Abstract
The leachability of uranium and vanadium ions was conducted from ferruginous siltstone using carbonate ions. The optimum alkaline leaching parameters for dissolving about 92.92% U(VI), and 94.82% V(V) were 70 mg/L Na2CO3, 150 mg/L NaHCO3, 1:3 solid:liquid (S:L) ratio, and 150 rpm stirring rate, for 5 h leaching time at 50 °C. Uranium and vanadium ions were extracted from the carbonate leach liquor using the prepared cetylpyridinium carbonate [(CP)2CO3] in kerosene. The maximum extraction of U(VI) was gained using 0.012 M/L (CP)2CO3 and 5% (w/v) tridecyl alcohol (TDA) in kerosene from the carbonate leach solution assaying 413 mg/L U(VI) at pH 11 and 3:1 aqueous:organic (A:O) phase. Moreover, the maximum extraction of V(V) was achieved using 0.012 M/L (CP)2CO3 and 5% (w/v) TDA in kerosene from the carbonate leach solution containing 336 mg/L V(V) at pH9, and 3:1 A:O phase ratio. Vanadium ions were scrubbed from the uranium-loaded solvent and then it was followed by uranium stripping while vanadium ions were stripped from the vanadium-loaded solvent. McCabe–Thiele diagrams for U(VI) and V(V) extraction and stripping were constructed to determine the theoretical stages of extraction and stripping. Finally, the optimum conditions were applied to gain the sodium diuranate and ammonium vanadate cakes from carbonate leach liquor with the appropriate purities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uranium is already in the environment due to nuclear influences testing and unexpected liberation from nuclear plants of electrical power generation in low concentrations. Furthermore, uranium or vanadium mining and other metals ions led to the use of uranium and vanadium vehicles in the environment. Moreover, vanadium has multiple and continuously progressing manufacturing applications. Today, up to 85% for vanadium production were consumed in stainless steel manufacture and production of ferrovanadium alloy (Khorfan et al. 2001). Uranium and vanadium ions were presented in the soil, rocks, water, and contaminated water (Altmaier and Vercouter 2012).
Ferruginous siltstone of Abu Zenima area, Southwestern Sinai, Egypt, is the promising locality for uranium recovery, and it contained clays, carbonates, and quartz. The mineralogical identification of siltstone suggested quartz, ettringite, chlorite, illite, dolomite, aragonite, plagioclase, calcite, goethite, alkali feldspar, siderite, hematite, mica–montmorillonite, carnotite, and lepidocrocite (El Aassy et al. 2011; Marshall and Brett 2016; El Mezayen et al. 2016). Anyway, uranium regularly paired with some transition metal ions (such as vanadium and molybdenum) in carbonate rocks (Atia et al. 2018).
The main conventional methods of uranium leaching were utilized by sulfuric acid for uranium ore processing (Reiller et al. 2011). Notwithstanding, this strategy is not cost-sufficient for the uranium carbonate rocks owing to extraordinary acid consumption. Recently, the methodologies on uranium and vanadium alkaline leaching were studied by many alkaline leaching agents such as hydroxide or carbonate and bicarbonate, whereas Na2CO3 and NaHCO3 mixture solution favored these types of deposits (Edwards and Oliver 2000; Du-Preez 1989). In the alkaline carbonate leaching, uranium (VI) leaching was selectivity higher than the most impurities which were precipitated. Nevertheless, vanadium (V) was related to the carbonate leach solution, together with uranium (VI) (Gajda et al. 2015). Uranium content in phosphorite was leached with ammonium carbonate and bicarbonate, and the uranyl carbonate solution was produced (Guzman et al. 1995).
Formerly, uranium and vanadium recovery from various leach liquors were broadly utilized by ion exchange, precipitation, and solvent extraction techniques (Cheira 2015; Hashad et al. 2011; Kumar et al. 2011; Menis and Iyer 1971). Amidst these, solvent extraction is extremely used particularly for uranium and vanadium extraction, and it is the most assuring method suggested for future study and construction; it is obliged to extract and separate uranium and vanadium from leach solutions (Sole et al. 2011). The extraction of uranium from the main and transition metal ion groups is relatively clear, while the U(VI)/V(V) extraction is complicated from carbonate solutions. Therefore, substituted amines had become to perform a critical role in the separation of uranium and additionally some transition metals (as vanadium) from the sulfuric leaching of uranium-bearing rocks (Moyer 2017). The main advantages of these extractants are ease of manufacture, excellent radiolytic and chemical stabilities and the plausibility of complete incineration that runs to fewer quantities of trash. Besides, the degradation products of amines recorded no interference during the separation processes and easily eliminated from the solutions.
In the mean time, some quaternary amines were prepared and utilized to separate uranium and vanadium ions from alkaline leach liquors by solvent extraction, but no quaternary amines were run well by hydrocarbon diluents due to the third phase formed. Aliquat 336 was utilized to separate uranium ions from carbonate leach solutions although the third phase composition was restricted by isodecanol that was used as a modifier in Shellsol D70 (Zhu et al. 2013). The extraction equilibrium and thermodynamic requirements of U(VI) from sulfate leach solution carrying impurities such as manganese, iron, aluminum, magnesium, and calcium were also examined by Alamine 336 into kerosene (Khanramaki et al. 2017). Uranium extraction from sulfate solution utilizing Alamine 336 melting in Exxsol D-100 merchant kerosene and tridecanol (5% v/v) as a modifying agent was done at the unity of aqueous/organic ratio (Avelar et al. 2017). U(VI) extraction was evaluated by LIX63/kerosene to acquire information about the conditions concerning the extraction degree of U(VI) and the consequent stripping rate of U(VI) to propose a mechanism for explaining the extraction rule from the uranium leach liquor of Egyptian monazite (Hussein et al. 2017). Stripping of U(VI) from U-Alamine 336 attained from sulfate leach liquor solution was investigated by employing various stripping agents (Na2CO3, (NH4)2CO3, and NH4Cl) in A:O phase ratio (0.5–5) and concentration extent (0.25–2.00 M/L) (Mir-mohammadi et al. 2018).
Recently, some quaternary amines were also applied as extractants to recover vanadium ions from several leach solutions. Alamine 336, and Primene 81R in kerosene were used to extract vanadium ions from sulfate solution (Lozano and Godi´nez 2003). Furthermore, Aliquat 336 and TBP in kerosene modified with 10% octanol were applied to separate vanadium (V) from the chloride liquor (Zhang et al. 2016). Vanadium ion extraction was evaluated from acid leachate by tertiary amine N235 (Ye et al. 2018). Extraction of Cr(III) and V(V) was carried out from ilmenite sulfate leach liquor by Aliquat 336 chloride (0.4 M/L) in kerosene (Nayl and Aly 2015). A two-stage process of extraction–stripping-NH4NO3 techniques were improved for vanadium precipitation, and it was established to apply on the alkaline leachate generated for the roasted coal (Long et al. 2014).
In the current study, the carbonate leaching of uranium and vanadium from El Sheikh Soliman ferruginous siltstone was obtained by sodium carbonate and sodium bicarbonate solutions. Several leaching parameters were investigated on the leaching processes at identical conditions. The solvent extraction system of U(VI) and V(V) was applied using the synthesized cetylpyridinium carbonate in kerosene from the carbonate leach liquor. Various experimental parameters were tested on the extraction of U(VI) and V(V) from the leach solution, and the stripping parameters were also studied from the loaded solvent. These parameters comprised of pH, cetylpyridinium carbonate concentration, agitating time, A:O ratio, and temperature in the extraction processes while the stripping parameters involved stripping concentration, contact time, phase ratio, and temperature.
Materials and methods
Initial processing of ferruginous siltstone
The studied sample is ferruginous siltstone of Um Bogma Formation lower member, Paleozoic age and it was attained from the El-Sheikh Soliman area, Southwestern Sinai, Egypt. The working sample was first managed within the crushing, milling and sieving pursuit by suitable quartering, and it was quantitatively analyzed after full dissolution utilizing the appropriate techniques.
