Abstract
Pollution caused by organic dyes is of serious environmental and health concern to the population. Dyes are widely used in textile coloring applications. In the present work, cotton textile was coated with a conducting polymer, polypyrrole (PPy), in situ during the oxidative polymerization of pyrrole. The resulting materials were utilized as easily separated and recyclable adsorbent for the removal of methylene blue (MB) as a model of cationic dyes in alkaline solutions. It showed also some affinity to remove Acid Green 25 as an anionic dye in acidic medium. The adsorption was assessed by monitoring the decrease in dye concentration by UV–Visible absorption spectroscopy. The influence of various parameters such as initial dye concentration, contact time, pH, temperature, and adsorbent dose on the adsorption process was studied. The pseudo-second-order kinetic model and Freundlich isotherm model were found to describe the adsorption process. The thermodynamic study revealed that the adsorption of MB by PPy was feasible, spontaneous, and exothermic process. Investigation of the substrate regeneration revealed that PPy deposited on cotton textile can be reused for dye adsorption several times with good efficiency and it allows for the recovery of MB for recycling purposes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Conducting polymers (CPs) (Lay et al. 2016), such as polypyrrole (PPy; Scheme 1), and organic dyes share some common features in the molecular structure. They both include the conjugated structure of single and double bonds that is responsible for optical absorption in the visible part of spectra and, consequently, they are colored. They both include benzene or heterocyclic rings. The basic difference lies in the presence of charge carriers (polarons) in CPs, and their absence in dyes, which are therefore virtually electronically non-conducting.
CPs and organic dyes are likely to interact. Conducting forms of polyaniline (PANI) (Bober et al. 2016) and PPy (Alekseeva et al. 2015) are organic salts where the polymer backbone is a polycation and its positive charges are balanced by counter-ions afforded by acids (Scheme 1). Many organic dyes include anionic sulfonic group that increases dye solubility in aqueous media. The ionic interaction between both species is thus the first possibility (Wang et al. 2015). Its nature is pH dependent because properties of CPs and many organic dyes are affected by the protonation. This is demonstrated by the salt–base transition between conducting and non-conducting forms of CPs (Blinova et al. 2007) and similar transition in dyes exploited as acid–base indicators. The interaction based on ionic interaction does not affect the conjugated system of both components, and the optical and electric properties then would have an additive contribution of both moieties.
The interaction of benzene or pyrrole rings in CPs with rings in dyes constitutes another type to be considered. It is expected to be based on π–π or donor–acceptor electron shifts in the electronic structure. If we accept the hypothesis that such interaction of conjugated systems takes place, it should manifest itself in the changes of optical absorption of both components or in the modification of electronic conduction, both these properties being closely related.
In the contrast to many organic dyes, CPs are insoluble in aqueous media. Recently, CPs have indeed been used for adsorption of dyes, such as PPy for Acid Orange 10 (Palanisamy et al. 2013), Acid Red G (Feng et al. 2014), or methylene blue (MB) (Ovando Medina et al. 2014). Polyaniline was similarly used for adsorption/removal of organic dyes, e.g., Acid Red G (Wang et al. 2015), Congo red (Debnath et al. 2015; Kumar et al. 2015), Coomassie Brilliant Blue (Liu et al. 2015b), MB (Ayad et al. 2013; Ayad and Zaghlol 2012; Ayad and El-Nasr 2010; Ayad et al. 2012), or Reactive Black 5 (Ballav et al. 2015), especially in environmental issues, such as water pollution treatment.
Many methods for the removal of dyes, including biodegradation, photocatalytic (Abdullah et al. 2016; Nassar et al. 2017a), photolytic, and oxidative degradation (Chen et al. 2003; Walker et al. 2003), flocculation, precipitation, photochemical oxidation, ozonation, and adsorption have been investigated (Balathanigaimani et al. 2009; Sharma 2009; Nassar et al. 2016; Xiong et al. 2014; Zhu et al. 2016). Because of the organic nature of dye molecules, most of the conventional treatment methods fail to recover the dyes from the effluents (McKay 1979). The adsorption has been recognized as one of the best methods for the removal of dyes from water (Ayad et al. 2014; Khare et al. 1987). The photocatalytic decomposition of dyes on CPs is an application aimed at similar target area. This was illustrated for PANI and a series of dyes (Riaz et al. 2014; Salavati and Kohestani 2013; Wang et al. 2014; Zhang et al. 2014). Also in this case, the interaction between the conducting polymer and a dye before its decomposition has to be considered and was indeed reported (Zhang et al. 2014).
