Abstract
Preparation, structural elucidation, Hirshfeld surface analysis, thermal analysis and impedance spectroscopy study are carried out for an interesting organic–inorganic hybrid compound (NH2C5H3ClNH)2ZnBr4·H2O. X-ray diffraction analysis reveals that the title compound belongs to the triclinic crystallographic system with the space group P-1 with Z = 2 and the following unit cell dimensions: a = 7.3209(9) Å, b = 8.4407(10) Å, c = 16.923(2) Å, α(°) = 82.975(6), β(°) = 85.118(6) and γ(°) = 74.398(6). The crystal structure is composed of two protonated 4-amino-2-chloropyridinium cations, tetrabromozincate anions and a water molecule which are held together by a number of hydrogen bonds forming infinite chains. In addition, crystal structure is stabilized with π…π interactions (Cg–Cg and Zn-Cl…Cg, with Cg is the 4-amino-2-chloropyridinium rings) which have also been investigated in terms of their corresponding Hirshfeld surface and the breakdown of fingerprint. To quantify intermolecular interactions in crystal lattice, FT-IR spectroscopy has been used to distinguish the different chemical functional groups and their environments in this molecule. The dielectric conductivity of this compound has been measured in the temperature range 298–438 K and the frequency range 209 Hz–5 MHz. The analysis of the experimental data of the impedance spectroscopy based on the activation energy shows that this material is an ionic–protonic conductor at low temperature and becomes an electronic one at high temperature. This work aims to reveal the thermal properties of a new zinc (II) based organic–inorganic hybrid and the conductivity properties that this compound exhibits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over decades of chemical research, preparations and characterizations of organic–inorganic metal-based bivalent cations are carried out by several researchers throughout the world attempting to combine desirable physical properties of both organic and inorganic components within a single molecular-scale composite. Among these compounds, we mention hybrids with cations. These compounds have attracted the attention of researchers and whetted their interest, since they exhibit different kinds of phase transitions generally related to the motion of the alkyl ammonium cations (Karâa et al. 2010, 2013). Moreover, halogenozincate compounds are essentially associated with their potential applications and interesting electrical properties (Chihaoui et al. 2016a, b, 2017).
The organic divalent zinc chloride has several applications in various domains such as catalysis, biochemistry, dielectric transition and magnetism material science (Chihaoui et al. 2016a, b, 2017). In addition, the study of such zinc-based materials has received much attention recently in the light of photovoltaic and multifunctional properties. Besides, the organization of the interactions in the crystal packing plays a major role to advance diverse applications in industry. For this reason, we attempt to seek a novel structural topologies and properties that can be useful in the field of luminescence and semiconductivity (Chihaoui et al. 2016a, b, 2017).
Having the motivations stated above and encouraged by this pioneering work, we attempt to present a novel hybrid compound: (4-amino-2-chloropyridinium)2 tetrabromozincate (II) monohydrate, (NH2C5H3ClNH)2ZnBr4·H2O. A detailed interpretation of the structural geometry of the crystal has been displayed. Further processes (Hirshfeld surface analyses) have been used to investigate the various contacts and the intermolecular interactions in the crystal packing analysis, which are derived by single-crystal XRD data and spectroscopic technique (FT-IR). Besides, thermal analysis (DSC and TGA) and careful examination of ionic conductivity of the compound have been performed. The electrical features of the material have been studied using complex impedance spectroscopy.
Experimental section
Synthesis and crystallization
All reagents were obtained commercially and used without further purifications. The first step of the preparation of the material was the preparation of 4-amino-2-chloropyridiniumbromide ((NH2C5H3ClNH)+, Br−) solution which was formed through the addition of an aqueous solution of HBr (10 ml) to 4-amino-2-chloropyridine (NH2C5H3ClN) (2.5 mmol). The dried precipitates were then washed with diethyl ether to remove unreacted substances. The stoichiometric amounts 2:1 of the resulting solution [(NH2C5H3ClNH)+, Br−] and ZnBr2 (1.25 mmol) were mixed with excess of HBr. The slow evaporation is the method used to obtain transparent crystals. The mixture was stirred and kept at room temperature for several days. Needle like transparent crystals were obtained a week later. A single crystal suitable for X-ray diffraction analysis was selected and studied.