Analytical methods
The mineralogical analysis of the ferruginous siltstone sample was examined utilizing the X-ray diffraction (XRD) method, employed to determine bulk mineral assemblies. The quantitative analysis of the studied sample after its digestion was then carried out for major oxides and trace element analysis. The major oxides Al2O3, SiO2, and TiO2 spectrophotometrically examined by UV/Vis spectrometer Unicam UV2-100 while K and Na oxides were assessed by the flame photometric technique on the sample and leach solutions. The MgO, Fe2O3, and CaO were titrimetrically determined (Shapiro and Brannock 1975). The estimated error for these major constituents was not more than ± 2%. In the meantime, the trace elements were determined within inductively coupled plasma optical emission spectrometry (ICP-OES). U(VI) in the leachate solution was spectrophotometrically determined using Arsenazo III at 650 nm (Marczenko and Balcerzak 2000) while atomic absorption spectrophotometer (AAS) was used to assess vanadium concentration. All analyses were repeated three times for each sample. Fourier transform infrared (FTIR) spectra were marked in the range 400–4000 cm−1 by Thermo Fischer Scientific Nicolet IZ10 equipment that was used for determining the functional groups of the synthesized cetylpyridinium carbonate.
Leaching exploration
The alkaline leaching operations were investigated and directed to attain the goal of dissolving most uranium and vanadium ingredients under optimum conditions with fewer dissolutions of the other unwanted materials. The leaching operations were performed in a 300-mL Teflon beaker at various alkaline types, several concentrations of sodium carbonate and bicarbonate mixture, solid:liquid ratio (1:1–1:6), leaching time (1.0–7.0 h), stirring degree (50–250 rpm), and temperature (25–80 °C). The conducted leaching solutions at different parameters were analyzed. At the ending of leaching, the deposit was separated and cleaned by distilled water. The obtained leach liquor was quantitatively analyzed. The following equations calculated uranium and vanadium leaching efficiencies.
Synthesis of cetylpyridinium chloride
Cetylpyridinium chloride (CPCl) (Rodriguez-Morales et al. 2005) was synthesized by refluxing of 25 g cetyl chloride (1-chlorohexadecane) (99%, Sigma-Aldrich), 70 g pyridine (99%, Sigma-Aldrich) and catalyst (0.5 g containing the mixture of formic acid, N,N bis(carboxymethyl) glycine, propyl ethylamine, and hydrochloric acid (99%, Loba Chemie, India)) into a three-necked flask and it was retained for 8 h at 95 °C. After the reaction completion, the bulk was cooled, separated and the collected product was washed with recovered methyl isobutyl ketone (99%, Loba Chemie, India) to get crystalline cetylpyridinium chloride (31.5 g) with 99.5% purity (Scheme 1).
Formation of cetylpyridinium carbonate
Conversion of 0.1 M/L cetylpyridinium chloride from chloride to the carbonate form was completed by washing three times with 5 M/L Na2CO3, at phase ratio 1:2, shaking at 150 rpm for 5 h each time. No chloride ions were found in the third aqueous raffinate using 0.1 M/L AgNO3 as an indicator and no emulsion appeared during this process. The reason was that the emulsification by a surfactant was proportional to the shaking speed and shaking time. The anion exchange equilibrium was gotten earlier, and thus the emulsification occurred (Scheme 2).
Extraction evaluation
The leach solution containing 413 mg/L uranium (VI) and 336 mg/L vanadium (V) was adjusted to the desired pH value by 1 M/L sulfuric acid and 1 M/L sodium hydroxide solution, the aqueous phase was equilibrated by stirring for a particular time and fitting organic phase volume in a separatory funnel. After equilibration, the solution contents were permitted and fixed to separate the aqueous phase from the organic phase. The U(VI) or V(V) concentrations in the gained aqueous phase were measured by the spectrophotometer and AAS techniques, respectively. The distribution ratio of extraction (D) was used for attaining extraction efficiency, and it was calculated by the following equation (Jeffery et al. 1995):
where D is the distribution ratio of extraction, Minitial is the initial concentration of U (VI) or V(V), and Maq is uranium or vanadium ion concentrations in aqueous phase after the extraction procedures. The extraction percentage (E) is evaluated from the following equation:
where Vaq and Vorg are volumes of aqueous and organic solutions, sequentially. The stripping experimentations were executed by shaking several volumes of metal ion-loaded organic phase after extraction and the aqueous solution volumes of varying concentrations of Na2CO3 + NaOH mixture under contact time ranging from 1 to 15 min at room temperature. After equilibration, the complete separation was performed by settling down of both the phases. Known aliquot portions were fully outgone from the aqueous stripping phase for estimating the tripped metal ion concentration. Extraction and stripping investigations were applied at ambient temperature (except for examining the temperature parameters). All experiments were replicated various times to confirm the correctness of the obtained results. The relative errors are no more than 2%.
Results and discussion
Characterization of ferruginous siltstone
The ferruginous siltstone sample was submitted to complete chemical analysis using the mentioned procedures, and the obtained results are shown in Table 1. From these results, it was evident that the concentrations of silica, alumina, Fe2O3, K2O, and CaO were assayed to be 43.91, 13.71, 11.23, 2.89, and 7.61%, respectively, while uranium and vanadium were at 1338 and 1065 mg/kg, respectively.
The XRD analysis of ferruginous siltstone in Fig. 1 reveals that the chief mineral constituents comprised quartz (SiO2), kaolinite (Al2Si2O5(OH)4), hematite (Fe2O3), dolomite (CaMg(CO3)2) and gypsum (CaSO4.2H2O) minerals and there is no definite uranium mineralization. It should be noted here that the ferruginous siltstone ore material does not show any uranium mineralization in a way to propose that the uranium (0.1338%) and vanadium (0.1065%) contents were possibly adsorbed on kaolinite and hematite minerals, as well as uranium and vanadium were suggested to exist as deposits replaced and altered by other elements in the rock sample (Atia et al. 2018). Siltstone of El Sheikh Soliman area was ferruginous siltstone of the lower member of Um Bogma formation, Paleozoic age. Sheikh Soliman area is the promising locality for uranium extraction where uranium is high and shows more excitability (El-Rayes and Arnous 2015).
Leaching data
The chemistry of uranium leaching by Na2CO3 and NaHCO3 was correlated with uranium ore materials, and they were the common leachate used industrially under optimum conditions illustrated in the chemical reactions. The alkaline leaching operation of the study sample attributed to the applicability of hexavalent uranium to make extremely soluble complexes within alkaline media. The major cations within the ore material were Na+, K+, Ca2+, and Mg2+. The major anions were uranyl tricarbonate or uranyl dicarbonate and vanadate (El-Sheikh et al. 2017; Gorman-Lewis et al. 2008).
In this regard, it had been reported that quaternary amine could act as strong base extractant which was capable of reactions in the high pH range, and it would extract uranium ions from carbonate solutions as follows:
In the leaching solution, the vanadium (V) has VO3− form at the pH value over 8.0 and the VO3− turns into anion of isopolyvanadic acid by the reduction of pH from 8.0 to 2.4; suddenly, the anions of isopolyvanadic acid convert to VO2+ at pH fewer than 2.4 (Li et al. 2017).
Sodium carbonate concentration
A series of leaching trials were achieved using different Na2CO3 concentrations (10–100 mg/L) to leach uranium(VI) and vanadium(V) from the ferruginous siltstone sample. The other leaching parameters were remained constant at the solid particle size of − 80 mesh size, solid to liquid ratio (1:3), the contact time of 4 h and the stirring rate of 150 rpm. The leaching efficiencies are shown in Fig. 2a. The data were precise that the Na2CO3 concentration increased from 10 to 70 mg/L, the uranium and vanadium dissolution efficiencies increased from 21.19 and 25.33 to 55.78 and 73.43%, respectively. By increasing Na2CO3 concentration above 70 mg/L, vanadium leaching efficiency remained constant, while uranium leaching efficiency was decreased due to the formation of NaOH which led to the precipitation of uranium ions as shown in the following equations:
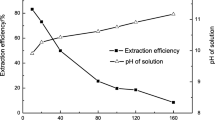
Leaching parameters on U(VI) and V(V) leaching efficiencies from ferruginous siltstone sample, a sodium carbonate concentration (1:3 S:L ratio, 4 h contact time, 150 rpm stirring speed, − 80 mesh size, room temperature), b solid: liquid ratio (70 mg/L Na2CO3, 4 h contact time, 150 rpm stirring speed, − 80 mesh size, room temperature), c sodium bicarbonate concentration (70 mg/L Na2CO3, 1:3 S:L ratio, 4 h leaching time, 150 rpm stirring speed, − 80 mesh size, room temperature), d leaching time (70 mg/L Na2CO3, 150 mg/L NaHCO3, 1:3 S:L ratio, 150 rpm stirring speed, − 80 mesh size, room temperature), e stirring rate (70 mg/L Na2CO3, 150 mg/L NaHCO3, 1:3 S:L ratio, 5 h leaching time, − 80 mesh size, room temperature), and f temperature (70 mg/L Na2CO3, 150 mg/L NaHCO3, 1:3 S:L ratio, 5 h leaching time, − 80 mesh size, 150 rpm)
However, 70 mg/L Na2CO3 attain 55.78 and 73.43% U(VI), and V(V) leaching efficiencies, which was the concentration selection employed in the following leaching operations.