The interaction manifests itself also by the dye incorporation into conducting polymer nanostructures during their preparation as illustrated for PPy and anionic dye, eriochrome cyanine R (Tavoli and Alizadeh 2014) or methyl orange (Kopecká et al. 2014). The presence of dye affected the morphology of PPy and improved its conductivity or electrochromic properties. The nature of interaction between CPs and dyes, however, requires additional analysis by physicochemical or physical methods. CPs and dyes have often been used in dye-sensitized solar cells (Wang et al. 2013). Such applications, however, do not require the direct interaction between the dye and conducting polymer, which serves as counter-electrode. Many dyes display antimicrobial properties. In a single case, dyes were covalently bound to nitrogen atoms in PANI (Jangid et al. 2014, 2015) and the resulting materials were found to have improved antimicrobial and antifungal properties compared to common drugs (Jangid et al. 2014).
CPs have often been deposited on cellulosic substrates and used for many applications. Cellulose was applied as a matrix component with PANI and PPy films for the purposes of circuitry, photo-current systems and supercacitors (Rußler et al. 2011; Dubal et al. 2011; Sasso et al. 2011; Babu et al. 2013). PANI-coated ligno-cellulose extracted from pine cones was synthesized and used as an adsorbent for the removal of some anionic dyes, such as Reactive Black 5 and Congo red, from wastewater. It is worth mentioning that the synthesis of these composites was complicated and time-consuming process (Ballav et al. 2015; Debnath et al. 2015). PPy/cellulosic agricultural waste composite was synthesized and used for adsorption of MB dye from water and the adsorption process required about 11 days to attain the equilibrium state, which makes PPy/cellulosic waste composite not promising for such applications (Ovando Medina et al. 2014).
Textiles fabricated from cellulose, viz. cotton, are considered as good substrates for coating with conducting polymers as they offer high surface area, provide a mechanical support and they have low weight. PPy coating on textiles was used as a promising material for fabricating flexible supercapacitors (Babu et al. 2013) or plant sensors (Bajgar et al. 2016). The interaction between PPy with cotton is stronger than the interaction with other types of textiles, e.g., linen or viscose. It has recently been reported that PPy coating on cotton textile possesses high surface conductivity and provides low impedance, and the prepared film was stable even after repeated chemical cleaning (Bober et al. 2015) or by the standard tape test and the abrasion test (Fan et al. 2017). This indicates that PPy coating on cotton textile can be considered as a suitable adsorbent for the removal of dyes. PPy deposited on cotton fabric prepared as described by (Bober et al. 2015) is demonstrated in the present study as an adsorbent for MB dye. Fan et al. (2017) reported the preparation of PPy-coated cotton fabric by in situ chemical polymerization using blends of anionic and cationic surfactants as soft template to enhance the PPy deposition onto the cotton fibers. They studied the effect of ionic strength, pH, contact time and initial dye concentration onto the MB uptake using a mixture of surfactants.
In the present paper, the dye adsorption kinetics, isotherm models and thermodynamic parameters for PPy-coated cotton textile without surfactants are reported. The comparison between the adsorption capacities of this adsorbent with other adsorbents reported in the literature is discussed.
MB dye has been selected as a model compound, as it is widely used in textile, pulp, and paper industries. The numerous studies published in the literature on the adsorption of this dye allow also for the comparison of efficiency of the individual adsorbents. The effect of various operating parameters, such as adsorbent amount, temperature, contact time, pH of the solution, and initial dye concentration, on MB dye removal has been investigated and is reported in the present paper.