Crystallography
A suitable single crystal with dimensions of 0.42 × 0.39 × 0.20 mm3 for (NH2C5H3ClNH)2ZnBr4·H2O was selected for single-crystal X-ray diffraction analysis. The intensity data for the single crystal compound investigations was collected at 293(2) (K) using BRUKER AXS (Kappa APEX II-CCD area detector). The sample was selected under polarising microscope and examined by oscillation method. The intensity data was collected with Graphite monochromated MoKα radiation (λ = 0.71073 Å). The Zinc, Bromide and Chloride atoms were located by Patterson methods using SHELXS-97 (Sheldrick 1997). However, the organic entities were found from successive Fourier calculations and refined through the full-matrix least-squares method using SHELXL-97 (Sheldrick 1997), implemented in the WinGX (Farrugia 1999) software package. Crystal parameters and details of data collection are presented in Table 1. The final atomic coordinates obtained from the single crystal refinement with Ueq are displayed in table S1. Anisotropic thermal displacement parameters were used to refine all non-hydrogen atoms, shown in table S2. Hydrogen atoms of the organic ring and water were fixed at their ideal positions. The final geometrical calculations and the graphical manipulations were carried out with ORTEP (Farrugia 1997), MERCURY (Spek 2003) and DIAMOND (Brandenburg 1998) programs. Selected bond lengths and angles are exhibited in Table 2. Data for the structure of (NH2C5H3ClNH)2ZnBr4·H2O can be obtained free of charge from the Cambridge Crystallographic Data Center http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Center, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk), with CCDC reference numbers 1577208 containing the supplementary crystallographic data for this paper.
Hirshfeld surfaces calculations
To further understand the atomic interactions in the crystal structure, we have determined the Molecular Hirshfeld surfaces (Spackman and McKinnon 2002) which are constructed on the basis of electron distribution calculated as the sum of spherical atom electron densities (Spackman and Byrom 1997). The CrystalExplorer 3.1 program (Wolff et al. 2012) was used to construct the three-dimensional Hirshfeld surfaces as well as the fingerprint plots.
The dnorm (normalized contact distance) is a symmetric function of distances which are the nearest nuclei internal and external to the Hirshfeld surface (di and de, respectively), relative to their respective van der Waals radii of the atoms internal or external to the surface (r vdwi /r vdwe ) which identifies the regions of particular importance to the intermolecular interactions given by the following equation:
with r vdw i and r vdw e being the van der Waals radii of the respective atoms which are internal and external to the molecular surface.
In the color scale, negative values of dnorm are detected through the red color, indicating contacts shorter than the sum of van der Waals radii. The white color denotes intermolecular distances close to van der Waals contacts with dnorm equal to zero. In turn, contacts longer than the sum of van der Waals radii with positive dnorm values are indicated by blue. Two colored properties (shape-index and curvedness) based on the local curvature of the surface can be specified (Koenderink and van Doorn 1992).
Thermal analysis
The DSC calorimetric measurement was carried out using a Perkin Elmer PYRIS 6 DSC calorimeter, by putting a powder sample (about 9.06 mg) in an aluminum capsule. The DSC was performed with a heating rate of 5 K/min up to 430 K under constant nitrogen flow.
Thermogravimetric analysis (TGA) was carried out using an ATG PYRIS 6 instrument with a heating rate of 5 K/min up to 430 K under a nitrogen atmosphere.
FT-IR spectroscopy
The infrared absorption spectrum (FT-IR) (Fig. 4) (spectrum in red) of the crystallized powder (NH2C5H3ClNH)2ZnBr4·H2O, was recorded on a Nicolet Impact 410 spectrometer in the 4000–400 cm−1 range at room temperature. The measurement is carried out on a pellet containing 95% of KBr and 5% of powder.
Electrical impedance spectroscopy
The impedance spectroscopy was performed on pellet discs of about 8 mm diameter and about 1.6 mm in thickness. Electrical impedances were measured in the frequency range from 209 Hz to 5 MHz with the TEGAM 3550 ALF automatic bridge over a temperature range of (298–438 K).
Results and discussion
Structure description
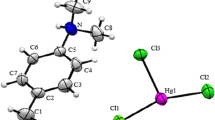
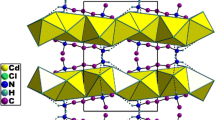
The structure of a new compound was developed in the centro-symmetric space group P-1 with (Z = 2), with the following unit cell dimensions: a = 7.3209(9) Å, b = 8.4407(10) Å, c = 16.923(2) Å, α(°) = 82.975(6), β(°) = 85.118(6), γ(°) = 74.398(6) and v = 998.2(2) Å3. The asymmetric unit of the structure drawn with 50% probability thermal ellipsoids and exposed in Fig. 1 shows the ORTEP drawing of the asymmetric unit together with an atom numbering scheme (Farrugia et al. 1997). As it can be noticed, the asymmetric unit of the title compound comprises two crystallographically independent organic cations denoted 4-amino-2-chloropyridinium [(NH2C5H3ClNH)+(I) and (NH2C5H3ClNH)+(II)] protonated cations ligand, one [ZnBr4]2− anion and one uncoordinated water molecule. The hybrid organic–inorganic compound (NH2C5H3ClNH)2ZnBr4·H2O naturally forms a periodic zero-dimensional structure which consists of alternating layers of organic and inorganic corrugated sheets stacked along b and a axis, as shown in Fig. 2. The graphical representation is provided with Diamond (Brandenburg 1998).