Solid:liquid ratio
To investigate the effect of solid: liquid ratio on the leaching efficiencies for V(V) and U(VI), several experiments were carried out using from 1:1 to 1:6 S:L phase ratio while the other parameters were kept constant at 70 mg/L sodium carbonate, − 80 mesh size particle size and 4 h leaching time at 25 °C. The results reported in Fig. 2b. The leaching efficiencies of uranium(VI) and vanadium(V) increased at solid:liquid ratio from 1:1 to 1:3, while increasing the volume of alkali by a solid:liquid rate from 1:4 of 1:6, only very slightly increased the vanadium leaching efficiency but it was decreased the uranium leaching efficiency also because of the formation NaOH which led to precipitate of uranium ions. So, 1:3 solid:liquid ratio was the ideal ratio.
Sodium bicarbonate concentration
The effect of bicarbonate (NaHCO3) concentration on the leaching efficiencies of U(VI) and V(V) species from ferruginous siltstone sample was investigated by varying from 25 to 200 g/L while the other leaching conditions were fixed at 70 mg/L sodium carbonate, − 80 mesh size, 4 h leaching time, and 1:3 solid:liquid ratio. The gained results are plotted in Fig. 2c. From the advanced data, it was noticeable that the leaching of both U(VI) and V(V) was increased with increasing the concentration of bicarbonate to 150 g/L and the uranium and vanadium leaching efficiencies increased from 51.32 and 73.55 to 82.42 and 88.55%, respectively, whereas the addition of NaHCO3 led to reacting with the formed NaOH and, therefore, the uranium dissolution increased because of the formation of sodium carbonate as in the following equation:
Nevertheless, bicarbonate concentration increased above 150 g/L; the U(VI) and V(V) leaching efficiencies increased slightly. Accordingly, 150 g/L bicarbonate concentration was considered a suitable concentration for the next procedures.
Leaching time
In these experiments, different leaching times (1–7 h) were tested. The other leaching conditions were kept constant at − 80 mesh ore particle size, 70 mg/L Na2CO3, 150 mg/L NaHCO3, 150 rpm stirring speed, and 1:3 solid:liquid ratio at 25 °C. From the results plotted in Fig. 2d, it was established that at 5 h leaching time, the obtained leaching efficiencies of U(VI) and V(V) were 88.59% and 90.66%, respectively. As the leaching time was increased to more than 5 h, no effect was realized in the leaching efficiencies of two metal ions. Consequently, it could be decided that 5 h of leaching time outlined the favored condition for the consequent uranium and vanadium ion dissolution experiments.
Stirring rate
The impact of the stirring speed was studied by the conditions of − 80 mesh particle size, 70 mg/L Na2CO3, 150 mg/L NaHCO3, and 1/3 solid/liquid ratio for 5 h leaching time at 25 °C. Stirring rate going from 50 to 250 rpm was examined. The results are shown in Fig. 2e. The leaching rates for uranium and vanadium ions increased with increased stirring rate to 150 rpm, reaching 88.59% and 90.66% leaching efficiencies, respectively, and then rested roughly constant above 150 rpm. Therefore, the preferred speed of 150 rpm was applied. It is worthy of mentioning that by increasing the values of the parameters as discussed above, the metal impurity dissolution was significantly increased.
Temperature
The alkaline leaching operations were conducted to explore the influence of temperature upon U(VI) and V(V) leaching efficiencies from ferruginous siltstone in the range from 25 to 80 °C and the other leaching parameters remained constant at the above optimum conditions. The attained data are specified in Fig. 2f, and it reveals that by increasing the temperature to 50 °C, the leaching efficiencies of uranium and vanadium increased to 92.92% and 94.82%, respectively. With a further increase to 80 °C, the uranium and vanadium leaching efficiencies were slightly extended to 93.92% and 95.97%, respectively. Increasing the temperature enhanced the solubility of unwanted impurities such as arsenide, sulfides, chlorites, silicates, and phosphates. Hence, it could be inferred that 50 °C leaching temperature would be satisfactory and deemed optimum for the leaching of the studied two metal ions.
Solvent extraction approaches
Characterization of CPCl and (CP)2CO3
The purity of the synthesized cetylpyridinium chloride (CPCl) and cetylpyridinium carbonate ((CP)2CO3) was confirmed by the melting point (Rodriguez-Morales et al. 2005), elemental analysis, and FTIR analysis. They were in the pure forms and as white crystalline powders at room temperature. The CPCl had a melting point of 78 °C, and the melting point of (CP)2CO3 is 85 °C. The yield of CPCl was 80.1% (31.5 g). The elemental analysis of CPCl: theoretical % for C21H38NCl: C: 74.19%, H: 11.27%, N: 4.12%, Cl: 10.44%. Found: C: 74.45 ± 0.52%, H: 10.75 ± 0.65%, N: 4.25 ± 0.25%, Cl: 10.51 ± 0.42%. However, the elemental analysis of (CP)2CO3 was as follows: theoretical % for C43H76N2O3: C: 77.19%, H: 11.47%, N: 4.18%, O: 7.17%. Found: C: 77.35 ± 0.51%, H: 10.82 ± 0.45%, N; 4.28 ± 0.25%, O: 7.27 ± 0.28%. Hence, molecular weights of CPCl and (CP)2CO3 are 339.99 and 668.99 g/mol, respectively.
The spectra of CPCl, (CP)2CO3, U-loaded (CP)2CO3, and V-loaded (CP)2CO3 indicated the characteristic absorption of band positions and intensities observed in FTIR spectra with wavenumber and intensities (Fig. 3). From the obtained data, the sharp peaks appearing at 2958–2857 cm−1 belonged to C–H of the CH2 and CH3 groups (James et al. 2009; Xiao-teng et al. 2019). FTIR of CPCl and (CP)2CO3 showed characteristic bands at 1022 and 1019 cm−1 that indicated the presence of C–N group, 1635 and 1623 cm−1 indicated the presence C=N group, respectively, but 1704 cm−1 designated the attendance of stretching of C=O group, and 1172 cm−1 designated the attendance of C–O–C group in (CP)2CO3 (Cheira et al. 2017). The results of FTIR studies showed solute–solute–solvent interaction via coordination bond formation through the quaternary amine group.
In the FTIR of U-loaded (CP)2CO3 complexes in Fig. 3c, there appeared new peaks which were not found in the spectrum of (CP)2CO3; these peaks were detected at 928 cm−1 and 480 cm−1 due to the formation of U–O and U–O–N groups, respectively (Hussein et al. 2017; Cheira et al. 2017); however, in the spectrum of V-loaded (CP)2CO3 complexes in Fig. 3d, the new bands appeared at 1571, 878 and 587 cm−1 because of the establishment V=O, V–O, and V–O–N bands, respectively (Anumula et al. 2013; Guerra et al. 2010). Some peaks of (CP)2CO3 were shifted toward redshift with 10–15 cm−1 after the adsorption of uranium carbonate and vanadate anions. From the previous data, (CP)2CO3 was indeed proved to have good affinity toward the U(VI) and V(V) ions in carbonate leach liquor.