Experimental
Coating of cotton textile with PPy
Cotton textile was coated by PPy as reported earlier (Bober et al. 2015; Bajgar et al. 2016; Maráková et al. 2017). PPy was deposited by in situ chemical oxidative polymerization of 0.2 M pyrrole (13.4 g in 500 mL deionized water) mixed with 0.5 M iron (III) chloride hexahydrate (135.1 g in 500 mL deionized water) on cotton textile at room temperature. Bleached plain weave 100% cotton textile (Mileta a.s., Czech Republic) (specific mass 120 g m−2, sett: 51.2 n/cm (warp), 28.0 n/cm (weft), yarn count 7.4/2 tex (warp), 14.5 tex (weft)) was used as received. Firstly, the textile sheet (4 g; 30 × 12 cm2) was immersed in the solution of pyrrole and left for 4 h to allow for the monomer impregnation/penetration. Then, an equal volume of iron (III) chloride hexahydrate (Sigma-Aldrich) solution was added. The reaction mixture (1 L) was gently stirred for 2 h, and then left to rest at room temperature for 24 h. The cotton textile turned dark brown as PPy was deposited. The textile coated with PPy was rinsed with 0.1 M hydrochloric acid to remove adhering PPy precipitate, then with ethanol, and dried in air. The content of PPy, 20.3 wt%, was calculated from the mass increase after its deposition.
Batch equilibrium studies
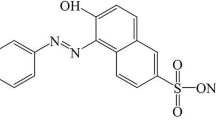
Methylene blue (3,7-bis(dimethylamino)phenazathionium chloride, Basic Blue 9; Scheme 2), is a water-soluble cationic dye used as the model sorbate in the present study, with optical absorption maximum at 664 nm. A stock solution was prepared by dissolving an appropriate quantity of MB in distilled water. The working solutions were prepared by successive dilution of the stock solution. Batch equilibrium adsorption studies were carried out in a set of conical flasks (100 mL) with 50 mL of MB solutions at various initial concentrations. About 50 mg of PPy-coated cotton textile was added to the dye solution, mixed, stirred for various time intervals at 25 °C at 500 rpm to attain equilibrium. The amount of dye adsorbed per unit mass of polymer adsorbent, Qe (mg g−1), and the dye removal percent (adsorptivity) A (%) were calculated from the two following equations:
where C0 is the initial dye concentration in liquid phase (mg L−1), Ce is the dye concentration at equilibrium (mg L−1), V is the volume of dye solution (L), and m is the mass of adsorbent (g).
All adsorption experiments have been carried out at neutral conditions, pH 7. Dye concentration was determined using a UV-double beam spectrophotometer (Labomed Inc., CA, USA) from absorption at wavelength 664 nm after calibration. The regeneration of the adsorbent was also studied and the same cotton textile coated with PPy was regenerated using acidic solution (pH 2).
Results and discussion
Adsorbent preparation
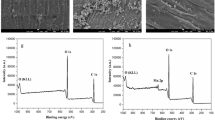
The coating of cotton textile with PPy is well documented by the conversion of originally white substrate to homogeneous brown-pigmented textile (Fig. 1). The scanning electron microscopy reveals that the coating of the textile with PPy was uniform (Fig. 2). This applies to the whole textile as well as to the individual threads. Only occasional clusters of PPy particles, i.e., residual of traces PPy precipitate that adhered to the textile after washing were still present. PPy has typical globular morphology and submicrometre particle size. Detailed characterization by infrared and Raman spectroscopies and other methods has been reported in separate papers (Bober et al. 2015; Maráková et al. 2017).
The molecular structure of PPy also contains a conjugated system of bonds (Scheme 1) which is similar to the structure of organic dyes. Dyes are colored because they absorb light in the visible spectrum (400–700 nm), by having at least one chromophore (color-bearing) group with a conjugated system of alternating double and single bonds (Abrahart 1977) as illustrated for MB dye structure (Scheme 2). The adsorption process is most likely based on the synergistic effect of π–π* interaction between the aromatic ring of PPy and MB. The electrostatic interaction is not likely because both PPy and MB are positively charged. As we are proposing an application for cotton textile coated with PPy to adsorb MB dye, the optimum conditions following order of pH value, dosage and temperature have been sought.
pH of the medium
In general, pH is an important variable affecting the adsorption or desorption of the dye as the solution pH influences the surface charge and functional groups of the adsorbent (Kamboh et al. 2011; Matheickal and Yu 1997). In strongly acidic media, the protons compete with MB ions for the active sites in PPy, which impedes the adsorption of cationic dye, whereas the competition from protons decreases with the increase in initial pH and the adsorption efficiency increases correspondingly (Dave et al. 2011; Yang et al. 2015). It is evident that alkaline conditions are beneficial for adsorption of MB.