The zinc atom is tetrahedrally coordinated by bromine atoms; present in a slight distorted tetrahedral geometry. These slight distortions are usually determined by the angles Br–Zn–Br, varying between 106.52(4) and 111.50(4). The Zn–Br bond lengths are in the range 2.4003(10)–2.4197(11) Å. A full list of bond lengths and angles is given in Table 2. The bond distances and angles fall in the range of those reported previously for compounds containing Zn–Br anions (Gao et al. 2007; Chihaoui et al. 2016a, b, c).
The organic part consists of two organic cations; (NH2C5H3ClNH)+, each of which with an N ring site protonated, and carbon atoms building up the pyridine rings. These organic cations exhibit a regular spatial configuration with normal distances C–C, C–N and angles C–C–C, C–C–N and have a good co-planarity with similar compounds in our previous study results (Karâa et al. 2010, 2013). The N-pyridine atom with its hydrogen atoms lie in the plane of the ring. Both H atoms of the amino group and the N-pyridine are involved in hydrogen bonds.
Water oxygen, instead, forms two N–H…OH-bonds. The N–H…OH-bonding geometries are N…O distances from 2.9721 to 2.7798 Å and N–H…O angles from 158° to 178°. The number of H-bonds per formula is eight (Fig. 3; Table 3).
The structural analysis reveals that the stabilization of the whole supra-molecular assembly has been built up by two classes of contacts: the hydrogen bonds and π–π interactions.
All hydrogen bondings are summarized in Table 3. A packing diagram (Fig. 3) of (NH2C5H3ClNH)2ZnBr4·H2O structure shows the presence of various hydrogen bonds between the organic cation and the bromine atoms of the inorganic part which are linked by classic bonds (N–H…Br) involving the hydrogen atoms of NH (pyridin) and NH2 (amine group). Other classical hydrogen bonds (C–H…Br) relate the carbon atom (α carbon: linked to the N-pyridine atom) to the bromine atoms of [ZnBr4]2−. It can be noted from Fig. 3 that water molecules play a significant role in stabilizing the crystal structure. First, they are connected to [ZnBr4]2− tetrahedral by O–H…Br bonding. Second, they are attached to the cationic part by N–H…O contact.
There are π–π stacking interactions between the 4-amino-2-chloropyridinium rings (Gillon et al. 2000, Ninković et al. 2012). The analysis of ring-interactions using PLATON (Table 4; Fig. 4) (Spek 2003), shows that the rings’ layers are parallel in the crystal connected with van der Waals forces. Stacking parameters which are taken into account are dCg1–Cg1 and dCg2–Cg2 (centroid–centroid distance), α (angle between planes containing aromatic rings), slipping angles β and γ (defined by the vector Cg–Cg and the normal to planes [Cg1 corresponding to (NH2C5H3ClNH)+(I)] and [Cg2 representing (NH2C5H3ClNH)+(II)]. Data of Table 4 reveals that (NH2C5H3ClNH)+ rings fall parallel (α = 0.00°) at 3.6673–5.1439 Å. However, there are four interactions ring–ring π–π between the (4-amino-2-chloropyridinium) of the title compound (Fig. 4) (Wheeler 2012). On the other side, data in Table 4 shows possibilities for such M–X…π stacking lone pair of X (bromide) and 4-amino-2-chloropyridinium rings, with X…Cg [(NH2C5H3ClNH)+(I)] distances of 3.8520 Å (Fig. 4; Table 4).
Hirshfeld surface analysis
In the present research, we used not only Hirshfeld surfaces and 2D fingerprint plots, but also Crystal Explorer (Spackman and Jayatilaka 2009), to identify intermolecular interactions and the environments of the components in the unit cell (two organic molecules, one isolated water molecule and one [ZnBr4]2− anion).
