, alloying new metals with Pt–Sn strongly influences the C−C bond cleavage and helps to remove intermediates from catalyst surfaces at low temperatures (Vigier et al. 2004; Lim et al. 2009; Wang et al. 2010). The incorporation of a third metal in Pt–Sn is said to change the electronic-structure and the d − band center, which contributes to the increase in catalytic activity (Beyhan et al. 2013b).
Recent investigations have shown that indium is a flexible metal with extraordinary physical properties that is commonly used to form alloys (Datta et al. 2020; Lou et al. 2020). Even small amounts of indium can cause an enormous difference in a compound and are often referred to as the "metal vitamin" (Kim et al. 2017). Moreover, some studies have been conducted to clarify the effect of indium addition on the activity of various binary alloy catalysts (Bazargan et al. 2017; Kim et al. 2020). The synergistic effect between indium and platinum has also been demonstrated by the decrease in the adsorption capacity of platinum in the presence of indium (Chu et al. 2012). Subsequently, in reforming and cracking the petroleum fraction, indium has been found to be impressive in cleaving C−C bonds (Frost and Hartley 2005). Kwon et al. also conducted adsorption studies on the effect of platinum changes on glycerol electrooxidation (Kwon et al. 2014). Passos et al., 1998 showed that indium has modified the atoms of Pt in a more homogeneous way than Sn, when indium is used as a promoter (Passos, F. B., Aranda, D. A., & Schmal 1998). Similarly, Knight et al. discovered that indium alloys can be formed with other metals by confirming their latter strength and durability (Knight and Rhys 1959). The semiconductor industry has repeatedly used In–Sn oxides for glass screens in smartphones, flat screens, LEDs, solar cells, optoelectronics, high-speed circuits, nanocomposites, etc., due to their electrical conductivity and transparency (Al-Kuhaili 2020; Datta et al. 2020; Jaiswal et al. 2020; Kim et al. 2020). Despite these interesting characteristics, research on the synthesis of trimetallic catalysts linked to indium is still limited, and the role of the third metal in terms of efficiency, compared to their bimetallic counterparts, remains unclear. In this study, carbon-supported monometallic (Pt/C), bimetallic (Pt–Sn/C and Pt–In/C) and trimetallic (Pt–Sn–In/C) electrocatalysts were synthesized by co-impregnation method to assess their electrocatalytic performance at MLEFC for the ethanol electrooxidation.
Experimental
Materials preparation
The metal precursors chloroplatinic acid hexahydrate (H2PtCl6.6H2O, from Aldrich), tin (II) chloride dehydrate (SnCl2.2H2O, from Alfa Aesar) and indium (III) chloride (InCl3.4H2O, from Alfa Aesar) were used to manufacture the electrocatalysts. Vulcan carbon (VC) XC-72 R (from Cabot Corp.) was applied as a catalyst support. Ethylene glycol (EG) (C2H6O2, from Merck) was used as a reaction solvent for reduction reactions. D521 Nafion™ (5 wt%—from DuPont) was utilized as a binder for the preparation of catalytic ink-gels. Ethanol (EtOH) (C2H5OH, from Merck), sodium percarbonate (Na2CO3.1.5H2O2, from Merck) and sulfuric acid (H2SO4, from Merck) in deionized (DI) water were used as fuels, oxidants and electrolytes in all electrooxidation reactions. Graphite plates (made of E-TEK) with a thickness of 1 mm were used in the form of catalyst adsorbents and as current collectors for the manufacture of the electrodes. Pt/C (40% by weight) was used as the cathodic catalyst. Only materials and chemicals of high analytical quality were used in this study.
Synthesis of Pt–Sn–In/C
The carbon-based electrocatalysts like Pt–Sn/C, Pt–In/C and Pt–Sn–In/C were manufactured by co-impregnation processes with various atomic ratios. Throughout the process, the metal powders along with VC XC-72 R were dispersed in a mixture containing an aqueous solution of EG at a ratio of 75:25 (v/v). They were thoroughly sonicated and refluxed at normal temperature for two hours, which were then heated to 120° C to reduce the metal. In its last phase, the residue was filtered, washed in DI water and dried. The metal loading of the catalyst was 40% by weight, the rest 60% by weight of carbon.
Preparation of working electrodes
The working electrodes were made with a catalytic ink-gel containing a mixture of 50 mg catalyst powder and 1 ml aqueous Nafion solution. 3 µl of the catalytic ink-gel was applied to a fresh glassy carbon electrode and dried at room temperature, which resulted in a total metal loading of 0.28 mg cm−2. Before each test, the working electrodes were prepared and used in the same way.
Physical characterization
The X-ray diffractograms were transcribed from a Rigaku RU-200 B diffractometer in a radiation source of Cu-Kα1 (λKα1 = 1.5406 Ao) in order to estimate the crystallite size and structure of the electrocatalysts. The 2-theta angle in the range from 20 to 90° was observed with a sweep frequency of five degree per minute. The tube current and the tube voltage were kept at 40 mA and 40 kV, respectively. The lattice constant and the crystallite dimensions were calculated from the diffraction peak of the 2 2 0 Pt plane. The shape and size of the electrocatalysts were determined with a CM 120 Philips transmission electron microscope (TEM), and the histogram of each catalyst was achieved from the images of the TEM by examining over 200 particles. The TEM particle size is confirmed with the crystallite size of XRD calculated according to the Scherrer equation (Eq.1) (Beyhan et al. 2013b).
where d is the mean crystallite size, 0.9 typically denotes the form factor constant of a crystallite and λKα1 the applicable X-ray wavelength. θmax is an angle of the peak in the maximum position and the peak width is ß2θ. The parameters of the crystal lattice were calculated according to Eq.2 (Monshi et al. 2012):
In Eq. 2, a stands for the lattice parameter; refer Eq. 1 for definitions of the remaining variables. The elemental compositions were analyzed with energy-dispersive X-ray spectroscopy (EDX).