Furthermore, in the solution of high pH, the PPy surface will become depronated to PPy base (Stejskal et al. 2016) and, thus, the cationic MB dye adsorption increased (Vadivelan and Kumar 2005). Similar trends were observed with different adsorbents, such as rice husk (Vadivelan and Kumar 2005), palm-kernel-shell-activated carbon (Jumasiah et al. 2005), and wheat shells (Bulut and Aydın 2006). Therefore, the adsorption of MB by the cotton textile coated with PPy was further examined under alkaline conditions at different pH values ranging from 7 to 10 (Fig. 3). It was observed that in the solution of high pH, PPy-coated cotton textile adsorbs MB dye faster, while under lower but still alkaline pH, the adsorption rate is slower. Although the rate of adsorption differs, the adsorption capacities are approximately the same in all cases; this may be attributed to the high effect of other types of interactions such as hydrogen bonding, van der Waals interactions and π–π stacking rather than the electrostatic interaction especially at lower pH. For that reason, we have studied the adsorption process of MB dye in neutral medium at pH 7.
Mass of textile substrate
The effect of PPy-coated textile dosages on the adsorption of MB dye from aqueous solution was evaluated by varying the dosage of the textile, which ranged from 50 to 470 mg and keeping constant initial MB dye concentration at 3.9 mg L−1 (Fig. 4). The adsorption rate of the MB increased with increasing amount of textile. This implies that the number of adsorption sites increases with increasing dosage of PPy coating during the adsorption as expected. The adsorption efficiencies are approximately the same in all cases.
Temperature
Temperature is one of the important parameters affecting dyes removal (Abramian and El-Rassy 2009; Ebrahimian and Saberikhah 2013). In the present work, adsorption capacity of MB dye decreased from 3.30 to 2.4 mg g−1 then to 2.23 then to 2.11 mg g−1 and finally to 2.01 mg g−1 due to increase in temperature gradually from 25 to 30 °C then to 35 °C then to 45 °C, and finally to 55 °C. The equilibrium adsorption capacity Qe of MB dye at different temperatures is reached its limit after ca. 300–400 min (Fig. 5). The decreasing trend confirms that adsorption of MB dye is exothermic process. This may be due to weakening of the residual forces between the active sites of the polymer and the dye, and also between adjacent dye molecules on the adsorbed phase (Al-Anber 2011; Ofomaja and Ho 2007). Further adsorption experiments were, therefore, carried out at 25 °C.
Dye retention
The adsorption of MB dye on cotton textile coated with PPy can be easily detected by naked eye as the blue solution becomes colorless after being in contact with the cotton textile (Fig. 6). Its difference with the uncoated cotton textile towards the adsorption of MB was also considered and discussed (Fig. 7).
Addition of 50 mg of PPy-coated textile to 50 mL of 3.9 mg L−1 MB solution led to noticeable decrease in the absorbance of MB with time (Fig. 7a). Similar experiment with uncoated textile under the same conditions revealed relatively small change in absorbance of MB (Fig. 7b). It can be concluded that the decisive role of PPy in adsorption of MB dye is apparent. Adsorptivity for the PPy-coated cotton textile reached about 96% after 24 h (Fig. 8a). In the case of uncoated textile, the adsorptivity was about 40% after 3 h and there was no significant change with time any more (Fig. 8b).
Effect of initial dye concentration
The experiment was carried out using different initial concentrations of MB. The dye solution (50 mL) was brought into contact with 50 mg of the PPy-coated textile substrate for 24 h to achieve the equilibrium. Two different pHs were used, pH 3.2 and pH 10.5. The amount of dye adsorbed per unit mass, Qe, increased with the increase of dye concentration at both pH levels. This means that the adsorption capacity is highly dependent on the initial dye concentration. At low concentration of dye, the ratio of the initial number of dye molecules to the available surface area is low. However, at high concentration the available sites of adsorption become fewer. Moreover, it is clearly indicated that the pH plays a great role. Under acidic pH 3.5, the dye adsorption is low, under alkaline pH 10.5, the adsorption capacity increased around three times (Fig. 9).