The study of the cationic environment by Hirshfeld surface analysis
The Hirshfeld dnorm surface, shape index, curvedness and the 2D fingerprint plots of the cationic part in the (NH2C5H3ClNH)2ZnBr4·H2O compound are depicted in Fig. 5. All interactions involving cationic parts in the crystal structure are displayed by the 2D fingerprint plots in Figs. 6 and 7. These analyses reveal the presence of different types of intermolecular interactions. It can be inferred that the dominant interactions are H…Br (25.1%), which are responsible for the appearance of deep red spots in the dnorm scheme. They are identified by N–H…Br and C–H…Br hydrogen bonds in the crystal lattice, where the molecules stand as donors (de > di). The second largest percentage (18.7%) can be attributed to the H…H interactions. Additionally, it is interesting to note that intermolecular H…Cl/Cl…H interactions are not detected by X-ray analysis but through Hirshfeld surface analysis, which accounts for 14.2% of the organic cation (intramolecular interactions). The contribution of H…N/N…H, H…O and H…Cl/Cl…H contacts to the total Hirshfeld surface is relatively small; 4.6, 3.8, and 14.2%, respectively.
The H–H interactions, appearing in the middle of scattered points in the 2D fingerprint map, comprise 18.7% of the total Hirshfeld surfaces. Nevertheless, they are obvious, because the majority of the surface of this molecular crystal is covered by H atoms. This proves that van der Waals interactions exert an important influence on the stabilization of the crystal lattice packing. We can also report the presence of H…C/C…H close contacts, which appear in the middle in the 2D fingerprint plot comprising 13.6% of the total Hirshfeld surface of the cationic molecules.
The presence of the adjacent red and blue triangles on the shape index surface (surrounded by a black circle in Fig. 5b demonstrates the presence of π…Br and π–π stacking interactions, which exist between two pyridin rings of the cationic part [NH2C5H3ClNH]+. This result has been confirmed by X-ray crystal structural analysis (Fig. 4).
Analysis of Hirshfeld surface shows the interactions between N…Br and C…Br with the percentages 1.5% and 1.3%, respectively of the Hirshfeld surface (Fig. 7). Again, very low percentages of N…C/C…N (3.6%) and C…C (4.1%) interactions are recorded in the molecule. Thus, π–π stacking interactions are not visible in the rotated dnorm map ([NH2C5H3ClNH]+(II)) owing to the corresponding C…C distances between pyridin rings which are longer than the sum of van der Waals radii, as it can be perceived in crystal structure determination.
The study of the anionic environment by Hirshfeld surface analysis
The Hirshfeld surface analysis of [ZnBr4]2− is displayed in Fig. 8, exhibiting the surfaces that have been mapped around the molecule by dnorm, shape index, curvedness and 2D fingerprint plots.
The decomposed fingerprint plots (Fig. 9) show that the intermolecular Br…H contacts present a major contribution to the anionic environment (80.4%), displaying a large circular depression on the dnorm surface. These contacts are visible in the fingerprint where one [ZnBr4]2− acts as an acceptor (di > de). Other visible spots in the Hirshfeld surfaces attributed to Br…Cl, are detected by a spike in the top left region of its corresponding fingerprint (de = 2.00Å, di = 2.00Å). Br…C and Br…N interactions participate in overall interactions with 2.5 and 2.9%, respectively.
The study of the water molecule environment by Hirshfeld surface analysis
Intermolecular contacts were analyzed around the water molecule in the asymmetric unit. The Hirshfeld surface analysis of the water molecule is depicted in Fig. 10, which is mapped by dnorm, shape index, curvedness and the 2D fingerprint plots. Figure 11 illustrates the decomposing of fingerprint plot. It is clear that H…H contacts are the most important interactions (30.9%), which refer basically to the abundance of hydrogen on the molecular surface. The H…O/O…H presents the next most abundant contacts. The proportion of these interactions comprises 28.5% of the total Hirshfeld surfaces for a water molecule.
The wings at the top left (di < de) correspond to the points on the surface around the O–H donor characteristic of O–H…Br. H…Cl interactions. The decomposition of the fingerprint plot shows that H…Br contacts correspond to 28.5% of the total Hirshfeld surface area for the molecule. The H…Cl interactions contribute to only 8.4% of the Hirshfeld surface.
Vibrational frequencies of the FT-IR spectroscopy
To gain more information on the crystal structure, an FT-IR spectroscopy study was performed at room temperature between 4000 and 400 cm−1 (2.5–25 μm). IR spectrum is shown in Fig. 12, and its corresponding assignments are depicted in Table 5. IR-spectroscopy has been successfully used in analysis and identification of the fundamental vibrations and associated rotational–vibrational structure. It is very difficult to attribute the vibration modes to organic and inorganic groups, the uses of analogous compounds are very necessary.