Extraction pH
The effect of pH on the extraction of uranium (413 mg/L) and vanadium (336 mg/L) from the alkaline leach solution using the studied cetylpyridinium carbonate ((CP)2CO3) was studied. Various experiments were achieved at pH values ranging from 8.6 to 11.6, and other parameters were constant at 0.012 M/L (CP)2CO3 and 5% (w/v) of tridecyl alcohol (TDA) in kerosene and 3:1 aqueous to organic phase ratio for 10 min contact time at room temperature. From the attained results in Fig. 4a, it was evident that the uranium and vanadium extraction increased from 10.77 and 77.65 to 97.7 and 79.85% with the increase in pH from 8.6 to 10.2. By increasing the pH until 11.2, the uranium extraction was slightly increased to 99.25%, but the vanadium extraction was decreased for vanadium to 35.06%, after that by increasing pH, the uranium extraction was reduced because the uranium ions started to precipitate as sodium diuranate and vanadium extraction was also reduced. Nevertheless, vanadium extraction was increased from 77.65 to 92.66% with the increase in pH from 8.6 to 9.2, and by increasing the pH to 11.6, the vanadium extraction is decreased to 21.24%. The U(VI) extraction with cetylpyridinium carbonate ((CP)2CO3) is as follows:
Parameters’ influence on uranium and vanadium extraction by cetylpyridinium carbonate extractant. a pH (413 mg/L U(VI) and 336 mg/L V(V), 0.012 M/L (CP)2CO3 and 5% (w/v) TDA in kerosene, 3:1 A:O phase, 10 min contact time), b (CP)2CO3 concentration (413 mg/L U(VI) [pH 11] and 336 mg/L V(V) [pH 9], 5% (w/v) TDA in kerosene, 3:1 A:O phase, 10 min contact time), c a design of log10D vs. log [(CP)2CO3] of U(VI) and V(V) extraction (413 mg/L U(VI) [pH 11] and 336 mg/L V(V) [pH 9], 5% (w/v) TDA in kerosene, 3:1 A:O phase, 10 min contact time), d contact time (413 mg/L U(VI) [pH 11] and 336 mg/L V(V) [pH 9]), 0.012 M/L (CP)2CO3 and 5% (w/v) TDA in kerosene, 3:1 A:O phase), e TDA concentration as a modifier (413 mg/L U(VI) [pH 11] and 336 mg/L V(V) [pH 9], 0.012 M/L (CP)2CO3 in kerosene, 3:1 A:O phase, 10 min contact time), and f A:O phase ratio (413 mg/L U(VI) [pH 11] and 336 mg/L V(V) [pH 9], 0.012 M/L (CP)2CO3 and 5% TDA as modifier in kerosene, 10 min contact time)
The increase in pH led to a rise in carbonate concentration by dissociation of HCO3− to form CO23. The formation of uranium–organic complexes was proportional to the concentration of UO2(CO3)4−3. Therefore, the increase of pH led to an increase in U(VI) extraction. At pH 10–11.2, carbonate ions were predominant, and its concentration almost approached maximum. Thus, a further increase in pH would not lead to an alteration in the carbonate concentration and it resulted in the relatively permanent uranium extraction over pH 11 (Fig. 4a). But the pH was reduced by acid addition; carbonate concentration was decreased by altering the carbonate to bicarbonate; hence, it performed the decrease of uranium extraction (Senol 2014).
The V(V) in the tested pH range should be in the form of VO3− and/or VO3(OH)2− which could convert each other and the V(V) extraction with cetylpyridinium carbonate ((CP)2CO3) follows the reactions:
By decreasing the pH to 9, VO3(OH)2− shifted to form more VO3− resulting in an increase in vanadium extraction as (R4N)(VO3). With an increase in pH ≥ 9, vanadium extraction was decreased due to the VO3− ions that would reduce via the formation of VO3(OH)2−. Hence, the maximum extraction of V(V) operated at pH 9, and maximum uranium extraction was done at pH 11. Finally, uranium was easily separated from vanadium at pH 11.
Effect of (CP)2CO3 concentration
The impact of (CP)2CO3 concentration in kerosene on the 413 mg/L U(VI) and 336 mg/L V(V) extraction was studied in the concentration range from 0.001 to 0.030 M/L (CP)2CO3 at 5% (w/v) of TDA, pH 11, for 10 min shaking time and (3:1) A:O phase ratio (Fig. 4b). The data exposed that the U(VI) and V(V) extraction was progressively increased from 48.44 and 41.61 to 99.41 and 92.99%, respectively, with increasing the concentration of cetylpyridinium carbonate from 0.001 to 0.012 M/L. Further, with an increase in the extractant concentration up to 0.030 M/L, the extraction efficiencies had not added any perceptible effect and displayed a plateau due to the excess of the free extractant. Hence, the 0.012 M/L (CP)2CO3 was the optimum concentration for the subsequent experiments.
To confirm that the compositions of the extracted complexes were dependent on the (CP)2CO3 to U(VI) and V(V) concentration ratios, the extraction of U(VI) and V(V) at pH 11 and pH 9, respectively, depended on their concentrations which were studied in the carbonate solution. log10D is the logarithm of the distribution ratio of U(VI) and V(V) extraction. Plotting the log10D which is the logarithm of distribution ratio of V(V) or U(VI) extraction versus the logarithm of (CP)2CO3 concentration at equilibrium condition was applied. The linear plot could explain the reaction mechanism between (CP)2CO3 and uranium or vanadium ions in the carbonate medium (Fig. 4c), it was shown R2 linear correlation (0.92 and 0.93) with a slope of 1.73 and 1.02 of U(VI) and V(V) that indicated the requirement of two moles and one mole of (CP)2CO3 for each one mole of uranyl and vanadium complexes.
Contact time
The effect of contact time was evaluated by varying the time from 2 to 20 min during the extraction of uranium (413 mg/L, pH 11) and vanadium (336 mg/L, pH 9) from the alkaline leach solution using the studied cetylpyridinium carbonate ((CP)2CO3), and other parameters were constant at 0.012 M/L (CP)2CO3 and 5% (w/v) of TDA in kerosene and 3:1 aqueous to organic phase ratio at room temperature. An increase in agitation time results in more contact inside the two phases; the mass transfer would be increased. The obtained results in Fig. 4d revealed that the U(VI) at pH 11 and V(V) at pH 9 extraction increased with increasing agitation time until 10 min, and subsequently extraction efficiencies were fixed. Hence, 10 min contact time was enough to achieve the maximum extractions. Therefore, the next operations were offered by shaking the two phases for 10 min.
Effect of modifier
The effect of tridecanol (TDA) concentration as modifier to prevent the third phase formation on the U(VI) and V(V) extractions (413 mg/L U(VI) at pH 11 and 336 mg/L V(V) at pH 9) was achieved in the concentration range from 1 to 10% (w/v) TDA at 0.012 M/L of (CP)2CO3 in kerosene, and (3:1) A:O phase ratio for 10 min shaking time at room temperature (Fig. 4e). The data exposed that the U(VI) and V(V) extractions were progressively increased from 43.24 and 9.55 to 99.25 and 35.65% at pH 11 with increasing the concentration of TDA from 1 to 5%, respectively. Further, with an increase in the modifier concentrations up to 10%, the extraction efficiencies had not recorded any perceptible effect and gave a plateau at pH 11. However, the U(VI) and V(V) extractions were gradually increased from 5.13 and 35.11 to 22.26 and 92.99% at pH 9 with increasing the concentration of TDA from 1 to 5%, respectively. Further, with an increase in the modifier concentrations up to 10%, the extraction efficiencies had not added any noticeable effect and exhibited a plateau at pH 9. Hence, the 5% TDA as modifier was the optimum concentration on the subsequent tests for U(VI) and V(V) extraction.
Effect of aqueous:organic phase ratio
Phase ratio (A:O) is an essential strategy that influences the extraction efficiency. The impact of A:O rate on U(VI) extraction at pH 11 and V(V) extraction at pH 9 was investigated in the range 7:1–1:4 using 0.012 M/L (CP)2CO3 and 5% (w/v) of TDA in kerosene for 10 min contact time at room temperature. The aqueous solutions assaying 413 mg/L U(V1) at pH 11 and so 336 mg/L V(V) at pH 9 were mixed with the organic phase and then it was followed by the separation of the two phases in which the uranium and vanadium concentrations were determined in the aqueous solution, and the obtained results are shown in Fig. 4f. From the established data, it was fairly directed that the maximum extraction efficiencies relatively remained constant at 3:1 until 1:4 A:O ratio. Therefore, the 3:1 A:O rate was the optimum phase ratio for the two metal ions.