Electrochemical characterization
Under normal temperature conditions, a standard cell with three electrodes was used for electrochemical analysis such as cyclic voltammetry (CV) and chronoamperometry (CA). In the CV experiment, an electro-analyzer (CHI-6005E SN1077, CH Instruments) was used to record the voltammograms with 0.5 M H2SO4 in the presence and absence of fuel (1 M ethanol). A silver-silver chloride (Ag/AgCl) electrode and a platinum electrode were used as the reference electrode and counter electrode. For the working electrode, a glassy carbon electrode coated with a catalyst with a diameter of 3.0 mm (electrode surface 0.071 cm2) was used. The scan rate of 50 mV s−1 was fixed in the range of 0.05 and 1.2 V for the entire CV study. The stability of the catalysts was determined for 30 min using chronoamperometry at 0.4 V.
Single-cell test
In this study, we developed a membraneless ethanol fuel cell in which fuel and oxidants can flow side by side without a physical barrier in between. Aqueous solutions such as ethanol, sodium percarbonate and sulfuric acid were used as fuel, oxidizing agent and electrolyte. Sodium percarbonate is an inexpensive, non-toxic and environmentally friendly substance that is primarily used as an active oxygen source in oxidizing agents (Arun et al. 2014). As shown in Eq. 3, sodium percarbonate is in the form of dimeric crystalline peroxo salts while providing hydrogen peroxide in aqueous solution (Cotton, F. A.; Wilkinson 1972; Karunakaran and Kamalam 2002).
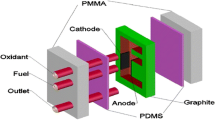
Two streams such as fuel and oxidant are allowed to flow side by side in a microfluidic channel containing an anode and a cathode in MLEFC. The cathode and anode are mounted on the side walls of the channel opposite each other (Fig. 1).
In the individual MLEFC test, different compositions of the catalysts were applied to the surface of graphite plates using the following method: First, the catalytic ink-gels were produced with constant stirring using 100 μL Nafion, 1 ml isopropanol and 15 mg catalyst powder. The gel was then applied to the graphite surface (in an area of 3 cm2) and dehydrated at 110 ºC for 25 min to achieve a total metallic load of approximately 2 mg cm−2. The electrocatalysts Pt100/C, Pt50In50/C, Pt50Sn50/C, Pt70Sn20In10/C, Pt70Sn15In15/C and Pt70Sn10In20/C were tested as an anode in the single cell. Pt100/C, which contains a metal load of about 2 mg cm−2, was used as the cathode for all experiments. In MLEFC, anolyte and catholyte can flow side by side, and the flow rate of each stream at room temperature is 0.3 mL min−1. The cell voltage measurements were recorded using an electro-analyzer and checked with a multimeter.
Results and discussion
Physical characterization
XRD analysis
The diffractograms of Pt50Sn50/C, Pt70Sn20In10/C, Pt70Sn15In15/C, Pt70Sn10In20/C together with Pt50In50/C and Pt/C catalysts, which reflect the fcc structure of platinum, are shown in Fig. 2. In comparison with the Pt peak, a significant peak shift to the lower value of 2θ is observed for the catalysts containing Sn. In addition, the lattice constants of Pt70Sn20In10/C, Pt70Sn15In15/C and Pt70Sn10In20/C are 0.3958, 0.3952 and 0.3949, respectively, which are between Pt50Sn50/C (0.3980) and Pt50In50/C (0.3945). This demonstrates that the integration of Sn in Pt enlarges the parameters of the fcc lattice according to the broad atomic radius of Sn (García-Rodríguez et al. 2009; Beyhan et al. 2013b), while a small shift of the peak toward a higher angle is observed for Pt50In50/C due to the partial substitution of Pt by In atom (Mingyuan et al. 2009; Godoi et al. 2010). The calculated values of the lattice and the average size of the crystallite of the electrocatalysts, based on the plane (2 2 0), are summarized in Table 1. As the results in Table 1 show, the Sn-containing Pt alloy catalysts have significantly increased Pt lattice parameters, which implies the formation of the Pt3sn and Pt–Sn phases. In addition to the Pt and In peaks, the Pt–Sn catalyst combination revealed new peaks at approximately 41°, 43° and 63° that belong to the Pt–Sn (1 0 2), (1 1 0) and (2 0 2) planes. The above observation is consistent with the incorporation of Sn into the Pt lattice and shows that Pt3Sn and Pt–Sn are the only crystalline phases existing in these catalysts.