We used the spectrum of free 4-amino-2-chloropyridine [NH2C5H3ClN] when assigning the FT-IR vibrational frequencies of the organic groups of the title compound bis (4-amino-2-chloropyridinium) tetrabromozincate(II), (NH2C5H3ClNH)2ZnBr4·H2O.
Based on FT-IR studies performed on compounds having similarities with our compound (Karaa et al. 2010, 2012, 2013), different bands for the observed organic groups (N–H), (NH2) C–N, C–N–C and C–Cl groups were assigned. Figure 12 shows the FT-IR spectra of the two compounds at room temperature.
Vibration of NH2 group The asymmetric vibrations of NH2 groups are observed in FT-IR spectrum as weak bands in the range 3300 cm−1 and the symmetric stretching vibrations of NH2 groups are observed in FT-IR around 3196 cm−1 for the (NH2C5H3ClNH)2ZnBr4·H2O. The bands at 1646 cm−1 in FT-IR are assigned to NH2 scissoring mode and the NH2 twisting and wagging bands are observed in the range 990–552 cm−1.
Vibration of NH group In the FT-IR, it is also interesting to indicate that v(N–H) mode appears at 3196 cm−1 for (NH2C5H3ClNH)2ZnBr4·H2O (and not observed in [NH2C5H3ClN]). The δ(N–H) bending vibration of the N–H group appears at 1582 cm−1 for (NH2C5H3ClNH)2ZnBr4·H2O (and not observed in [NH2C5H3ClN]). This result confirms that C–N–C groups of 4-amino-2-chloropyridinium cation are protonated. The protonation of the pyridine ring produces dramatic changes in frequencies observed for bands originating from vibrations of ring.
Vibrations of water molecules
The band characteristics of water molecules exist at about 3758 and 3516 cm−1.
Vibrations of C–N, C–C, C–N–C and N–C–N groups Hence, in the present investigation, FT-IR bands observed at 1364 and 1414 cm−1 of (NH2C5H3ClNH)2ZnBr4·H2O are assigned to the side chain (C–N) stretching mode of vibrations. By referring to the above literature value (4-amino-2-chloropyridine), the bands that appear at 1528-1512 cm−1 in the FT-IR spectrum of the (NH2C5H3ClNH)2ZnBr4·H2O are assigned to stretching (C–C) vibrations. The C–N–C and C–C–C out-of-plane and C–N–C and C–C–C in-plane bending vibrations are strongly coupled vibrations and occur in the region between 552 and 436 cm−1 for the (NH2C5H3ClNH)2ZnBr4·H2O.
Impedance analysis
Impedance spectroscopy experiments were carried out in the frequency range between 209 Hz and 5 MHz at different temperatures 298 K ≤ T≤438 K. The impedance spectra for (NH2C5H3ClNH)2ZnBr4·H2O sample at several different temperatures are plotted in Fig. 13, elucidating the presence of a single semicircle arc, which indicates departure from the Cole–Cole behavior (Nefzi et al. 2013).
The variation of real (Z′) and imaginary (Zʺ) parts of impedance with frequency at various temperatures are depicted in Fig. 14a, b. In the higher frequency region (> 104Hz), the merging of real part of the impedance curves can be attributed to the exit of space charge (James and Srinivas 1999; Selvasekarapandian and Vijaykumar 2003). Furthermore, the frequency dependence of the imaginary (Zʺ) parts of impedance at several temperatures show that Zʺ values achieve a maximum (Zʺmax), shifting to higher frequencies with increasing temperature. This conductivity marks the presence of relaxation in the system.
Dielectric studies
The mechanisms of conductivity of the materials have been investigated using the dependence of the frequency on electrical conductivity. Besides, the dielectric relaxation studies are of extreme significance in terms of specifying the nature and the origin of dielectric losses, which in turn may be useful in the determination of the structure. The complex dielectric function is expressed as (Böhmer et al. 1993; Hodge et al. 2005):
where ε′ and εʺ are the real and imaginary parts of the dielectric constant, respectively.
Figure 15a, b show the variation of the real ε′ and imaginary εʺ parts of the dielectric constant as a function of frequency at several temperatures. It is clear from theses plots that the values of ε′ and εʺ decrease with increasing frequency at various temperatures, while they remain nearly constant at sufficiently high frequencies. Then, the high values at low frequencies can be attributed to the possible interface polarization mechanisms since interface states cannot follow the electric conductivity (ac) signal at high frequencies (Parlakturk et al. 2008; Park et al. 1998). Interface polarization reaches a constant value owing to the fact that beyond a certain frequency of the external field, the electron hopping cannot follow the alternative field. The resulting dispersions in ε′ and εʺ with frequency may be assigned to Maxwell–Wagner (Bidault et al. 1994) and space charge polarization (Fanggao et al. 1996).
