Abstract
The solvent extraction behavior of cobalt and lithium from mixed sulfate solution using di(2-ethylhexyl)phosphoric acid (D2EHPA)/kerosene as extractant system has been investigated. The effect of different process parameters such as pH of feed solution, extractant concentration, cobalt and lithium ion concentrations in the feed solution have been studied. Extraction equilibrium constants have been calculated and found to be log Kex Co = –2.01 and log Kex Li = –2.42. The highest separation factor of 292 was achieved using 1.59 M D2EHPA at pH 1.85 from mixed sulfate solution. 93.9% of cobalt and 11.4% lithium was co-extracted from 0.01 M cobalt and lithium sulfate solution. Extraction of cobalt-lithium by D2EHPA is affected by cation exchange mechanism, cobalt is extracted as [Co(HA2)2] and [CoA2] depending on the metal concentration in the feed solution while the lithium is extracted as [Li(A2H)]. Quantitative extraction of cobalt was achieved in two-stage counter-current batch extraction (with McCabe–Thiele plot) using 0.477 M D2EHPA at an O : A phase ratio of 1. The data obtained from loaded organic and raffinate indicate a composition which reveals nearly complete extraction of cobalt and rejection of lithium resulting significant separation of these elements from mixed sulfate solution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
High power lithium-containing batteries are extensively used as electrochemical power sources in modern-life equipments. Lithium-ion batteries have been widely used in all portable electronic applications [1]. From the viewpoints of environmental concerns recovery of major valuable components for the provision of raw materials, the recycling of spent LIBs is highly desirable [2]. Recovery of cobalt and lithium is one of the primary objectives in the recycling of spent LIBs [3] because cobalt is a precious rare metal, and is a relatively expensive material compared with the other constituents of LIBs, and lithium is also vitally important in many industrial applications. Several processes for the recovery of cobalt and lithium from spent lithium-ion batteries have been developed [4–7]. The leach solution contains large amount of cobalt, lithium and small amount of nickel, aluminium and iron. The demand for higher purity metals and recent trends towards environmental friendly technology have drawn attention to solvent extraction which appears to meet the requirements for performance and economics to replace the conventional separation processes [8]. Several studies have been carried out to develop suitable solvent extraction separation of cobalt and lithium with Cyanex 272 (bis(2,4,4-trimethylpentyl)phosphinic acid) as extractant [9–13]. Though these processes have significant advantages like low energy consumption and good separation effect. But it still has the disadvantage like operational conditions and expensive solvents affect the economics of the process when it is scaled-up in industry. Therefore choosing appropriate and economical solvent extractants would become the most important in order to decrease the treatment cost [14]. Bis(2-ethylhexyl)phosphoric acid (better known as D2EHPA) has been reported to be an effective extractant in the hydrometallurgical processes for the separation and purification of a number of metals, due to its chemical stability and high selectivity [15, 16]. Moreover, it is cheap, commercially available and well-known for the extraction of cobalt [17, 18]. The use of synergic solvent extraction technique consisting of D2EHPA and MEHPA (mono-2-ethylhexylphosphoric acid) with TBP (tri-n-butylphosphate), respectively, to extract lithium from geothermal spring water consisting of Na+, K+, Mg2+, and Ca2+ was reported by Hano [17].
Zhang [18] suggested a hydrometallurgical process for the separation and recovery of cobalt and lithium from spent lithium ion batteries in chloride medium. This process consists of acid leaching of lithium cobalt oxide, separation of cobalt from the leach liquor by solvent extraction, and precipitation of lithium carbonate. However, the detailed literature survey suggests that limited works are reported on work on the separation of cobalt and lithium from sulfate medium solutions generated during recycling of spent lithium ion batteries employing D2EHPA/kerosene extraction system. Hence, a detailed, planned and systematic study was carried out to improve the knowledge about the solvent extraction separation of cobalt and lithium from their mixed sulfate solutions by D2EHPA. The effects of pH of the feed solution, extractant concentration, metal ions concentrations, and acid concentration on the stripping of cobalt have been investigated. At this end of this work, a novel viewpoint is proposed to recovery the lithium from actual leach solution in order to decrease the treatment cost.