TEM analysis
Particle size, morphology and size distribution have a major impact on the performance of electrocatalysts. Against this background, a TEM analysis was carried out, and the TEM images and the associated histograms are displayed in Fig. 3. As reflected in the pictures, each electrocatalyst exhibits uniform distribution on carbon supports of size 5 nm and below. The particles obtained in this analysis were observed to be spherical and slightly aggregated. Aggregation may be due to the rapid reduction in the co-impregnation process (Rousseau et al. 2006). The average particle size of the individual electrocatalysts was determined by examining over 300 particles in TEM images. Compared to the bimetallic catalysts, the mean particle sizes of the trimetallic catalysts were smaller because their surface-to-volume ratio is higher, as shown in Table 1, which is quite similar in both TEM and XRD cases. The slight difference between the XRD and TEM data for Pt50In50/C catalyst can, however, be due to the presence of microstructures that are not very precisely defined (Table 1).
EDX analysis
The elementary distributions of the individual Pt, Sn and In catalysts were examined with the aid of energy-dispersive X-ray spectroscopy. Figure 4 shows the EDX analysis of the Pt100/C, Pt50In50/C, Pt50Sn50/C and Pt70Sn10In20/C electrocatalysts, and their results are presented in Table 1. The EDX results for electrocatalysts are close to the target values, indicating that the carbon-supported nanoparticles were dispersed without evident losses.
Electrochemical characterization
Cyclic voltammetry (CV)
Figure 5 displays the voltammograms of the electrocatalysts, namely Pt100/C, Pt50In50/C, Pt50Sn50/C, Pt70Sn20In10/C, Pt70Sn15In15/C and Pt70Sn10In20/C in 0.5 M H2SO4 in the absence of ethanol. The prepared samples were applied to a glassy carbon electrode using the drop casting technique. As shown in Fig. 5, the Pt/C has the properties of polycrystalline platinum electrodes with regard to the hydrogen adsorption/desorption (Hads/des) region (0–0.4 V) and the double layer region (0.4–0.8 V vs. Ag/AgCl) (Tayal et al. 2012). However, due to the influence of the catalysts on their surface composition, the voltammograms of all Pt alloy catalysts examined do not have a precisely defined Hads/des charges, as is observed with Pt alloys (Lamy et al. 2004; Purgato et al. 2009), while the current for the carbon-supported Pt alloy catalysts in the double layer region is greater than that of pure Pt/C, which reflects the properties of binary and ternary alloy catalysts comprising transition metals (Ribeiro et al. 2008; Cunha et al. 2011; Dai et al. 2018).
The electrochemically active surface (SEAS) can be calculated from the Coulomb charge required to form a hydrogen monolayer on Pt (Carl H. Hamann, Andrew Hamnett 2007). Accordingly, the SEAS of each catalyst is derived from the voltammograms by solidifying the anode current corresponding to the Hads/des charges. Equation (4) is used to determine the SEAS values (Ribadeneira and Hoyos 2008; Bonesi et al. 2010).
In Eq. 4, QH is the total charge in relation to the H+ adsorption on the integrated peak area of the Hads/des; [Pt] is the platinum load (mg cm−2) on the surface of the working electrode; 210 µC cm−2 is the charge required for complete formation of an H monolayer on Pt and 0.77 for the hydrogen monolayer (Biegler et al. 1971; Beyhan et al. 2013b). The electrode roughness is calculated by dividing the resulting SEAS value by the plain surface area as shown in Table 2. It can be concluded from these values that trimetallic electrocatalysts have the largest electrochemically active area.
The electrocatalytic performance of Pt100/C, Pt50In50/C, Pt50Sn50/C, Pt70Sn20In10/C, Pt70Sn15In15/C and Pt70Sn10In20/C catalysts for the electrooxidation of EtOH was analyzed by means of CV at room temperature (Fig. 6).
It is observed that three oxidation peaks against Ag/AgCl appeared on the Pt100/C catalyst, including a double peak in the forward scan and a singular peak in the backward scan. The forward scan peak represents the oxidation of ethanol, and the backward scan peak represents the oxidation of the intermediates derived from the fractional oxidation of ethanol. The consolidated CV results related to the oxidation of ethanol such as onset potential, positive peak potential and peak current density are summarized in Table 3.
The CV results show that trimetallic catalysts have a significantly improved catalytic activity for the electrooxidation of ethanol compared to bimetallic catalysts in the low potential range. In particular, the onset potentials for the electrooxidation of ethanol were considerably lower for all Pt70Sn10In20/C, Pt70Sn15In15/C, Pt70Sn20In10/C and Pt50Sn50/C catalysts than for the Pt50In50/C and Pt100/C catalysts (Table 3). This shows that the characteristic oxophilic property of Sn increases the efficiency of the Pt alloy catalysts (Colmati et al. 2007; Neto et al. 2007; Herranz et al. 2012). The reduction of the overpotential in the oxidation of ethanol is owing to the stimulation of H2O molecules at the interface, which germinate the OH groups at the lower anode potential than the Pt and lead to a complete oxidation of ethanol. The Pt70Sn10In20/C catalyst apparently has the best electrochemical activity in the oxidation of ethanol with a low starting potential of 0.18 V, and its maximum current density has been found to be more than 2.5 times that of the Pt50Sn50/C catalyst. This can be due to the appearance of modifications in the platinum lattice, as well as the electronic effect and the bifunctional mechanism (Spinacé et al. 2005; Dos Anjos et al. 2008). In addition, tin oxide species removed the co-intermediate formed when the C−C bond was broken (Colmenares et al. 2006; Wang et al. 2010). Similar results were seen in the oxidation of ethanol by Chu et al. (Chu et al. 2012). These observations suggest that the presence of surface oxides could play an important role in the release of OH species to eliminate the adsorbed CO (COad) in the oxidation of ethanol.