Adsorption kinetics
In order to study the adsorption kinetics, three different kinetic models have been applied: pseudo-first order (Namasivayam and Sumithra 2005; Yagub et al. 2014), pseudo-second order (Anirudhan and Radhakrishnan 2008; Tan et al. 2008) and intraparticle diffusion (Liu et al. 2015a) models:
where Qt refers to the amount of dye adsorbed at equilibrium at time t, k1, k2 and ki are rate constants for pseudo-first order, pseudo-second order and intraparticle diffusion kinetic models, respectively. The validity of the models was verified by the linear equation analysis of log (Qe − Qt) vs t, (t/Qt) vs t, and Qt vs t1/2, respectively. The adsorption of MB onto PPy-coated cotton textile was found to obey pseudo-second-order kinetic model with the highest correlation coefficient R2 and good agreement between experimental and calculated Qe values compared to pseudo-first-order kinetic model (Table 1). This assumes the sorption process, which is accompanied by electron sharing or electron transfer in the adsorption. All adsorption kinetics models are shown in Fig. 10 and their parameters are summarized in Table 1. Additionally, intraparticle diffusion model plot (Fig. 10c) gives a multi-linear curve, which suggests that the adsorption occurs in more than one step. The initial steeper section represents surface or film diffusion, the second linear section represents a gradual adsorption step, where intraparticle diffusion is rate limiting (Nassar et al. 2016, 2017b). From the slope of the second linear part the intraparticle diffusion rate constant ki equals 0.127 mg g−1 min−1. The value of the intercept of the second part section c equals 0.738 mg g−1; the intercept value suggests that surface diffusion has a larger role as the rate-limiting step.
Adsorption isotherms
The distribution of dye molecules between the liquid phase and the adsorbent is a measure of the position of equilibrium in the adsorption process and can generally be expressed by one or more series of isotherm models. The adsorption isotherm models are fundamental for description of the interactive behavior between adsorbate and adsorbent and for the investigation of adsorption mechanism. Isotherm data should accurately fit into different isotherm models to find the suitable model that can be used for adsorption process (Gil et al. 2011). In the present study, Langmuir (Langmuir 1916), Freundlich (Allen et al. 2004), and Temkin (Temkin and Pyzhev 1940) isotherm models have been used to describe the adsorption equilibrium data derived from the adsorption of MB dye on PPy coating on cotton textile. These adsorption isotherms can be expressed as follows:
where Ce is the equilibrium concentration of the adsorbate, KL is the Langmuir constant, Qm is the maximum adsorption capacity of monolayer, KF and n for Freundlich one, and KT and B for Temkin model. The equilibrium data obtained were fitted to the above three isotherm equations separately and the parameters were evaluated (Table 2) along with the correlation coefficients for each fit (Fig. 11a–c).
The linear correlation coefficient of Freundlich model with R2 above 0.99 is higher than that for the Langmuir or Temkin isotherm models suggesting that the Freundlich isotherm model best fits the experimental data. This means that MB is adsorbed onto energetically heterogeneous binding sites, with different kinds of binding sites of adsorbent. The adsorption process is not restricted to the formation of monolayer, n value which is more than 1 indicates the favorable adsorption of MB onto the adsorbent surface.
Thermodynamic parameters
Temperature strongly affects the adsorption of MB on PPy-coated cotton (Fig. 5). From these previously stated data, some thermodynamic parameters can be calculated (Table 3) as Gibbs free energy change (ΔG°), enthalpy of adsorption (ΔH°) and entropy change (ΔS°):
where Kd is the thermodynamic equilibrium constant, which is the ratio of the concentration of the dye on adsorbent at equilibrium Q e to the remaining concentration of the dye in solution at equilibrium C e (Nassar et al. 2017c, d).
The negative values of ΔG° indicated the feasibility of the process and the spontaneous nature of the adsorption under the used experimental conditions (Ertaş et al. 2010). ΔH° and ΔS° can be determined from the slope and the intercept of the linear plot of ln Kd vs. 1/T (Fig. 12, Table 3).
The data confirmed that reaction is not an exclusive physisorption; generally, the process is considered as physisorption when ΔH° values are between 0 and − 20 kJ mol−1 (Zhang et al. 2017). The negative value for enthalpy change ΔH° (−26.96 kJ mol−1) confirms the adsorption of MB dye is an exothermic process; this matches the results of the effect of temperature discussed above that MB dye adsorption rate and capacity decreased by increasing the temperature. The negative value of ΔS° (−85.14 J mol−1 K−1) reflects the decrease in the disorder of the system at the solid–solution interface, and no significant change occurred in the internal structure of the adsorbent during the adsorption process; this reflects the strong type of interaction between the dye and the adsorbent surface.