THEORY
The mechanism of metal ion (Mn+) is extraction from an aqueous solution using D2EHPA was largely described by Ritcey et al. [19]. This extracting agent contains two functional groups: hydroxy (–OH) and phoshoryl (P=O) and thus behaves as liquid cation exchanger. According to Ritcey, the extraction at the higher metal loading is expressed as follows:
where HA represents D2EHPA. At low metal loading in the organic phase, metal extraction affected as indicated in following equation:
The equilibrium constants (Kex) is expressed as follow:
The distribution coefficient D is defined as:
Taking logarithms of both sides, using the relation pH = −log[H+] and re-arranging equation (4) takes the form as indicated below:
Analyzing the experimental value of distribution ratio D as a function of equilibrium pH and extractant concentration at constant value of either parameters allows estimation of the number of extractant molecules associated with the extracted metal complex:
and
The separation factor (β) is calculated using following equation:
where \({{D}_{{{{{\text{M}}}_{1}}}}}\) is the distribution coefficient of the more extractable metal ion and \({{D}_{{{{{\text{M}}}_{2}}}}}\) is the distribution coefficient of the comparatively less extractable metal.
EXPERIMENTAL
Solutions and Reagents
Di(2-ethylhexyl)phosphoric acid (D2EHPA) obtained from Fluka AG, Switzerland was used as received. Stock solutions of 1.0 M cobalt(II) and 1.0 M lithium(I) sulfate were prepared by dissolving suitable amounts of analytical grade reagents CoSO4 · 7H2O and Li2SO4 · H2O in ultrapure water. The synthetic aqueous solutions containing cobalt and lithium were used for each solvent extraction by diluting the stock solutions to the desired concentration and mixing them properly. Kerosene (bp 180–270°C) and tri-n-butylphosphate (TBP) from Fluka were used as a diluent and phase modifier, respectively for all sets of experiments. TBP was added at 1 vol % concentration in the organic solution. The addition of TBP as a modifier to D2EHPA can improve phase separation. The stock solutions of D2EHPA organic phase were prepared by diluting D2EHPA in kerosene at a predetermined weight to volume ratio. Aqueous and organic phases are assumed to be immiscible, so no volume variation is considered in the model.
Batch Extraction Studies
The synthetic aqueous feed phase (10 mL) containing cobalt(II) and lithium(I) was equilibrated with an equal volume of D2EHPA in kerosene in glass flasks using a mechanical shaker at 25.0 ± 0.2°C. The equilibration time of 15 min was found to be sufficient to attain the equilibrium which was verified in preliminary tests, which showed that even about 5 min shaking is sufficient to attain the equilibrium. The initial pH of the aqueous phase was controlled by adding dilute H2SO4 or NH4OH before equilibration. The mixture was then transferred to a separating funnel and allowed to settle for at least 1hour for a complete phase separation. Then, after separation of both phases, the equilibrium pH and metal concentrations in the aqueous phase were measured.
Analytical Methods
The concentrations of cobalt in the aqueous phases is measured by atomic absorption spectrophotometry AAS, Varian Spectra-400 after suitable dilution, while the concentrations of lithium is determined by AAS, Perkin Elmer 2380. Matrix problems by AAS measurement were negligible. Cobalt and lithium in the organic phases were stripped with sulfuric acid, and the metal concentration in the acidic solutions was analyzed by AAS. The process was proved to be quantitative; the mass balance for the metal was always checked with the extraction-stripping procedure and found to be less than 3%. The pH values of aqueous solutions were measured with HANNA pH meter 211. The extraction efficiency or extractability (%) and metal distribution coefficient were calculated using standard formulas.
RESULTS AND DISCUSSION
Effect of Equilibrium pH
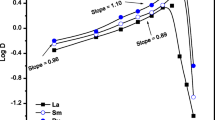
The effect of equilibrium solution pH on the extraction of cobalt and lithium was evaluated by changing the pH feed while the concentration of extractants and metals ions are kept constant, 0.477 and 0.01 M, respectively. Figure 1 shows the effects of the pH on the distribution ratios (D) of the metal ions. Straight lines with different slopes as per the valences of the metal ions suggest that the extraction progresses via a cation exchange mechanism. D2EHPA possess high extractability for Co2+ compared to Li+. The log D vs. equilibrium pH for cobalt is linear in nature and having slope close to 2. It could be presumed from Eq. (2) that the extraction of cobalt occurs through the exchange of 2 mol of H+ with 1 mol of cobalt. The slope of the log D vs. equilibrium pH for lithium is linear nature as shown in Fig. 1. The slope for lithium shows the association of 0.72 mol of extractant with 1 mol of lithium.