Extraction isotherm
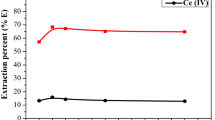
At equilibrium, only metallic complexes anions were partially transferred from aqueous to the cationic organic phase. Consequently, several stages of contact should be used to recover the maximum values of metallic species. In this study, McCabe–Thiele constructions were performed to determine the number of theoretical stages required for achieving the separation of U(VI) at pH 11 and V(V) at pH 9. The equilibrium curves were generally gotten by shaking of different ratios of aqueous leach liquor and the suitable organic solvent. In this work, the corresponding McCabe–Thiele diagrams were then constructed using a 3:1 A:O volume ratio for U(VI) at pH 11 and/or V(V) at pH 9. The numbers of stages were revealed in Fig. 5a, b. From the attained data, it was evident that three stages were quite adequate to practically saturate the organic phase, and to run down the mother leach liquor of U(VI) at pH 11, while five stages were somewhat passable to soak the organic phase virtually and to run down the leach liquor of V(V) at pH 9 on (CP)2CO3 extractant with 3:1 A:O ratio.
a McCabe–Thiele diagram for U(VI) extraction using (CP)2CO3 extractant (413 mg/L U(VI), pH 11, 0.012 M/L (CP)2CO3 and 5% TDA in kerosene, 10 min contact time), b McCabe–Thiele diagram for V(V) extraction using (CP)2CO3 extractant (336 mg/L V(V), pH 9, 0.012 M/L (CP)2CO3 and 5% TDA in kerosene, 10 min contact time), c influence of temperature on U(VI) and V(V) extractions using (CP)2CO3 extractant at pH 11 and pH 9 (413 mg/L U(VI) and 336 mg/L V(V), 0.012 M/L (CP)2CO3 and 5% TDA in kerosene, 3:1 A:O phase, 10 min contact time), and d plot of logD contrasted with 1/T of U(VI) and V(V) extractions using (CP)2CO3 at pH 11 and pH 9 (413 mg/L U(VI) and 336 mg/L V(V), 0.012 M/L (CP)2CO3 and 5% TDA in kerosene, 3:1 A:O phase, 10 min contact time)
Influence of temperature
The influence of temperature on U(VI) extraction at pH 11 and V(V) extraction at pH 9 was studied in the range 25–55 °C using the above optimum conditions. From the obtained results plotted in Fig. 5c, it was found that the U(VI) and V(V) extractions were decreased with increasing temperature from 25 to 55 °C. Thus, the maximum uranium and vanadium extraction efficiencies on the prepared (CP)2CO3 in kerosene were 99.21 and 93.17%, respectively, which are equivalent to 0.012 M/L (8.028 g (CP)2CO3/1 L organic solvent). In other words, the investigational uranium extraction capacity on (CP)2CO3 extractant was 153.3 mg(U)/g (extractant) at pH 11 while the practical vanadium extraction capacity on (CP)2CO3 extractant was 116.96 mg (V)/g (extractant) at pH 9. However, the extraction capacities of (CP)2CO3 were equivalent to 153.3 g uranium ions per 1 kg of (CP)2CO3 extractant and 116.96 g vanadium ions per 1 kg of (CP)2CO3 extractant. Accordingly, the room temperature is the most reliable extraction temperature.
Thermodynamic studies
Several experiments were applied to manage the analogous thermodynamic parameters of the examined methods. The solvent extraction behaviors were set by the reaction temperature, either endothermic or exothermic, and it may slow down or accelerate the reaction. The metal ion extraction with the organic solvent requires significant variations in enthalpy and entropy, managing to significant temperature influences (Mortimer 2008; Cheira et al. 2018).
The U(VI) at pH 11 and V(V) at pH 9 extractions on (CP)2CO3 were employed by varying the temperature from 298 to 328 K, while the other influences were constant at the above conditions. The deviations of solvent extraction data with temperature for uranium and vanadium extractions were utilized to appraise the thermodynamic parameters that comprise of standard free energy (∆G°, kJ/mol), enthalpy (∆H°, kJ/mol), and entropy (∆S°, J/(mol · K)). These parameters were measured using Van’t Hoff equations (Mortimer 2008) as follows:
where D is the distribution coefficient, R is the universal gas constant (8.314 J/(mol · K)). The corresponding data of logD against 1/T for uranium and vanadium extractions were straight lines that have correlation coefficients of 0.99 and 0.96, respectively (Fig. 5d). Consequently, the quantities of both the ΔH° and the ΔS° were spontaneously assessed from the −ΔH°/2.303R and ΔS°/2.303R of the design, respectively. The obtained values of ΔG°, ΔH°, and ΔS° for the U(VI) and V(V) extractions are listed in Table 2. The obtained data indicated that the negative value of the ΔG° validated the spontaneous and the feasibility nature, and the negative value of ΔH° exhibited the exothermic of extraction processes while the negative value of ΔS° implied an improvement in randomness through the U(VI) and V(V) extraction, and it also supported the stability of the extracted complexes by chelation operation.
Scrubbing vanadium from the uranium-loaded (CP)2CO3
The organic solvent consisting of 0.012 M/L (CP)2CO3 and 5% (w/v) tridecanol (TDA) was loaded with uranium (413 mg/L) and vanadium (336 mg/L) from their leach liquor using an A:O ratio of 3:1 at pH 11, 10 min contact time and room temperature. The loaded (CP)2CO3 contained 1.23 g/L U and 0.359 g/L V. So, attempts to scrub vanadium from uranium to vanadium-loaded (CP)2CO3 by different concentrations of scrubbing agents that were NaOH (0.1–1.2 M/L), Na2CO3 (0.1–1.2 M/L), and mixture of Na2CO3 (0.1–1.2 M/L) and 0.1 M/L NaOH, besides mixture of NaOH (0.1–1.2 M) and 0.1 M/L Na2CO3 were only partially successful at 1:4 A:O ratio, 10 min shaking time and 25 °C (Table 3). The best results were 1 M/L Na2CO3 + 0.1 M/L NaOH, which removed 95.3% of the vanadium (and 1.73% of the uranium) in a single contact at an aqueous to the organic ratio of 1:4. However, the residual vanadium contamination of the extract was 1.32% vanadium ions based on uranium ions in the loaded organic solvent after scrubbing processes.
To study the effect of the A:O phase ratios on V(V) scrubbing from the uranium-loaded (CP)2CO3 in kerosene (0.359 mg/L V), various experiments of scrubbing were checked at A:O ratios varying from 1:20 to 5:1 a mixture of 1 M/L Na2CO3 + 0.1 M/L NaOH as scrubbing agent for 10 min contact at 25 °C. From the data in Fig. 6a, it was assumed that the maximum scrubbing of vanadium was achieved at a 1:4 A:O ratio and remained constant after that. Hence, vanadium was completely scrubbed from the loaded (CP)2CO3 in kerosene (95.3%) with no significant loss of uranium. The McCabe–Thiele diagram of vanadium scrubbing was constructed (Fig. 6b). The 99% vanadium ions were scrubbed using A:O ratio of 1:4, and only 1.73% U(VI) were lost into the loaded scrubbing liquor. It was indicated that using an A:O ratio of 1:4, the co-extracted 0.359 g/L V in the loaded (CP)2CO3 was wholly removed by two stages of scrubbing.
Uranium stripping exploration
Stripping agent concentration
The organic solvent after scrubbing was loaded with 1.209 g/L of uranium ions that existed in the extract as uranium–carbonate quaternary complexes along with excess quaternary carbonate and/or bicarbonate. Uranium (VI) was stripped very effectively from the solvent with HNO3 and HCl solutions, and a mixture of sodium carbonate + sodium hydroxide solution. Sulfuric acid, sodium carbonate, and sodium hydroxide solutions were considerably less efficient stripping agents. To investigate the concentrations of stripping agents, the HNO3, HCl, H2SO4, sodium hydroxide, sodium carbonate, and mixture of sodium carbonate + sodium hydroxide with different concentrations were examined from 0.1 to 1.5 M/L while the other stripping conditions were fixed at 1:4 A:O ratio for 10 min contact time. From the data provided in Table 4, for the treatment of uranium-loaded solvent with HNO3, or HCl, the uranium and carbonate ions were displaced with nitrate or chloride:
The stripping efficiencies of uranium (VI) from 0.012 M/L (CP)2CO3 were 99.51% for 1 M/L HNO3 and 98.25% for 1 M/L HC1 (Table 4). In contrast, the stripping efficiency of uranium (VI) for 1.2 M/L H2SO4 was only 76.23%. Although the stripping efficiency of U(VI) was very effective with nitric and hydrochloric acids solutions, but the processes use of these reagents seemed unfortunate because of the formation of quaternary nitrate or chloride (cetylpyridinium nitrate or chloride) that required to be regenerated and converted to the carbonate forms before recycling to avoid serious interferences from nitrate or chloride in the extraction steps of uranium, whereas the removal of the nitrate and chloride from their cetylpyridinium complexes by carbonate was difficult. Hence, the stripping methods were more useful with alkaline solutions.
With 1 M/L Na2CO3, the stripping efficiency of uranium, 65.37%, was obtained from 0.012 M/L cetylpyridinium carbonate + 5% TDA in kerosene at 1:4 A:O ratio for 10 min contact time. With NaOH, uranium ions were stripped from cetylpyridinium carbonate and simultaneously precipitated as sodium diuranate by contact with caustic solutions (Table 4). At 1 M/L NaOH, U(VI) stripping efficiency (78. 98%) was not complete even after several successive contacts with fresh strip solution, and most of the uranium did not precipitate but remained in solution. The precipitate showed little tendency to collect at the organic–aqueous interface or to cause emulsions but settled fairly rapidly in the aqueous solution.