Regeneration of adsorbent
Many adsorbents have been reported to be regenerated, but they showed poor regeneration ability with high treatment cost. The facile regeneration and reusability test of cotton textile coated with PPy is quite important for an industrial application (Fig. 13).
50 mg of the coated cotton textile was used for removal of 3.9 mg L−1 MB dye solution. After the adsorption, the dye-loaded textile was treated with acidic solution (pH 2) to allow for desorption to occur. Fast release of MB from the surface of PPy-coated cotton textile indicates the presence of electrostatic interaction between MB and the adsorbent. The adsorbent was then reactivated by 0.1 M ammonium hydroxide and rinsed twice in water and reused again with a new solution of MB dye. The adsorption percentage of MB using the regenerated PPy-coated cotton textile (the second, third and fourth runs) was tested under similar conditions and compared to the first run. The adsorption percent of MB dye onto PPy-coated cotton textile decreased a little with increasing number of recycles (Fig. 14). After three cycles, the MB dye removal efficiency was still above 86%.
Alternative regeneration of adsorbent
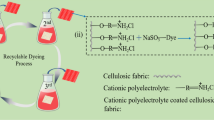
The efficiency of adsorption/desorption of MB and Acid Green 25 (AG) dyes from aqueous solutions by PPy-coated cotton textile were also investigated for the alternative removal by tuning the solution pH value. PPy-coated cotton textile can be easily protonated/deprotonated upon acid/base treatments (Stejskal et al. 2016). Depending on the proposal that the interaction is dependent on the electrostatic interactions between dyes molecules and the adsorbent, the deprotonated form is very efficient to adsorb MB. Moreover, the release process can be readily conducted by shifting the pH to acidic region. In the contrast, the protonated form can easily attract the AG anionic molecules and then can be released by shifting the pH to basic (Fig. 15).
In this regard, 0.05 g of the deprotonated PPy-coated cotton textile was immersed in MB dye solution (3.9 mg L−1, 50 mL) with an adsorption efficiency of 96%. After release of MB dye at pH 2, the latter process resulted in the formation of protonated form that was used as productive adsorbent for the removal of AG (20 mg L−1, 50 mL) with an adsorption efficiency of 94%. The regeneration of PPy-coated cotton textile can be successfully achieved by treatment in 0.1 M ammonium hydroxide and washed twice in water and reused again with MB dye.
Comparison with other adsorption systems
Table 4 compares the adsorption capacities of different adsorbents towards MB. The adsorption of MB onto either conventional PPy or PANI was found to be undetectable (Ayad et al. 2013; Ayad and Zaghlol 2012; Ayad and El-Nasr 2010; Ayad et al. 2012; Li et al. 2013), which indicates that both of these CPs have almost no adsorption tendency toward MB dye. Furthermore, the adsorption of MB dye onto PANI nanotubes was found to be much higher than the adsorption onto conventional PANI (Ayad and El-Nasr 2010) due to the high surface area that renders PANI nanotubes as an efficient adsorbent. Therefore, the morphology, polymer-chain ordering, and specific surface area of the conducting polymer play a crucial role in the enhancement of the adsorption.
In the current study, the coating of cotton textile with PPy increases the surface area and hence it affords multiple adsorption sites for MB dye. This enhances the adsorption properties unlike the conventional PPy (Li et al. 2013) and many other adsorbents (Table 4). The improvement of MB adsorption was also evident after coating sawdust of with PPy (Ansari and Mosayebzadeh 2010) due to the increment of the adsorption sites.
The adsorbent characterized in the current study has additional advantages, such as it is a low-cost and environmental friendly material with good regeneration ability. This renders PPy-coated cotton textile as a promising adsorbent for the removal of cationic dyes from wastewater.
Concluding remark
The data published in the literature as well as the present study document that CPs are efficient adsorbents of water-soluble dyes. The large number of papers published on this topic is due to the ease of the experimental setup based on the determination of the decrease in optical absorption, which is also supported by the naked-eye observation. It should be realized that other organic chemicals constituting water pollution, such as various drugs including antibiotics, herbicides and pesticides, might be similarly and efficiently removed from water. The studies in this direction, however, are missing because of the need to apply more complex analytical tools for determination of pollutant concentration.