2Chronoamperometry (CA)
The stability effect of Pt/C, Pt–In/C, Pt–Sn/C and Pt–Sn–In/C electrocatalysts for the oxidation of ethanol was investigated by means of chronoamperometry at 0.4 V for 30 min under constant operating conditions. The chronoamperograms for the various electrocatalysts produced using the co-impregnation process are shown in Fig. 7. A sharp drop in current density was observed in the first few minutes, which becomes relatively stable after a while. This behavior can be explained by the fact that ethanol initially adsorbs at low potentials around 150 mV at Pt in a series of dehydrogenation steps (Tayal et al. 2012). While the Pt surface is completely covered by COad species at a potential of approx. 220 mV, this prevents further absorption of ethanol. At higher potentials, COad is oxidized to CO2 by the nucleation of OH species, which releases Pt sites for further ethanol adsorption and oxidation (Torrero et al. 2016). In accordance with the CV results, it is found that the current density for ethanol oxidation in ternary catalysts is significantly greater than in binary and Pt/C catalysts due to the supporting synergistic effect between tin and indium. Moreover, the influence of the electrode potential on the efficiency of the catalyst has various promoting effects for the electrooxidation of ethanol.
Performance test in single cells
The catalytic activity of Pt100/C, Pt50In50/C, Pt50Sn50/C, Pt70Sn20In10/C, Pt70Sn15In15/C and Pt70Sn10In20/C catalysts was tested as an anodic catalyst in a MLEFC for the electrooxidation of ethanol. The polarization and power density curves of various catalysts that arise under a load of 2 mg cm−2 are shown in Fig. 8. At room temperature, fuel (1.0 M ethanol) and oxidant (0.1 M sodium percarbonate) in 0.5 M H2SO4 were used as anodic and cathodic feed in MLEFC at a rate of 0.3 mL min−1 in each stream. The comparison of the performance of Pt–Sn-based catalysts in terms of open circuit potential (OCP), current density and peak power density is shown in Table 4. As can be seen from Fig. 8, the OCP (0.48 V) obtained for Pt100/C was considerably lower than the reversible OCP (1.2 V) due to the poor catalytic activity in the electrooxidation of ethanol, whereas that for Pt70Sn10In20/C, the OCP obtained was 0.71 V.
To find out the type of voltage drop in the MLEFC, the polarization curve is usually divided into three regions. In the first region, the voltage drops sharply due to activation losses. In the second region, the voltage decreases relatively linearly with current and more slowly, which is an indication of ohmic losses. In the third region, there is a rapid voltage drop with a higher current due to the loss of concentration (Rismani-Yazdi et al. 2008). In this study, the internal resistance is mainly due to the ohmic losses as the linear portion of voltage and current is dominant. The main reason for ohmic losses is possibly the poisoning of the catalyst, the formation of oxide layer and the mixed potential of the catalyst surface (Shaegh et al. 2011; Wnuk et al. 2019; Ullah and Zeshan 2020).
The current density of Pt100/C is also very low (50.9 mA cm−2) compared to Pt70Sn10In20/C (242.5 mA cm−2). The current adapted by the MLEFC was practically identical for all catalysts with the exception of Pt100/C in the low-current discharge area. However, if the voltage reached around 0.25 V, the Pt70Sn10In20/C generated more current than other catalysts (Fig. 8). In the tested catalysts, the power density achieved with Pt70Sn10In20/C (38 mW cm−2) was significantly higher than that of Pt50Sn50/C (22 mW cm−2) and Pt50In50/C (14 mW cm−2) catalysts. This clearly shows that the addition of In to the Pt–Sn /C catalyst can promote the oxidation reaction of ethanol in response to half-cell measurements. Overall, the significant increase in the catalytic activity of these catalysts is mainly due to the water molecules that are activated at low potentials.
Single-cell durability test
The durability tests for the Pt100/C, Pt50In50/C, Pt50Sn50/C, Pt70Sn20In10/C, Pt70Sn15In15/C and Pt70Sn10In20/C catalysts were carried out in a single MLEFC for the electrooxidation of ethanol. The results showed high cell voltage stability for ternary anode catalysts compared to binary anode catalysts (Fig. 9a). In particular, it turns out that Pt70Sn10In20/C catalyst shows the highest stability on ethanol electrooxidation among all samples, consistent with CV results. Furthermore, a minimal decrease in current density from 242 to 157 mA cm−2 (nearly 35%) in six hours showed successful anchoring of the Pt70Sn10In20/C catalyst, good durability and reduced power loss of the cell during the trial period (Fig. 9b). This result agrees with the results of Mingyuan et al. and Chu et al. (Mingyuan et al. 2009; Chu et al. 2012). The performance of the catalysts in terms of potential and current density of the durability test is shown in Table 5. The results suggest that the atomic ratio of Pt70Sn10In20/C could be the optimal atomic ratio for the electrooxidation of ethanol in membraneless fuel cells.