With sodium carbonate + sodium hydroxide solutions, uranium ions (94%) was stripped by a 10 min contact of uranium-loaded cetylpyridinium carbonate with 1 M/L Na2CO3 + 0.5 M/L NaOH at an aqueous:organic phase ratio of 1:4 while uranium ions (99%) was stripped (as sodium diuranate precipitate) with 0.5 M/L Na2CO3 + 1 M/L NaOH (Table 4). In the mixture solutions containing 0.5 M/L Na2CO3 + 1.0 − 1.5 M/L NaOH, the precipitation of stripped uranium ions was > 99% complete. In all tests, the phases separated in 10 min, with the precipitate settling fairly fast in the aqueous solution. The stripping and precipitation reactions can be expressed as the following:
Hence, the 0.5 M/L Na2CO3 + 1 M/L NaOH mixture gave the maximum uranium stripping and precipitated of about 99.25% from the loaded cetylpyridinium carbonate.
Stripping contact time
The effect of contact time on U(VI) stripping efficiency from the loaded cetylpyridinium carbonate in kerosene was studied in the range from 1 to 20 min at the fixed conditions of 0.5 M/L Na2CO3 + 1 M/L NaOH and 1:4 A:O ratio at room temperature. From the data in Fig. 7a, it was pointed out that the stripping efficiency of uranium ions increased from 65.17 to 99.31%, with increasing the contact time from 1 to 10 min. After that, a contact time beyond 10 min did not perceptibly affect the U(VI) stripping and precipitation from the loaded extractant. Thus, a 10 min shaking time could be reflected adequately for accessible stripping and precipitation of uranium (as sodium diuranate).
a Effect of contact time on U(VI) stripping and precipitated from the loaded extractant (0.5 M/L Na2CO3 + 1 M/L NaOH, 1:4 A:O ratio, room temperature), b effect of A:O phase ratio on U(VI) stripping from the loaded cetylpyridinium carbonate (0.5 M/L Na2CO3 + 1 M/L NaOH, 10 min contact time, room temperature), and c McCabe–Thiele diagram for U(VI) stripping and precipitating from the loaded extractant (0.5 M/L Na2CO3 + 1 M/L NaOH solution, 10 min contact time, room temperature)
Effect of aqueous to organic ratio
To study the influence of the A:O phase ratios on U(VI) stripping from U-loaded 0.012 M/L cetylpyridinium carbonate mixed with 5% TDA in kerosene, several experiments of stripping were checked at A:O ratios varying from 1:20 to 3:1 at 0.5 M/L Na2CO3 + 1 M/L NaOH as stripping mixture for 10 min contact time. From the data in Fig. 7b, it was clear that the maximum stripping was realized at 1:4 A:O ratio and remained constant thereafter.
Stripping isotherm of uranium
The number of theoretical stripping stages required for stripping and precipitating of U(VI) from the loaded 0.012 M/L cetylpyridinium carbonate was checked by mixing with 5% TDA in kerosene; a series of stripping experiments were applied at the above optimum conditions. The obtained data were used to construct the corresponding McCabe–Thiele stripping diagram plotted to obtain the equilibrium isotherm and proper operating line (Fig. 7c). From the gained data, it would seem that the probability for the number of precipitated uranium ions could be attainable via two countercurrent stages and 1:4 A:O ratio with 0.5 M/L Na2CO3 + 1 M/L NaOH solution.
Vanadium stripping examination
Effect of stripping concentration
The organic solvent after extraction at pH 9, 0.012 M/L cetylpyridinium carbonate + 5% (w/v) TDA in kerosene was loaded with 0.938 g/L of vanadium and 0.2 mg/L uranium. Vanadium ions existed in the loaded extract as a vanadate–cetylpyridinium complex associated with overflowing quaternary carbonate and uranium tricarbonate–cetylpyridinium complexes. To check the effect of stripping agent concentration on the V(V) stripping, according to the previous study of uranium stripping, it was truly demonstrated that the mixture of sodium carbonate and sodium hydroxide as a stripping agent was applied for V(V) stripping. For this purpose, different concentrations of sodium carbonate from 0. 1 to 1.5 M/L mixed with 0.3 M/L NaOH were selected and examined while the other stripping conditions were constant at 1:4 A:O ratio for 10 min contact time. From the obtained results given in Fig. 8a, it was clear that 1 M/L Na2CO3 + 0.3 M/L NaOH solution provided the maximum vanadium stripping efficiency of about 96.5% from the loaded cetylpyridinium complexes.
a Effect of sodium carbonate concentration on U(V) stripping from the loaded extractant (0.3 M/L NaOH, 1:4 A:O ratio, 10 min contact time, room temperature), b stripping contact time on V(V) stripping efficiency from the loaded cetylpyridinium complexes (1 M/L Na2CO3 + 0.3 M/L NaOH, 1:4 A:O ratio, room temperature), c effect of A:O phase ratio on V(V) stripping efficiency from the loaded cetylpyridinium complexes (1 M/L Na2CO3 + 0.3 M/L NaOH, 10 min contact time, room temperature), and d McCabe–Thiele diagram for V(V) stripping from the loaded cetylpyridinium complexes (1 M/L Na2CO3 + 0.3 M/L NaOH, 10 min contact time, room temperature)
Stripping contact time
The effect of stripping time on V(V) stripping efficiency in the range from 1 to 20 min was studied by 1 M/L Na2CO3 + 0.3 M/L NaOH solution from the loaded 0.012 M/L cetylpyridinium carbonate mixed with 5% (w/v) TDA in kerosene at the fixed conditions of 1:4 A:O ratio at room temperature. From the data revealed in Fig. 8b, it was indicated that the stripping efficiency of vanadium increased from 74.22 to 98.5%, with increasing the contact time from 1 to 10 min. Afterward, a contact time beyond 10 min did not perceptibly increase the V(V) stripping efficiency from the loaded extractant beyond 98.5%. Thus, a 10 min shaking time would be considered sufficient for quantitative stripping.
Effect of aqueous to organic ratio
To evaluate the influence of the A:O phase ratios on V(V) stripping efficiency from the loaded cetylpyridinium complexes in kerosene, various experiments of stripping were tested at A:O ratios varying from 1:20 to 3:1 at 1 M/L Na2CO3 + 0.3 M/L NaOH mixture as stripping agent for 10 min contact time. From the data in Fig. 8c, it was given that the supreme stripping efficiency (96.5%) was reached at 1:4 A:O ratio, and afterward, it remained constant.
Stripping isotherm for vanadium
To set the number of theoretical stripping stages required for V(V) stripping from the loaded cetylpyridinium complexes in kerosene, a series of stripping trials were employed at the preceding optimum settings. The gained data were utilized to construct the identical McCabe–Thiele stripping diagram for calculating the acquired equilibrium isotherm and to which a suitable working line was built in Fig. 8d. From the latter, it might be supposed that the possibility of quantitative stripping steps of V(V) could be possible through two countercurrent theoretical steps and an A:O ratio 1:4 with 1 M/L Na2CO3 + 0.3 M/L NaOH solution mixture for 10 min stripping time.
Extractant regeneration and reusability
For the regeneration and reusability of the studied cetylpyridinium carbonate after extraction–stripping processes, the used organic phase was contacted by the same volume of 1 M/L sodium carbonate solution for 25 min followed by equal volume de-ionized water. The phases were separated, and the pH of the aqueous solution was determined. The obtained organic phase was also contacted with the repeated number of contacts using fresh de-ionized water up to there was no change in the pH of the separated water. The organic phase (cetylpyridinium carbonate in kerosene) was then reused for extraction.
Uranium and vanadium ion recovery
Based on the results as mentioned earlier for uranium and vanadium extraction and stripping, the applicability of the prepared (CP)2CO3 organic extractant mixed with TDA in kerosene for U(VI) and V(V) extraction was applied on carbonate leach solution that was obtained from 5 kg ferruginous siltstone sample using 15 L of 70 mg/L Na2CO3, 150 mg/L NaHCO3, and 1:3 S:L ratio, 5 h leaching time, − 80 mesh size, and 150 rpm at 50 °C. The attained leach liquor was found to assay 413 mg/L U(VI) and 336 mg/L V(V), indicating leaching efficiencies of 92.92% and 94.82%, respectively. From the obtained data in Table 5, the leach liquor contained diverse concentrations of associated ions. Therefore, it was recommended that the leach liquor required further purification or addition of some masking agents to obtain a high grade of the studied metal ions.