Conclusions
PPy-coated cotton textile can be used as an adsorbent for the removal of MB, cationic dye model, from aqueous solutions. The PPy-coated cotton textile had a higher adsorption capacity compared to the neat cotton textile. Alkaline conditions favoured the dye adsorption. The amount of the dye adsorbed was found to increase with contact time and also with the amount of adsorbent. Freundlich isotherm model is more convenient to describe the adsorption process with the highest correlation coefficient. Furthermore, the adsorption kinetics were successfully fitted by pseudo-second-order kinetics. Thermodynamic parameters indicate that the adsorption process is feasible, efficient, and spontaneous with negative ΔH° value. The PPy-coated cotton textile can be easily regenerated and reused for dye adsorption several times with good efficiency and high durability, which allows for the recycling of both the adsorbent and the adsorbed MB dye.
References
Abramian L, El-Rassy H (2009) Adsorption kinetics and thermodynamics of azo-dye Orange II onto highly porous titania aerogel. Chem Eng J 150:403–410. https://doi.org/10.1016/j.cej.2009.01.019
Abdullah H, Kuo DH, Kuo YR, Yu FA, Cheng K-B (2016) Facile synthesis and recyclability of thin Nylon film-supported n-type ZnO/p-type Ag2O nano composite for visible light photocatalytic degradation of organic dye. J Phys Chem C 120:7144–7154. https://doi.org/10.1021/acs.jpcc.5b12153
Abrahart EN (1977) Dyes and their intermediates. Edward Arnold Ltd., London
Al-Anber MA (2011) Thermodynamics approach in the adsorption of heavy metals. In: Moreno-Pirajan JC (ed) Thermodynamics—interaction studies—solids, liquids and gases. InTech, Rijeka, Croatia, pp 737–764. https://doi.org/10.5772/21326
Alekseeva E, Bober P, Trchová M, Šeděnková I, Prokeš J, Stejskal J (2015) The composites of silver with globular or nanotubular polypyrrole: the control of silver content. Synth Met 209:105–111. https://doi.org/10.1016/j.synthmet.2015.07.003
Allen SJ, Mckay G, Porter JF (2004) Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems. J Colloid Interface Sci 280:322–333. https://doi.org/10.1016/j.jcis.2004.08.078
Anirudhan T, Radhakrishnan P (2008) Thermodynamics and kinetics of adsorption of Cu (II) from aqueous solutions onto a new cation exchanger derived from tamarind fruit shell. J Chem Thermodyn 40:702–709. https://doi.org/10.1016/j.jct.2007.10.005
Ansari R, Mosayebzadeh Z (2010) Removal of basic dye methylene blue from aqueous solutions using sawdust and sawdust coated with polypyrrole. J Iran Chem Soc 7:339–350. https://doi.org/10.1007/BF03246019
Ayad MM, El-Nasr AA (2010) Adsorption of cationic dye (methylene blue) from water using polyaniline nanotubes base. J Phys Chem C 114:14377–14383. https://doi.org/10.1021/jp103780w
Ayad M, Zaghlol S (2012) Nanostructured crosslinked polyaniline with high surface area: synthesis, characterization and adsorption for organic dye. Chem Eng J 204:79–86. https://doi.org/10.1016/j.cej.2012.07.102
Ayad MM, El-Nasr AA, Stejskal J (2012) Kinetics and isotherm studies of methylene blue adsorption onto polyaniline nanotubes base/silica composite. J Ind Eng Chem 18:1964–1969. https://doi.org/10.1016/j.jiec.2012.05.012
Ayad M, El-Hefnawy G, Zaghlol S (2013) Facile synthesis of polyaniline nanoparticles; its adsorption behavior. Chem Eng J 217:460–465. https://doi.org/10.1016/j.cej.2012.11.099
Ayad M, Salahuddin N, Fayed A, Bastakoti BP, Suzuki N, Yamauchi Y (2014) Chemical design of a smart chitosan–polypyrrole–magnetite nanocomposite toward efficient water treatment. Phys Chem Chem Phys 16:21812–21819. https://doi.org/10.1039/c4cp03062a