Conclusions
The primary aim of this study was the development of an energetic Pt–Sn–In/C anode catalyst for the electrooxidation of ethanol in a membraneless ethanol fuel cell. Against this background, well-dispersed Pt alloy catalysts such as Pt100/C, Pt50In50/C, Pt50Sn50/C, Pt70Sn20In10/C, Pt70Sn15In15/C and Pt70Sn10In20/C were manufactured using the co-impregnation process. XRD studies showed that Sn-containing Pt alloy catalysts implied the formation of the Pt3sn and Pt–Sn phases with an increase in the parameter of the Pt lattice. The electrocatalytic performance of the catalysts was examined in terms of cyclic voltammetry and chronoamperometry. Ternary catalysts have been found to have more dynamic efficiency in oxidizing ethanol than the rest of the catalysts. The onset potential of the ternary catalysts for this reaction was in the range of 0.2 V vs. Ag/AgCl, which suggests that the activation on the electrode surface is due to a synergistic ligand effect between Sn and In and to the reduced size of the particle. In the individual MLEFC tests, an increase in the catalytic activity of the catalysts was found in the following order: Pt70Sn10In20/C > Pt70Sn15In15/C > Pt70Sn20In10/C > Pt50Sn50/C > Pt50In50/C > Pt100/C, which corresponds to the measurements of half the cells. Adding indium to the Pt–Sn/C catalyst is found to increase the efficiency of the cell by helping to break the C − C bond. However, the lower indium content in Pt70Sn20In10/C hinders the oxidation of intermediate products, which has a negative effect on cell performance. As observed in the single cell tests, the maximum power densities for Pt70Sn20In10/C, Pt70Sn15In15/C and Pt70Sn10In20/C catalysts are 27, 33 and 38 mW cm−2, respectively. From the discussions above, it can be concluded that Pt70Sn10In20/C is the best anode catalyst for the electrooxidation of ethanol in MLEFC among the catalysts tested.
References
Al-Kuhaili MF (2020) Electrical conductivity enhancement of indium tin oxide (ITO) thin films reactively sputtered in a hydrogen plasma. J Mater Sci Mater Electron 31:2729–2740. https://doi.org/10.1007/s10854-019-02813-9
Antolini E (2009) Carbon supports for low-temperature fuel cell catalysts. Appl Catal B Environ 88:1–24. https://doi.org/10.1016/j.apcatb.2008.09.030
Antolini E, Colmati F, Gonzalez ER (2007) Effect of Ru addition on the structural characteristics and the electrochemical activity for ethanol oxidation of carbon supported Pt-Sn alloy catalysts. Electrochem commun 9:398–404. https://doi.org/10.1016/j.elecom.2006.10.012
Arun A, Gowdhamamoorthi M, Kiruthika S, Muthukumaran B (2014) Analysis of membraneless methanol fuel cell using percarbonate as an oxidant. J Electrochem Soc 161:F311–F317. https://doi.org/10.1149/2.067403jes
Arun A, Gowdhamamoorthi M, Ponmani K, Kiruthika S, Muthukumaran B (2015) Electrochemical characterization of Pt-Ru-Ni/C anode electrocatalyst for methanol electrooxidation in membraneless fuel cells. RSC Adv 5:49643–49650. https://doi.org/10.1039/c5ra04958j
Bazargan AM, Sharif F, Mazinani S, Naderi N (2017) A high quality ITO/PET electrode for flexible and transparent optoelectronic devices. J Mater Sci Mater Electron 28:2962–2969. https://doi.org/10.1007/s10854-016-5881-7
Beyhan S, Coutanceau C, Léger JM, Napporn TW, Kadirgan F (2013a) Promising anode candidates for direct ethanol fuel cell: Carbon supported PtSn-based trimetallic catalysts prepared by Bönnemann method. Int J Hydrogen Energy 38:6830–6841. https://doi.org/10.1016/j.ijhydene.2013.03.058
Beyhan S, Léger JM, Kadirgan F (2013b) Pronounced synergetic effect of the nano-sized PtSnNi/C catalyst for ethanol oxidation in direct ethanol fuel cell. Appl Catal B Environ 130–131:305–313. https://doi.org/10.1016/j.apcatb.2012.11.007
Beyhan S, Léger JM, Kadirgan F (2014) Understanding the influence of Ni Co, Rh and Pd addition to PtSn/C catalyst for the oxidation of ethanol by in situ Fourier transform infrared spectroscopy. Appl Catal B Environ 144:66–74. https://doi.org/10.1016/j.apcatb.2013.07.020
Biegler T, Rand DAJ, Woods R (1971) Limiting oxygen coverage on platinized platinum; Relevance to determination of real platinum area by hydrogen adsorption. J Electroanal Chem 29:269–277. https://doi.org/10.1016/S0022-0728(71)80089-X
Bonesi AR, Moreno MS, Triaca WE, Luna AMC (2010) Modified catalytic materials for ethanol oxidation. Int J Hydrogen Energy 35:5999–6004. https://doi.org/10.1016/j.ijhydene.2009.12.093
Carl H. Hamann, Andrew Hamnett WV (2007) Electrochemistry, 2nd, Completely Revised and Updated Edition