Temperature dependence of the real part ε′ and the imaginary part εʺ of the dielectric constant are illustrated in Fig. 16a, b, which are measured at selected frequencies 1, 5, 10, 15 and 100 kHz. It is inferred from these evaluations, the presence of three anomalies localized at T = 328, 380 and 418 K, respectively. These evaluations confirm the results found by the simultaneous calorimetric study (DSC) and thermogravimetric analysis (TGA), which are reported in Fig. 17. The first peak signals the evaporation of water surface, while the second signal indicates the evaporation of water structure. The anomaly detected at T = 418 K may be associated with the phase transition (not detected in TGA), which is connected to the movement and the dynamic disorder of cationic parts. It should be also noted that the dielectric and thermal properties of our novel compound are very similar to those of the iso-structural compound (Karâa et al. 2013).
Conductivity analysis
The log–log plot of the electrical conductivity (σac) versus frequency at different temperatures of (NH2C5H3ClNH)2ZnBr4·H2O compound is exhibited in Fig. 18. Departing from this plot, we notice the presence of four distinct regimes. The σac(f) curves are found to be merging suggesting less defect mobility and low conductivity in the material. The phenomenon of the conductivity dispersion in the materials is generally analyzed using Jonscher’s power law (Jonscher 1999):
where σdc is the frequency independent conductivity which can be obtained by extrapolating the low frequency plateau to zero frequency, A is a constant that depends on temperature and determines the strength of polarization and n is an exponent less than or equal to unity which represents the degree of interaction between mobile ions and its surrounding lattices (Dyre and Schrøder 2000).
In Fig. 19, conductivity data (dc) is represented in Arrhenius format as ln(σdc) versus 1000/T showing Arrhenius-type behavior that is described by:
where B is the pre-exponential factor, Ea is the activation energy, and K is the Boltzmann’s constant. This curve indicates the presence of four linear regions on each side of the anomalies (T = 324, 380 and 416 K). This behavior is in agreement with the previously observed DSC curve. The values of the activation energies determined only in the two regions II and IV are 0.3 and 0.4 eV, respectively. Based on thermal analysis results (DSC and ATG) and referring to the bibliography (Weng et al. 2011; Mesbeh et al. 2016; Karâa et al. 2010; 2012), we realize that the first region in temperature range 298–324 K corresponds to the beginning of evaporation of water surface, which is activated as soon as the heating has started. Region II in the temperature range 324–380 K corresponds to the ionic–protonic conduction. The value of the activation energy results in the hopping of proton H+. Region III in the temperature range 380–416 K was detected also in the thermal property by TGA and DSC technical which could be accounted for the disappearance of the water molecule of the structure (construction water) and finally the region IV in the temperature range 416–438 K may refer to electronic conduction which will be more and more activated.
Conclusion
To sum up, in this research work a novel Zn(II) hybrid compound has been synthesized and structurally characterized. The most prominent features of our structure refer basically to the presence of zero-dimensional (0D) anionic network built up of organic parts and water molecules. Hirshfeld surface analysis shows different types of intermolecular interactions including hydrogen bonding, and π–π(Cg–Cg) stacking and Zn–Br…Cg interactions. This paper highlights the usefulness of the Hirshfeld analysis in terms of understanding the intricacies of interactions occurring in crystallized solids.
Differential scanning, calorimetric and electric measurements were carried out. They showed the evaporation of water surface at the temperature range 298–324 K and structural water structural (construction water) at the temperature range 380–416 K. The activation energy issued from the impedance spectra suggests an ionic–protonic conductivity for this material. It is assigned by the mechanism of hopping in the temperature range 416–438 K which may be due to electronic conduction which will be more and more activated.
The value of the activation energy reveals a significant electrical property of the molecule confirming that this material is a good candidate to superconductor applications in energy conversion, miniaturized sensors and imaging devices, solar cells, LED and detectors.