(a) pH equilibrium dependency of log D of cobalt and lithium, organic phase: 0.477 M D2EHPA, 1 vol % TBP; (b) plot of log D vs. log[D2EHPA], organic phase: 0.095–1.59 M D2EHPA, 1 vol % TBP ; 1 and 2—cobalt at low and high loading organic phase respectively, 3—lithium; aqueous phase: [Co2+] = 0.01 M; [Li+] = 0.01 M.
Effect of Extractant Concentration
Figure 1 shows the effect of D2EHPA concentration on the extraction of cobalt and lithium from mixed solution of sulfate media. The D2EHPA concentration varies from 0.095 to 1.59 M. The cobalt extraction yield increases fast to reach 100% with the extractant concentration above an equal to 0.477 M. The extraction of lithium starts to increase appreciably with an extractant concentration higher than 0.3 M due to the decrease in effective concentration of cobalt in the aqueous phase. The loading capacity of 0.477 M D2EHPA is 58.9 g/L for cobalt and 1.59 g/L for lithium at in these conditions. The separation factors β were calculated and is also plotted in Fig. 2. The separation factor increases with respect to increase in extractant concentration. The highest separation factor of 292 is achieved with 1.59 M D2EHPA at equilibrium pH 1.85. Cobalt is quantitavely extracted from mixed sulfate solution in one step. The extractant concentration has a significant effect on the separation factor and it is clearly demonstrated that D2EHPA is suitable for cobalt-lithium separation. The results suggest the effect of the lithium co-extraction can be minimized by using a relatively large extractant concentration for a small metal concentration.
Figure 2 shows the effect of extractant concentration, log[D2EHPA] on the distribution ratio, log D. The plots are straight lines with slopes range from 2.08 to 2.3 for the high and low loading, respectively. The extraction equilibrium of cobalt is expressed by the following equation:
This structure was based on slope analysis. The extracted species was suggested to be 1 : 2 metal : ligand complex at low loading in the pH range. Whereas at higher metal loading, the available evidence supports the view that extraction occurs according to the following equation:
which suggests that the effects of dimerization of the D2EHPA are apparent only with that low metal concentration and that maybe the dimerization effect are not significant as the metal loading increasing [17].
In the case of the lithium ion extraction, straight line with slope of 0.85 was obtained whatever the concentration of D2EHPA. The extraction equilibrium of this metal ion is expressed by the following equation:
Table 1 presents the distribution ratios (D), the extraction equilibrium constants (logKex), the corresponding extraction yields (E, %) and the equilibrium constants. The value of the distribution ratio (D) of cobalt is high >100 for 1 : 1 phase ratios. The equilibrium constants for the extraction of cobalt from sulfate media with D2EHPA found in the literature ranged between –2.96 [19] and –5.40 [20]. In the case of lithium, logKex = –2.42 was obtained. It should be noted this value is close to that obtained by Hanol et al. [19]. However, it is evident that the values of logKex changes with the aqueous compositions or/and the organic diluents [21].
Effect of Metal Ion Concentration
Figure 3 shows the effect of the initial cobalt sulfate concentration on the separation cobalt–lithium from mixed sulfate solutions. The experiments were carried out using 0.477 M of DEHPA at initial pH of 5.93. The concentration of cobalt ranges between 0.001 and 0.4 M and the lithium sulfate concentration is constant at 0.01 M. It is observed that the concentration of cobalt in the organic phase increases quickly with increasing cobalt concentration in the aqueous phase. However, with further increasing cobalt concentration in the aqueous phase to 0.07 M, the increase of cobalt concentration in the organic phase is limited and becomes constant because the cobalt–D2EHPA complex is saturated in the organic phase. In the case of lithium, the concentration in the organic phase decreases with the increase of cobalt concentration in the aqueous phase and the extraction of lithium sulfate becomes insignificant. This may be attributed to the better selectivity of cobalt by D2EHPA. The extraction of cobalt is independent of the lithium concentration in the aqueous phase within the range tested. Table 2 shows the separation factor calculated from the experimental results shown in Fig. 4. For cobalt, the β value increases reaching the highest value 179 for a cobalt concentration equal to 0.005 M and decreases as the concentration of cobalt increases in the feed solution. At cobalt concentration higher than 0.01 M, β value decreases with the increase of cobalt concentration in feed solution. The decrease of separation factor is due to the decrease in free extractant availability which led to decrease in percentage cobalt extraction.