Choban ER, Markoski LJ, Wieckowski A, Kenis PJA (2004) Microfluidic fuel cell based on laminar flow. J Power Sources 128:54–60. https://doi.org/10.1016/j.jpowsour.2003.11.052
Chu D, Li Z, Yuan X, Li J, Wei X, Wan Y (2012) Electrocatalytic properties of carbon nanotubes supported ternary PtSnIn catalysts for ethanol electro-oxidation. Electrochim Acta 78:644–648. https://doi.org/10.1016/j.electacta.2012.06.092
Colmati F, Antolini E, Gonzalez ER (2007) Ethanol oxidation on a carbon-supported Pt75Sn25 electrocatalyst prepared by reduction with formic acid: Effect of thermal treatment. Appl Catal B Environ 73:106–115. https://doi.org/10.1016/j.apcatb.2006.06.013
Colmenares L, Wang H, Jusys Z, Jiang L, Yan S, Sun GQ, Behm RJ (2006) Ethanol oxidation on novel, carbon supported Pt alloy catalysts-Model studies under defined diffusion conditions. Electrochim Acta 52:221–233. https://doi.org/10.1016/j.electacta.2006.04.063
Corradini PG, Santos NA, Perez J (2018) Pt-Sn-Eu/C catalysts: application of rare earth metals as anodes in direct ethanol fuel cells. Fuel Cells 18:73–81. https://doi.org/10.1002/fuce.201700129
Cotton, F. A.; Wilkinson G (1972) Advanced Inorganic Chemistry.pdf
Cunha EM, Ribeiro J, Kokoh KB, De Andrade AR (2011) Preparation, characterization and application of Pt-Ru-Sn/C trimetallic electrocatalysts for ethanol oxidation in direct fuel cell. Int J Hydrogen Energy 36:11034–11042. https://doi.org/10.1016/j.ijhydene.2011.06.011
Dai S, Huang TH, Yan X, Yang CY, Chen TY, Wang JH, Pan X, Wang KW (2018) Promotion of ternary pt-sn-ag catalysts toward ethanol oxidation reaction: revealing electronic and structural effects of additive metals. ACS Energy Lett 3:2550–2557. https://doi.org/10.1021/acsenergylett.8b01632
Datta RS, Syed N, Zavabeti A, Jannat A, Mohiuddin M, Rokunuzzaman M, Yue Zhang B, Rahman MA, Atkin P, Messalea KA, Ghasemian MB, Della Gaspera E, Bhattacharyya S, Fuhrer MS, Russo SP, McConville CF, Esrafilzadeh D, Kalantar-Zadeh K, Daeneke T (2020) Flexible two-dimensional indium tin oxide fabricated using a liquid metal printing technique. Nat Electron 3:51–58. https://doi.org/10.1038/s41928-019-0353-8
Dos Anjos DM, Hahn F, Léger JM, Kokoh KB, Tremiliosi-Filho G (2008) Ethanol electrooxidation on Pt-Sn and Pt-Sn-W bulk alloys. J Braz Chem Soc 19:795–802. https://doi.org/10.1590/s0103-50532008000400026
Drzymała E, Gruzeł G, Depciuch J, Pawlyta M, Donten M, Parlinska-Wojtan M (2020) Ternary Pt/Re/SnO2/C catalyst for EOR: Electrocatalytic activity and durability enhancement. Nano Res 13:832–842. https://doi.org/10.1007/s12274-020-2704-1
Ferrigno R, Stroock AD, Clark TD, Mayer M, Whitesides GM (2002) Membraneless vanadium redox fuel cell using laminar flow - Supporting Information. J Am Chem Soc 124:12930–12931
Frost C, Hartley J (2005) New applications of indium catalysts in organic synthesis. Mini Rev Org Chem 1:1–7. https://doi.org/10.2174/1570193043489006
García-Rodríguez S, Somodi F, Borbáth I, Margitfalvi JL, Peña MA, Fierro JLG, Rojas S (2009) Controlled synthesis of Pt-Sn/C fuel cell catalysts with exclusive Sn–Pt interaction. Appl Catal B Environ 91:83–91
Godoi DRM, Perez J, Villullas HM (2010) Alloys and oxides on carbon-supported Pt-Sn electrocatalysts for ethanol oxidation. J Power Sources 195:3394–3401. https://doi.org/10.1016/j.jpowsour.2009.12.037
Gowdhamamoorthi M, Arun A, Kiruthika S, Muthukumaran B (2014) Perborate as novel fuel for enhanced performance of membraneless fuel cells. Ionics (Kiel) 20:1723–1728. https://doi.org/10.1007/s11581-014-1142-z
Herranz T, García S, Martínez-Huerta MV, Peña MA, Fierro JLG, Somodi F, Borbáth I, Majrik K, Tompos A, Rojas S (2012) Electrooxidation of CO and methanol on well-characterized carbon supported Pt xSn electrodes. effect of crystal structure. Int J Hydrogen Energy 37:7109–7118. https://doi.org/10.1016/j.ijhydene.2011.11.131
Jacob JM, Corradini PG, Antolini E, Santos NA, Perez J (2015) Electro-oxidation of ethanol on ternary Pt-Sn-Ce/C catalysts. Appl Catal B Environ 165:176–184. https://doi.org/10.1016/j.apcatb.2014.10.012
Jaiswal N, Pandey CM, Solanki S, Tiwari I, Malhotra BD (2020) An impedimetric biosensor based on electrophoretically assembled ZnO nanorods and carboxylated graphene nanoflakes on an indium tin oxide electrode for detection of the DNA of Escherichia coli O157:H7. Microchim Acta 187:1–8. https://doi.org/10.1007/s00604-019-3921-8