The best optimum parameters of U(VI) and V(V) extraction and stripping were applied on carbonate leach liquor. The extraction process was carried out on 15 L of the prepared leach liquor which contained 413 M/L U(VI) (6.195 g total content of uranium ions in the aqueous solution), 336 M/L V(V) (5.04 g entire content of vanadium ions in the aqueous solution) and their associated ions using 5 L of 0.012 M/L (CP)2CO3 in kerosene at the optimum conditions of pH 9 for vanadium and/or pH 11 for uranium of aqueous solutions for 10 min shaking time.
From the obtained data, the U(V) content on the (CP)2CO3 mixed with 5% (v/v) TDA in kerosene was 6.15 g uranium ions in 5 L organic phase (40.01 g of (CP)2CO3 in 5 L in kerosene) after three stages while the V(V) content on the (CP)2CO3 in kerosene was 4.7 g vanadium ions in 5 L organic phase (40.01 g of (CP)2CO3 in 5 L in kerosene) after five stages. The working uranium loaded organic solution (5 L) was stripped and precipitated by agitation with 1.25 L of 0.5 M/L Na2CO3 + 1 M/L NaOH solution for 10 min contact time at room temperature by two stages. Sodium diuranate (Na2U2O7 · xH2O) was gained and precipitated. The uranium precipitate was dried at 110 °C to attain the final product, which weighed 8.19 g. Otherwise, the working vanadium-loaded organic solution (5 L) was stripped using 1.25 L of 1 M/L Na2CO3 + 0.3 M/L NaOH for 10 min contact time at room temperature by two stages. The vanadium content was found to assay 4.7 g V ions in 1.25 L aqueous phase. The vanadium precipitation process was carried out by stirring of the 0.5 L (30%) ammonium chloride solution for 2 h to precipitate vanadium as NH4VO3 · xH2O (11.25 g) which was dried at 110 °C to obtain ammonium vanadate (NH4VO3) that was weighed 9.55 g.
The obtained sodium diuranate and ammonium vanadate cakes were also identified using the scanning electron microscope (SEM), and their corresponding EDX spectrum (Figs. 9 and 10). Furthermore, they were quantitatively examined using the ICP-OES technique to determine their chemical constituents of the sodium diuranate and ammonium vanadate cakes (Table 6). According to the obtained data, the uranium and vanadium contents were found to assay 71.23 and 53.65%.
Conclusion
The existing examinations had recorded in the resignation of a useful technique for alkaline leaching, extraction, and separation of U(VI) and V(V) using the prepared cetylpyridinium carbonate extractant for making more powerful extraction capacities under several performing conditions. The ferruginous siltstone was subjected to a selective alkali leaching agent for uranium and vanadium. The optimum leaching parameters were 70 mg/L Na2CO3, 150 mg/L NaHCO3, 1:3 S:L ratio, 5 h leaching time, 150 rpm stirring rate, − 80 mesh size, 50 °C for dissolving about 92.92% of the uranium (VI), and 94.82% of vanadium (V). Using these parameters, a stock leach liquor assaying 413 mg U/L and 336 mg V/L was prepared for U(VI) and V(V) extraction via the solvent extraction by cetylpyridinium carbonate as an extractant in kerosene. More than 99% of uranium extraction was obtained using 0.012 M/L (CP)2CO3 and 5% (w/v) TDA in kerosene from a carbonate leach solution containing 413 mg/L U at pH 11. Furthermore, the maximum vanadium extraction (93.17%) was achieved using 0.012 M (CP)2CO3 and 5% (w/v) TDA in kerosene from a carbonate leach solution containing U 336 mg/L V at pH 9 and room temperature. The extraction capacities of (CP)2CO3 were equivalent to 153.3 g uranium ions per 1 kg (CP)2CO3 extractant at pH 11 and 116.96 g vanadium ions per 1 kg of (CP)2CO3 extractant at pH 9.
Moreover, the V(V) was scrubbed from working U(VI)-loaded (CP)2CO3 using scrubbing mixture 1.0 M/L Na2CO3 + 0.1 M/L NaOH, 10 min contact, 1:3 A:O phase ratio, and 25 °C. After the scrubbing process, the U(VI) was stripped from U(VI)-loaded (CP)2CO3 by precipitation as sodium diuranate using a mixture of 0.5 M/L Na2CO3 + 1 M/L NaOH, 1:4 A:O ratio, 10 min stirring time, and room temperature. Additionally, the V(V) was also stripped from V(V) loaded (CP)2CO3 using a mixture 1 M/L Na2CO3 + 0.3 M/L NaOH, 10 min contact time, and 1:4 A:O ratio. Extraction, scrubbing, and stripping stages were evaluated by adopting the McCabe–Thiele diagram. Furthermore, the negative value of ΔG° validated the spontaneous and the feasibility nature while a negative value of ΔH° exhibited the exothermic of extraction processes and also the negative value of ΔS° implied the improvement in randomness through the extraction of U(VI) and V(V) ions. The suggested technique for U(VI) and V(V) extraction using (CP)2CO3 in kerosene is easy to use, simple, and selective for the proper extraction and separation of U(VI) and V(V) from carbonate leach liquor with high efficiencies.
References
Altmaier M, Vercouter T (2012) Aquatic chemistry of the actinides: aspects relevant to their environmental behavior. In: Poinssot C, Geckeis H (eds) Radionuclide behaviour in the natural environment science, impacts and lessons for the nuclear industry, vol 42. Woodhead Publishing Limited, Oxford, pp 44–69. https://doi.org/10.1533/9780857097194.1.44
Anumula R, Nookaraju M, Selvaraj K (2013) A novel vanadium n-propylamino phosphate catalyst synthesis, characterization and applications. Mater Res 16(1):181–189. https://doi.org/10.1590/S1516-14392012005000161
Atia BM, Gado MA, Cheira MF (2018) Kinetics of uranium and iron dissolution by sulfuric acid from Abu Zeneima ferruginous siltstone, Southwestern Sinai, Egypt. Euro-Mediterr J Environ Integr 3:39. https://doi.org/10.1007/s41207-018-0080-y
Avelar ÉC, Alvarenga CLG, Resende GPS, Morais CA, Mansur MB (2017) Modeling of the solvent extraction equilibrium of uranium (vi) sulfate with Alamine 336. Braz J Chem Eng 34(1):355–362. https://doi.org/10.1590/0104-6632.20170341s20150301
Cheira MF (2015) Synthesis of pyridylazo resorcinol—functionalized amberlite XAD-16 and its characteristics for uranium recovery. J Environ Chem Eng 3:642–652. https://doi.org/10.1016/j.jece.2015.02.003
Cheira MF, Atia BM, Kouraim MN (2017) Uranium(VI) recovery from acidic leach liquor by Ambersep 920U SO4 resin: kinetic, equilibrium and thermodynamic studies. J Rad Res Appl Sci 10:307–319. https://doi.org/10.1016/j.jrras.2017.07.005
Cheira MF, Orabi AS, Atia BM, Hassan SM (2018) Solvent extraction and separation of thorium(IV) from chloride media by a schiff base. J Solut Chem 47:611–633. https://doi.org/10.1007/s10953-018-0740-1
Du-Preez JGH (1989) A review of industrial processes involving uranium-from the ore to the reactor. Rad Prot Dosim 26:7–13. https://doi.org/10.1093/oxfordjournals.rpd.a080375
Edwards CR, Oliver AJ (2000) Uranium processing: a review of current methods and technology. JOM 52(9):12–20. https://doi.org/10.1007/s11837-000-0181-2
El Aassy IE, El Galy MM, Nada AA, El Feky MG, Abd El Maksoud TM, Talaat SM, Ibrahim EM (2011) Effect of alteration processes on the distribution of radionuclides in uraniferous sedimentary rocks and their environmental impact, Southwestern Sinai, Egypt. J Radioanal Nucl Chem 289:173–184. https://doi.org/10.1007/s10967-011-1059-1
El Mezayen AM, Abu Bakr MA, Sherif HMY, El Nahas HA, Ali HH (2016) Geology and radioactivity of the basement rocks of wadi El-Sahu area, Southwestern Sinai, Egypt. Greener J Geol Earth Sci 4(1):1–22. https://doi.org/10.15580/GJGES.2016.1.021716041