(a) Effect of concentration of cobalt on separation extraction of cobalt and lithium. Aqueous phase: [Co2+] = 0.001–0.4 M, [Li+] = 0.01 M, pH 5.93; (b) effect of concentration of lithium on separation extraction of cobalt and lithium, aqueous phase: [Co2+] = 0.01 M, [Li+] = 0.001–0.4 M; organic phase: 0.477 M D2EHPA, 1 vol % TBP, pH 5.93.
Figure 3 shows the effect of the initial lithium concentration on the separation cobalt–lithium in mixed sulfate solutions. The experiments were carried out using 0.477 M of DEHPA at initial pH of 5.93. The concentration of sulfate lithium ranges between 0.001 and 0.4 M while the cobalt sulfate concentration is constant at 0.01 M. The lithium concentration in the organic phase increased with increasing lithium concentration in the aqueous phase while the concentration of cobalt in the organic phase was found unaffected by the variations in lithium concentration in the feed solution. This result suggests that cobalt could not be separated using D2EHPA in mixed solution of cobalt and lithium sulfate when the concentration of lithium is higher than that of cobalt in feed solution. This indicates that the step of separation of lithium sulfate with D2EHPA could be carried out only with a feed solution which have high concentration of cobalt that of lithium. Thus it is applicable to the solution generated by recycling of spent lithium ion batteries.
Cobalt and Lithium Purification
Extraction isotherm and stripping. An extraction isotherm was obtained for a feed solution containing 0.01 M cobalt and lithium using 0.477 M D2EHPA in kerosene as an extractant and the results are depicted in the Fig. 4. The McCabe–Thiele plot for a feed solution containing 0.01 M of cobalt indicated two stages at a phase ratio O/A = 1 for the complete extraction of cobalt under these conditions. In order to achieve quantitative extraction of cobalt, a two-stage counter-current batch simulation study was carried out using 0.477 M D2EHPA at an O : A phase ratio of 1. Representative results for loaded organic and raffinate obtained have the compositions shown in Table 3 indicating nearly complete separation of cobalt and lithium.
It should be noted that the effect of acid concentration in the range 0.09–0.72 M on the stripping of cobalt from the cobalt and lithium-loaded organic phase has also been investigated using H2SO4. Sufficient amount of loaded organic was generated from mixed sulfate solution containing cobalt and lithium of 0.4 M (23.572 g/L) at an equilibrium pH 1.93 using 0.477 M D2EHPA in kerosene. The resulting organic solution contained 21 g/L cobalt. Cobalt stripping studies at Organic : Aqueous (O : A) ratio of 2 : 1 were carried out. Figure 5 shows that the stripping of cobalt increased with H2SO4 concentration from 0.018 M (7.25%) to 0.54 M (98.75%); 0.54 M H2SO4 achieves for a quantitative stripping of cobalt.
Process flowsheet for recovery of cobalt and lithium. Based on the above data and previous studies, a process flowsheet can be proposed in the recycling of a actual sulfate leaching solution containing Co2+, Fe3+, Li+, and Al3+ with pH 3. Pranolo et al. [13] show a mixed solvent extractant system containing Ionquest 801 and Acorga M5640 and the Cyanex 272 organic system can be used in a first solvent extraction system in order to extract all iron and aluminium. The raffinate would contain only cobalt and lithium. The specificity of this novel viewpoint is to recovery directly the sulfate lithium in aqueous phase with the extraction of cobalt–D2EHPA–TBP in a second step. In this case, the use of ion-exchange resins, which generate solid waste, can be avoided [22, 23]. The process flowsheet is shown in Fig. 6.
CONCLUSION
The solvent extraction separation cobalt and lithium from mixed sulfate solution using di(2-ethylhexyl)phosphoric acid (D2EHPA) as extractants in kerosene has been investigated with the following results.