Jayasayee K, Van VJAR, Manivasagam TG, Celebi S, Hensen EJM, de Bruijn FA (2012) Oxygen reduction reaction (ORR) activity and durability of carbon supported PtM (Co, Ni, Cu) alloys: Influence of particle size and non-noble metals. Appl Catal B Environ 111–112:515–526. https://doi.org/10.1016/j.apcatb.2011.11.003
Karunakaran C, Kamalam R (2002) Structure-reactivity correlation of anilines in acetic acid. J Org Chem 67:1118–1124. https://doi.org/10.1021/jo0158433
Kim GW, Shin D, Yang M (2020) Optical property change of silver nanowire thin films in laser patterning process. Int J Precis Eng Manuf 21:301–308. https://doi.org/10.1007/s12541-019-00091-3
Kim YJ, Hwang CW, Jeong MH, Hwang TS (2017) Design of flow through continuous deionization system for indium recovery. Sep Purif Technol 176:200–207. https://doi.org/10.1016/j.seppur.2016.11.066
Knight JR, Rhys DW (1959) The systems palladium - indium and palladium - tin. J Less-Common Met 1:292–303. https://doi.org/10.1016/0022-5088(59)90005-0
Kwon Y, Hersbach TJP, Koper MTM (2014) Electro-oxidation of glycerol on platinum modified by adatoms: activity and selectivity effects. Top Catal 57:1272–1276. https://doi.org/10.1007/s11244-014-0292-6
Lamy C, Rousseau S, Belgsir EM, Coutanceau C, Léger JM (2004) Recent progress in the direct ethanol fuel cell: Development of new platinum-tin electrocatalysts. Electrochim Acta 49:3901–3908. https://doi.org/10.1016/j.electacta.2004.01.078
Lee E, Murthy A, Manthiram A (2011) Effect of Mo addition on the electrocatalytic activity of Pt-Sn-Mo/C for direct ethanol fuel cells. Electrochim Acta 56:1611–1618. https://doi.org/10.1016/j.electacta.2010.10.086
Lim DH, Choi DH, Lee WD, Lee HI (2009) A new synthesis of a highly dispersed and CO tolerant PtSn/C electrocatalyst for low-temperature fuel cell; its electrocatalytic activity and long-term durability. Appl Catal B Environ 89:484–493. https://doi.org/10.1016/j.apcatb.2009.01.011
Lou J, Wang G, Xia Y, Liang C, Huang H, Gan Y, Tao X, Zhang J, Zhang W (2020) Achieving efficient and stable interface between metallic lithium and garnet-type solid electrolyte through a thin indium tin oxide interlayer. J Power Sources 448:227440. https://doi.org/10.1016/j.jpowsour.2019.227440
Mingyuan Z, Gongquan S, Shiyou Y, Huanqiao L, Qin X (2009) Preparation, structural characterization, and activity for ethanol oxidation of carbon-supported PtSnIn catalyst. Energy Fuels 23:403–407. https://doi.org/10.1021/ef800726b
Monshi A, Foroughi MR, Monshi MR (2012) Modified Scherrer equation to estimate more accurately nano-crystallite size using XRD. World J Nano Sci Eng 02:154–160. https://doi.org/10.4236/wjnse.2012.23020
Neto AO, Dias RR, Tusi MM, Linardi M, Spinacé EV (2007) Electro-oxidation of methanol and ethanol using PtRu/C, PtSn/C and PtSnRu/C electrocatalysts prepared by an alcohol-reduction process. J Power Sources 166:87–91. https://doi.org/10.1016/j.jpowsour.2006.12.088
Passos FB, Aranda DA, Schmal M (1998) Characterization and catalytic activity of bimetallic Pt-In/Al2O3 and Pt-Sn/Al2O3 catalysts. J Catal 178:478–488. https://doi.org/10.1139/v84-246
Ponmani K, Durga S, Gowdhamamoorthi M, Kiruthika S, Muthukumaran B (2014) Influence of fuel and media on membraneless sodium percarbonate fuel cell. Ionics (Kiel). https://doi.org/10.1007/s11581-014-1118-z
Ponmani K, Kiruthika S, Muthukumaran B (2015) Investigation of nanometals (Ni and Sn) in platinum-based ternary electrocatalysts for ethanol electro-oxidation in membraneless fuel cells. J Electrochem Sci Technol 6:95–105. https://doi.org/10.5229/JECST.2015.6.3.95
Ponmani K, Nayeemunisa SM, Kiruthika S, Muthukumaran B (2016) Electrochemical characterization of platinum-based anode catalysts for membraneless fuel cells. Ionics (Kiel). https://doi.org/10.1007/s11581-015-1555-3
Priya M, Kiruthika S, Muthukumaran B (2017) Synthesis and characterization of Pt–Sn–Ce/MC ternary catalysts for ethanol oxidation in membraneless fuel cells. Ionics (Kiel). https://doi.org/10.1007/s11581-016-1940-6
Purgato FLS, Olivi P, Léger JM, de Andrade AR, Tremiliosi-Filho G, Gonzalez ER, Lamy C, Kokoh KB (2009) Activity of platinum-tin catalysts prepared by the Pechini-Adams method for the electrooxidation of ethanol. J Electroanal Chem 628:81–89. https://doi.org/10.1016/j.jelechem.2009.01.010
Queiroz MAR, Ribeiro J (2019) Catalysts of PtSn/C modified with ru and ta for electrooxidation of ethanol. Catalysts. https://doi.org/10.3390/catal9030277