References
Böhmer R, Ngai KL, Angell CA, Plazek DJ (1993) Nonexponential relaxations in strong and fragile glass formers. J. Chem. Phys. 99:4201–4209. https://doi.org/10.1063/1.466117
Bidault O, Goux P, Kchikech M, Belkaoumi M, Maglione M (1994) Space charge relaxation in perovskites. J Phys Rev B 49:7868–7873. https://doi.org/10.1103/physrevb.49.7868
Brandenburg K (1998) Diamond version 2.0 impact GbR. Bonn, Germany
Chihaoui N, Hamdi B, Salah AB, Zouari R (2016a) A new mononuclear complex: structure, vibrational (FT-IR and Raman), Hirshfeld surfaces analysis, electrical properties and equivalent circuit. J Phys Chem Biophys 6:216–226. https://doi.org/10.4172/2161-0398.1000216
Chihaoui N, Hamdi B, Dammak T, Zouari R (2016b) Molecular structure, experimental and theoretical spectroscopic characterization and non-linear optical properties studies of a new non-centrosymmetric hybrid material. J Mol Struct 1123:144–152. https://doi.org/10.1016/j.molstruc.2016.06.031
Chihaoui N, Hamdi B, Zouari R (2016c) Structural study, intermolecular interactions in view of X-ray and Hirshfeld surface analysis, DSC characterization, and electrical properties of bis-(4-benzylpyridinium) tetrabromozincate. J Ionics 23:1173–1186. https://doi.org/10.1007/s11581-016-1926-4
Chihaoui N, Hamdi B, Zouari R (2017) Structural study, spectroscopic analysis and dielectric proprieties of new hybrid organic-inorganic compound. J Mol Struct 1147:48–55. https://doi.org/10.1016/j.molstruc.2017.06.087->
Dyre JC, Schrøder TB (2000) Universality of ac conduction in disordered solids. J Rev Modern Phy 72:873–892. https://doi.org/10.1103/revmodphys.72.873
Farrugia LJ (1997) ORTEP-3 for Windows - a version of ORTEP-III with a Graphical User Interface (GUI). J Appl Crystallogr 30:565. https://doi.org/10.1107/S0021889897003117
Farrugia LJ (1999) WinGX suite for small-molecule single-crystal crystallography. J Appl Crystallogr 30:837–838. https://doi.org/10.1107/s0021889899006020
Fanggao C, Saunders GA, Lambson EF, Hampton RN, Carini G, Di Marco G, Lanza M (1996) Temperature and frequency dependencies of the complex dielectric constant of poly(ethylene oxide) under hydrostatic pressure. J Polym Sci: Part B: Polym Phys 34:425–433. https://doi.org/10.1002/(SICI)1099-0488(199602)34:3%3c425::AID-POLB3%3e3.0.CO;2-S
Gao FX, Gu W, Yang YS, Qian J, Yan SP (2007) Bis(tetra-n-butylammonium) tetrabromidozincate (II). J Acta Cryst E 63:m1621. https://doi.org/10.1107/s160053680702140x
Gillon AL, Lewis GR, Orpen AG, Rotter S, Starbuck J, Wang X-M, Rodriguez-Martin Y, Ruiz-Pérez C (2000) Organic–inorganic hybrid solids: control of perhalometallate solid state structures. J Chem Soc Dalton Trans. https://doi.org/10.1039/b005036i
Hodge IM, Ngai KL, Moynihan CT (2005) Comments on the electric modulus function. J Non-Cryst Solids 351:104–115. https://doi.org/10.1016/j.jnoncrysol.2004.07.089
James AR, Srinivas K (1999) Low temperature fabrication and impedance spectroscopy of PMN-PT ceramics. J Mater Res Bull 34:1301–1310. https://doi.org/10.1016/s0025-5408(99)00127-0
Jonscher AK (1999) Dielectric relaxation in solids. J Phys D Appl Phys 32:R57–R70. https://doi.org/10.1088/0022-3727/32/14/201
Karaa N, Hamdi B, Ben Salah A, Zouari R (2012) Synthesis, infra-red, MAS-NMR characterization, structural study and electrical properties of the new compound [C5H6ClN2]2Cd3Cl8. J Mol Struct 1013:168–176. https://doi.org/10.1016/j.molstruc.2011.12.053
Karâa N, Hamdi B, Oueslati A, Ben Salah A, Zouari R (2010) Preparation, infra-red, MAS-NMR and structural characterization of a new copper based inorganic–organic hybrid compound: [C5H6N2Cl]2CuCl4. J Inorg Organomet Polym 20:746–754. https://doi.org/10.1007/s10904-010-9409-y
Karâa N, Hamdi B, Ben Salah A, Zouari R (2013) Synthesis, infra-red, CP/MAS-NMR characterization, structural study and electrical properties of the bis(4-amino-2-chloropyridinium) tetrachlorozincate (II) monohydrate. J Mol Struct 1049:48–58. https://doi.org/10.1016/j.molstruc.2013.06.003