–Extraction equilibrium constants have been calculated, log Kex Co = –2.01 and log Kex Li = –2.42. Extraction of cobalt-lithium by D2EHPA involves cation exchange mechanism, cobalt is extracted as [Co(HA2)2] and [CoA2] versus the metal concentration while the lithium as [Li(A2H)].
–The highest separation factor of 292 was achieved with 1.59 M D2EHPA at equilibrium pH 1.85 from mixed sulfate solution of 0.01 M cobalt and lithium. About 93.9% of cobalt was extracted with 11.4% lithium co-extraction at this condition.
–The extraction of cobalt is independent of the lithium concentration in the aqueous phase within the range tested. The cobalt could not be separated using D2EHPA in mixed solution of cobalt and lithium sulfate when the concentration of cobalt is higher than that of lithium in feed solution.
–The McCabe–Thiele extraction isotherm for the extraction of cobalt reveals that two stages are required for quantitative extraction of cobalt. A simulation study shows a nearly complete separation of cobalt (94%) and a total rejection of lithium (99.9%).
This investigation is a preliminary step to obtain necessary information before studying the extraction using membrane contactors.
REFERENCES
C. K. Lee and K. I. Rhee, Hydrometallurgy 68, 537 (2003).
S. M. Shin, N. H. Kim, J. S. Sohn, D. H. Yang, and Y. H. Kim, Hydrometallurgy 79, 172 (2005).
M. Contestabile, S. Panero, and B. Scrosati, J. Power Sources 92, 65 (2001).
P. Zhang, T. Yokoyama, O. Itabashi, T. M. Suzuki, and K. Inoue, Hydrometallurgy 47, 259 (1998).
C. K. Lee and K. I. Rhee, J. Power Sources 109, 17 (2002).
D. S. Kim, J. S. Sohn, C. K. Lee, K. I, K. S. Han, and Y. I. Lee, J. Power Sources 132, 145 (2004).
J. M. Nan, D. M. Han, M. J. Yang, M. Cu, and X. L. Hou, Hydrometallurgy 47, 75 (2006).
B. Swain, J. Foeng, J. C. Lee, and G. H. Lee, Hydrometallurgy 84, 130 (2006).
B. Swain, J. Jeong, J. C. Lee, G. H. Lee, and J.-S. Sohna, J. Power Sources 167, 536 (2007)
Y. Pranolo and C. Y. Cheng, CSIRO Minerals Report No. DMR 2598 (2005), p. 1.
B. Swain, J. Jeong, J.-C. Lee, and G. H. Lee, Sep. Purif. Technol. 63, 360 (2008).
B. Swain, J. Jeong, K. Yoo, and J. C. Lee, Hydrometallurgy 101, 20 (2010)
Y. Pranolo, W. Zhang, and C. Y. Cheng, Hydrometallurgy 102, 37 (2010)
J. Xu, H. R. Thomas, R. W. Francis, K. R. Lum, and J. W. B. Liang, J. Power Sources 177, 512 (2008).
H. Svedsen, G. Schei, and M. Osman, Hydrometallurgy 25, 197 (1990).
G. L. Tulasi and S. Kumar, AIChE J. 45, 2534 (1999).
T. Hano, M. Matsumoto, T. Ohtake, N. Egashira, and F. Hori, Solvent Extract. Ion Exchange 10, 195 (1992).
P. Zhang, T. Y. Osamu, I. Tshishige, M. Suzuki, and K. Inoue, Hydrometallurgy 47, 259 (1998).
G. M. Ritcey and A. W. Ashbrook, Solvent Extraction, Principles and Applications to Process Metallurgy, Part 1 (Elsevier, Amsterdam, 1984).
M. L. Brisk and W. J. McManamey, J. Appl. Chem. 19, 103 (1969).
R. S. Juang and J. Y. Su, Ind. Eng. Chem. Res. 31, 239 (1992).
W. P. C. Duyvesteyn, D. A. Neudorf, and E. M. Weenink, US Patent No. 6350420B1 (2002).
P. Zhang, T. Yokoyama, T. M. Suzuki, and K. Inoue, Hydrometallurgy 61, 223 (2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamina Boukraa Extraction of Cobalt and Lithium from Sulfate Solution Using Di(2-ethylhexyl)phosphoric Acid/Kerosene Mixed Extractant. Russ. J. Phys. Chem. 94, 1136–1142 (2020). https://doi.org/10.1134/S0036024420060321
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024420060321