El-Rayes AE, Arnous MO (2015) A novel approach in hydrogeochemical exploration for uranium mineralization: an example from West Central Sinai, Egypt. Acta Geologica Sinica 89:1895–1913. https://doi.org/10.1111/1755-6724.12606
El-Sheikh EM, El Aassy IE, Abdel-Rahman A, Ayad MI, Fathy WM, Taha MN, Kassab WA (2017) Recovery of uranium and associated elements from ferruginous gibbsite bearing shale of Dabbet Abu Thor locality, SW Sinai, Egypt. Intern J Adv Res 5(12):1445–1459. https://doi.org/10.21474/IJAR01/6105
Gajda D, Kiegiel K, Zakrzewska-Koltuniewicz G, Chajduk E, Bartosiewicz I, Wolkowicz S (2015) Mineralogy and uranium leaching of ores from Triassic Peribaltic sandstones. J Radioanal Nucl Chem 303:521–529. https://doi.org/10.1007/s10967-014-3362-0
Gorman-Lewis D, Burns PC, Fein JB (2008) Review of uranyl mineral solubility measurements. J Chem Thermodyn 40:335–349. https://doi.org/10.1016/j.jct.2007.12.004
Guerra EM, Cestarolli DT, Da Silva LM, Oliveira HP (2010) Synthesis, characterization and electrochemical behavior of the vanadium pentoxide/cetyl pyridinium chloride hybrid material. J Solid State Electrochem 14:305–312. https://doi.org/10.1007/s10008-009-0877-3
Guzman ETR, Regil EO, Malagon GP (1995) Uranium leaching from phosphate rock. J Radioanal Nucl Chem 201:313–320. https://doi.org/10.1007/BF02164050
Hashad AH, Afifi SY, Cheira MF, Ghonaim AKH (2011) Extraction and determination of vanadium and its application on geological samples with 8-hydroxy quinolone. Arab J Nucl Sci Appl 44:27–42. https://inis.iaea.org/search/searchsinglerecord.aspx?recordsFor=SingleRecord&RN=42108080
Hussein GM, Muhammad SS, Gomaa NA, Shehata MR, Hosny WM (2017) A study on the extraction of uranium(VI) from sulphate leach liquor using LIX63. J Disp Sci Tech 38:866–875. https://doi.org/10.1080/01932691.2016.1214837
James D, Venkateswaran G, Rao TP (2009) Removal of uranium from mining industry feed simulant solutions using trapped amidoxime functionality within a mesoporous imprinted polymer material. Microporous Mesoporous Mater 119:165–170. https://doi.org/10.1016/j.micromeso.2008.10.011
Jeffery GH, Bassett J, Mendham J, Denney RC (1995) Vogel’s textbook of quantitative chemical analysis, 5th edn. John Wiley and sons Inc., New York
Khanramaki F, Shirani AS, Safdari J, Torkaman R (2017) Equilibrium and kinetics of uranium(VI) extraction from a sulfate leach liquor solution by Alamine 336 using single drop technique. Chem Eng Res Des 125:181–189. https://doi.org/10.1016/j.cherd.2017.07.026
Khorfan S, Wahoud A, Reda Y (2001) Recovery of vanadium pentoxide from spent catalyst used in the manufacture of sulphuric acid. Period Polytech Ser Chem Eng 45:131–137
Kumar JR, Kim JS, Lee JY, Yoon HS (2011) A brief review on solvent extraction of uranium from acidic solutions. Sep Purif Rev 40:77–125. https://doi.org/10.1080/15422119.2010.549760
Li M, Zheng S, Liu B, Wang S, Dreisinger DB, Zhang Y, Du H, Zhang Y (2017) A clean and efficient method for recovery of vanadium from vanadium slag: nonsalt roasting and ammonium carbonate leaching processes. Min Proc Ext Metal Rev 38:228–237. https://doi.org/10.1080/08827508.2017.1288117
Long S, Feng Q, Zhang G, He D (2014) Recovery of vanadium from alkaline leaching solution from roasted stone coal. Sci Asia 40:69–72. https://doi.org/10.2306/scienceasia1513-1874.2014.40.069
Lozano LJ, Godı́nez CC (2003) Comparative study of solvent extraction of vanadium from sulphate solutions by Primene 81R and alamine 336. Miner Eng 16:291–294. https://doi.org/10.1016/S0892-6875(03)00009-8
Marczenko Z, Balcerzak M (2000) Separation, preconcentration and spectrophotometry in inorganic analysis. Elsevier Science B.V, Amsterdam, p 521
Marshall N, Brett CE (2016) Siltstone: a key to cyclicity in mixed siliciclastic–carbonate successions: example from upper ordovician kope formation (Ohio, Indiana, Kentucky). Can J Earth Sci 53(8):823–835. https://doi.org/10.1139/cjes-2015-0207
Menis O, Iyer CSP (1971) Spectrophotometric determination of vanadium and iron with β isopropyltropolone. Anal Chim Acta 55:89–95. https://doi.org/10.1016/S0003-2670(01)82744-6
Mir-mohammadi SL, Mallah MH, Torkaman R, Safdari J (2018) A new study on uranium (VI) stripping from loaded alamine 336 using some of alkaline solutions. Prog Nucl Energy 103:229–235. https://doi.org/10.1016/j.pnucene.2017.12.001
Mortimer RG (2008) Physical chemistry, 3rd edn. Elsevier Inc., London, p 1092
Moyer BA (2017) Ion exchange and solvent extraction: a series of advances, vol 19, 1st edn. CRC Press, Boca Raton, p 673. https://doi.org/10.1201/9781420059700(First published 2009)
Nayl AA, Aly HF (2015) Solvent extraction of V(V) and Cr(III) from acidic leach liquors of ilmenite using Aliquat 336. Trans Nonferrous Met Soc China 25:4183–4191. https://doi.org/10.1016/S1003-6326(15)64021-3
Reiller PE, Marang L, Jouvin D, Benedetti MF (2011) Uranium (VI) binding to humic substances: speciation, estimation of competition, and application to independent data. In: Merkel B, Schipek M (eds) The new uranium mining boom, challenge and lessons learned. Springer, Berlin, pp 565–572. https://doi.org/10.1007/978-3-642-22122-4
Rodriguez-Morales S, Compadre RL, Castillo R, Breen PJ, Compadre CM (2005) 3D-QSAR, synthesis, and antimicrobial activity of 1-alkylpyridinium compounds as potential agents to improve food safety. Eur J Med Chem 40:840–849. https://doi.org/10.1016/j.ejmech.2005.02.012
Senol A (2014) Optimization of extractive removal of uranium(VI) from aqueous acidic solutions using commercial amines: linear solvation energy relation based modeling. Sep Purif Tech 131:35–49. https://doi.org/10.1016/j.seppur.2014.04.034
Sole KC, Cole PM, Feather AM, Kotze MH (2011) Solvent extraction and ion exchange applications in Africa’s resurging uranium industry: a review. Sol Ext Ion Exch 29:868–869. https://doi.org/10.1080/07366299.2011.581101
Xiao-teng Z, Dong-mei J, Yi-qun X, Jun-chang C, Shuai H, Liang-shu X (2019) Adsorption of uranium(VI) from aqueous solution by modified rice stem. J Chem. https://doi.org/10.1155/2019/6409504
Shapiro L, Brannock WW (1975) Rapid analysis of silicate, carbonate and phosphate rocks. US Geol Surv Bull 1144A:1–56. https://doi.org/10.3133/b1144A
Ye G, Hu Y, Tong X, Lu L (2018) Extraction of vanadium from direct acid leaching solution of clay vanadium ore using solvent extraction with N235. Hydrometallurgy 177:27–33. https://doi.org/10.1016/j.hydromet.2018.02.004
Zhang G, Chen D, Zhao W, Zhao H, Wang L, Li D, Qi T (2016) A novel synergistic extraction method for recovering vanadium (V) from high-acidity chloride leaching liquor. Sep Purif Tech 165:166–172. https://doi.org/10.1016/j.seppur.2016.04.008
Zhu Z, Pranolo Y, Cheng CY (2013) Uranium solvent extraction and separation from vanadium in alkaline solutions. Sep Sci Tech 48:1402–1408. https://doi.org/10.1080/01496395.2012.738277
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cheira, M.F. Solvent extraction of uranium and vanadium from carbonate leach solutions of ferruginous siltstone using cetylpyridinium carbonate in kerosene. Chem. Pap. 74, 2247–2266 (2020). https://doi.org/10.1007/s11696-020-01073-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-020-01073-w