Ramos SG, Calafiore A, Bonesi AR, Triaca WE, Castro Luna AM, Moreno MS, Zampieri G, Bengio S (2012) Supported catalysts for alcohol oxidation synthesis and analysis of their catalytic activity. Int J Hydrogen Energy 37:14849–14853. https://doi.org/10.1016/j.ijhydene.2012.01.171
Ribadeneira E, Hoyos BA (2008) Evaluation of Pt-Ru-Ni and Pt-Sn-Ni catalysts as anodes in direct ethanol fuel cells. J Power Sources 180:238–242. https://doi.org/10.1016/j.jpowsour.2008.01.084
Ribeiro J, Dos Anjos DM, Léger JM, Hahn F, Olivi P, De Andrade AR, Tremiliosi-Filho G, Kokoh KB (2008) Effect of W on PtSn/C catalysts for ethanol electrooxidation. J Appl Electrochem 38:653–662. https://doi.org/10.1007/s10800-008-9484-8
Rismani-Yazdi H, Carver SM, Christy AD, Tuovinen OH (2008) Cathodic limitations in microbial fuel cells: an overview. J Power Sources 180:683–694. https://doi.org/10.1016/j.jpowsour.2008.02.074
Rousseau S, Coutanceau C, Lamy C, Léger JM (2006) Direct ethanol fuel cell (DEFC): electrical performances and reaction products distribution under operating conditions with different platinum-based anodes. J Power Sourc 158:18–24. https://doi.org/10.1016/j.jpowsour.2005.08.027
Shaegh SAM, Nguyen NT, Chan SH (2011) Air-breathing membraneless laminar flow fuel cell with flow-through anode. ASME 2011 9th Int Conf Fuel Cell Sci Eng Technol Collocated with ASME 2011 5th Int Conf Energy Sustain Fuelcell 2011: 217–222 . https://doi.org/10.1115/FuelCell2011-54918
Silva JCM, De Souza RFB, Romano MA, D’villa-Silva M, Calegaro ML, Hammer P, Neto AO, Santos MC, (2012) PtSnIr/C anode electrocatalysts: promoting effect in direct ethanol fuel cells. J Braz Chem Soc 23:1146–1153. https://doi.org/10.1590/S0103-50532012000600021
Spinacé EV, Linardi M, Neto AO (2005) Co-catalytic effect of nickel in the electro-oxidation of ethanol on binary Pt-Sn electrocatalysts. Electrochem commun 7:365–369. https://doi.org/10.1016/j.elecom.2005.02.006
Tayal J, Rawat B, Basu S (2012) Effect of addition of rhenium to Pt-based anode catalysts in electro-oxidation of ethanol in direct ethanol PEM fuel cell. Int J Hydrogen Energy 37:4597–4605. https://doi.org/10.1016/j.ijhydene.2011.05.188
Thilaga S, Durga S, Selvarani V, Kiruthika S, Muthukumaran B (2017) Multiwalled carbon nanotube supported Pt–Sn–M (M = Ru, Ni, and Ir) catalysts for ethanol electrooxidation. Ionics (Kiel). https://doi.org/10.1007/s11581-017-2349-6
Torrero J, Pérez-Alonso FJ, Peña MA, Domínguez C, Al-Youbi AO, Al-Thabaiti SA, Basahel SN, Alshehri AA, Rojas S (2016) In situ infrared study of the electrooxidation of ethanol and acetaldehyde in acid electrolyte. ChemElectroChem 3:1072–1083. https://doi.org/10.1002/celc.201600136
Ullah Z, Zeshan S (2020) Effect of substrate type and concentration on the performance of a double chamber microbial fuel cell. Water Sci Technol 81:1336–1344. https://doi.org/10.2166/wst.2019.387
Vigier F, Coutanceau C, Hahn F, Belgsir EM, Lamy C (2004) On the mechanism of ethanol electro-oxidation on Pt and PtSn catalysts: electrochemical and in situ IR reflectance spectroscopy studies. J Electroanal Chem 563:81–89. https://doi.org/10.1016/j.jelechem.2003.08.019
Wang F, Zheng Y, Guo Y (2010) The promoting effect of europium on PtSn/C catalyst for ethanol oxidation. Fuel Cells 10:1100–1107. https://doi.org/10.1002/fuce.200900158
Wang ZB, Yin GP, Zhang J, Sun YC, Shi PF (2006) Investigation of ethanol electrooxidation on a Pt-Ru-Ni/C catalyst for a direct ethanol fuel cell. J Power Sourc 160:37–43. https://doi.org/10.1016/j.jpowsour.2006.01.021
Wnuk P, Jurczakowski R, Lewera A (2019) Electrochemical characterization of low-temperature direct ethanol fuel cells using direct and alternate current methods. ChemRxiv. https://doi.org/10.26434/chemrxiv.8787311.v1
Zeng Y, Ji B, Lv Z, Zheng X, Yang X, Cui P, Dong Y, Zhang X, Jiang J (2020) Rapid synthesis of porous Pt-Ni-Cu coatings with a wide composition range, tunable structures and enhanced electrocatalytic properties. J Alloys Compd 835:155402. https://doi.org/10.1016/j.jallcom.2020.155402
Zhou WJ, Li WZ, Song SQ, Zhou ZH, Jiang LH, Sun GQ, Xin Q, Poulianitis K, Kontou S, Tsiakaras P (2004) Bi- and tri-metallic Pt-based anode catalysts for direct ethanol fuel cells. J Power Sour 131:217–223. https://doi.org/10.1016/j.jpowsour.2003.12.040
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Selvarani, V., Kiruthika, S., Gayathri, A. et al. Enhanced electrochemical performance of Pt–Sn–In/C nanoparticles for membraneless fuel cells. Chem. Pap. 75, 3521–3533 (2021). https://doi.org/10.1007/s11696-021-01598-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-021-01598-8