Koenderink JJ, van Doorn AJ (1992) Surface shape and curvature scales. Image Vis Comput 10:557–564. https://doi.org/10.1016/0262-8856(92)90076-f
Mesbeh R, Hamdi B, Zouari R (2016) (H2pdcCuBr 2)2∙2(MH +)2Br − (M = Melamine, H2pdc = Pyridine-2,6-Dicarboxylic Acid): crystal structure, Hirshfeld surface analysis, vibrational and thermal studies. J Inorg Organomet Polym 26:744–755. https://doi.org/10.1007/s10904-016-0371-1
Nefzi H, Sediri F, Hamzaoui H, Gharb N (2013) Electric conductivity analysis and dielectric relaxation behavior of the hybrid polyvanadate (H3N(CH2)3NH3)[V4O10]. J Mater Res Bull 48:1978–1983. https://doi.org/10.1016/j.materresbull.2013.02.003
Ninković DB, Janjić GV, Zarić SD (2012) Crystallographic and ab initio study of pyridine stacking interactions. Local nature of hydrogen bond effect in stacking interactions. J Cryst Eng Commun 12:2113–2121. https://doi.org/10.1021/cg201389y
North ACT, Phillips DC, Mathews FS (1968) A semi-empirical method of absorption correction. Acta Cryst A24:351–359. https://doi.org/10.1107/S0567739468000707
Park BH, Hyun SJ, Moon CR, Choe BD, Lee J, Kim CY, Jo W, Noh TW (1998) Imprint failures and asymmetric electrical properties induced by thermal processes in epitaxial Bi4Ti3O12 thin films. J Appl Phys 84:4428–4435. https://doi.org/10.1063/1.368666
Parlakturk F, Altındal S, Tataroglu A, Parlak M, Agasiyev A (2008) On the profile of frequency dependent series resistance and surface states in Au/Bi4Ti3O12/SiO2/n-Si(MFIS) structures. J Microelectron Eng 85:81–88. https://doi.org/10.1016/j.mee.2007.03.012
Selvasekarapandian S, Vijaykumar M (2003) The ac impedance spectroscopy studies on LiDyO2. J Mater Chem Phys 80:29–33. https://doi.org/10.1016/s0254-0584(02)00510-2
Sheldrick GM (1997a) SHELXL-97. Program for crystal structures refinement, University of Gottingen. Electron Crystallogr. https://doi.org/10.1007/978-94-015-8971-0_18
Sheldrick GM (1997b) SHELXS-97. Program for the solution of crystal structures, University of Gottingen. Electron Crystallogr. https://doi.org/10.1007/978-94-015-8971-0_18
Spackman MA, Byrom PG (1997) A novel definition of a molecule in a crystal. J Chem Phys Lett 267:215–220. https://doi.org/10.1016/s0009-2614(97)00100-0
Spackman MA, Jayatilaka D (2009) Hirshfeld surface analysis. J Cryst Eng Commun 11:19–32. https://doi.org/10.1039/b818330a
Spackman MA, McKinnon JJ (2002) Fingerprinting intermolecular interactions in molecular crystals. J Cryst Eng Commun 4:378–392. https://doi.org/10.1039/b203191b
Spek AL (2003) Single-crystal structure validation with the program PLATON. J Appl Cryst 36:7–13. https://doi.org/10.1107/s0021889802022112
Weng DF, Wang BW, Wang ZM, Gao S (2011) Polymorphism of (H2mela)2[CuCl5] Cl (mela = melamine): structures, transformation and magnetic properties. Cryst Eng Commun 13:4683–4688. https://doi.org/10.1039/c1ce05143a
Wheeler SE (2012) Understanding substituent effects in noncovalent interactions involving aromatic rings. Acc Chem Res 46(4):1029–1038. https://doi.org/10.1021/ar300109n
Wolff SK, Grimwood DJ, McKinnon JJ, Turner MJ, Jayatilaka D, Spackman MA (2012) CrystalExplorer 3.1. University of Western Australia, Crawley
Acknowledgements
The authors would like to thank the members of the unit of common services, in particular Mr Tarek GARGOURI, at the University of Sfax for their useful assistance as far as the measurements of X-ray diffraction is concerned.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hamdi, B., Zouari, R. & Ben Salah, A. Preparation, molecular structure, thermal properties, electrical conductivity analysis and dielectric relaxation of a new hybrid compound (NH2C5H3ClNH)2ZnBr4·H2O. Chem. Pap. 72, 2795–2811 (2018). https://doi.org/10.1007/s11696-018-0521-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-018-0521-8