Babu KF, Subramanian SS, Kulandainathan MA (2013) Functionalisation of fabrics with conducting polymer for tuning capacitance and fabrication of supercapacitor. Carbohydr Polym 94:487–495. https://doi.org/10.1016/j.carbpol.2013.01.021
Bajgar V, Penhaker M, Martinková L, Pavlovič A, Bober P, Trchová M, Stejskal J (2016) Cotton fabric coated with conducting polymers and its application in monitoring carnivorous plant response. Sensors 16:498. https://doi.org/10.3390/s16040498
Balathanigaimani M, Shim WG, Park KH, Lee JW, Moon H (2009) Effects of structural and surface energetic heterogeneity properties of novel corn grain-based activated carbons on dye adsorption. Microporous Mesoporous Mater 118:232–238. https://doi.org/10.1016/j.micromeso.2008.08.028
Ballav N, Debnath S, Pillay K, Maity A (2015) Efficient removal of Reactive Black from aqueous solution using polyaniline coated ligno-cellulose composite as a potential adsorbent. J Mol Liq 209:387–396. https://doi.org/10.1016/j.molliq.2015.05.051
Blinova NV, Stejskal J, Trchová M, Prokeš J, Omastová M (2007) Polyaniline and polypyrrole: a comparative study of the preparation. Eur Polym J 43:2331–2341. https://doi.org/10.1016/j.eurpolymj.2007.03.045
Bober P, Stejskal J, Šeděnková I, Trchová M, Martinková L, Marek J (2015) The deposition of globular polypyrrole and polypyrrole nanotubes on cotton textile. Appl Surf Sci 356:737–741. https://doi.org/10.1016/j.apsusc.2015.08.105
Bober P, Zasonska BA, Humpolíček P, Kuceková Z, Varga M, Horák D, Babayan V, Kazantseva N, Prokeš J, Stejskal J (2016) Polyaniline–maghemite based dispersion: electrical, magnetic properties and their cytotoxicity. Synth Met 214:23–29. https://doi.org/10.1016/j.synthmet.2016.01.010
Bulut Y, Aydın H (2006) A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 194:259–267. https://doi.org/10.1016/j.synthmet.2016.01.010
Chakrabarti S, Dutta BK (2005) On the adsorption and diffusion of methylene blue in glass fibers. J Colloid Interface Sci 286:807–811. https://doi.org/10.1016/j.jcis.2005.01.035
Chen KC, Wu JY, Liou DJ, Hwang SCJ (2003) Decolorization of the textile dyes by newly isolated bacterial strains. J Biotechnol 101:57–68. https://doi.org/10.1016/S0168-1656(02)00303-6
Chen J, Feng J, Yan W (2016) Influence of metal oxides on the adsorption characteristics of PPy/metal oxides for methylene blue. J Colloid Interface Sci 475:26–35. https://doi.org/10.1016/j.jcis.2016.04.017
Dave PN, Kaur S, Khosla E (2011) Removal of Eriochrome black-T by adsorption on to eucalyptus bark using green technology. Indian J Chem Technol 18:53–60 (WOS:000288733300007)
Debnath S, Ballav N, Maity A, Pillay K (2015) Development of a polyaniline-lignocellulose composite for optimal adsorption of Congo red. Int J Biol Macromol 75:199–209. https://doi.org/10.1016/j.ijbiomac.2015.01.011
Dubal DP, Patil SV, Kim WB, Lokhande CD (2011) Supercapacitors based on electrochemically deposited polypyrrole nanobricks. Mater Lett 65:2628–2631. https://doi.org/10.1016/j.matlet.2011.05.114
Ebrahimian A, Saberikhah E (2013) Biosorption of methylene blue onto Foumant tea waste: equilibrium and thermodynamic studies. Cell Chem Technol 47:657–666 (WOS: 000326418100017)
Ertaş M, Acemioğlu B, Alma MH, Usta M (2010) Removal of methylene blue from aqueous solution using cotton stalk, cotton waste and cotton dust. J Hazard Mater 183:421–427. https://doi.org/10.1016/j.jhazmat.2010.07.041
Fan L, Wei C, Xu Q, Xu J (2017) Polypyrrole-coated cotton fabrics used for removal of methylene blue from aqueous solution. J Textile Inst 108:1847–1852. https://doi.org/10.1080/00405000.2017.1296989
Feng J, Li J, Lv W, Xu H, Yang H, Yan W (2014) Synthesis of polypyrrole nano-fibers with hierarchical structure and its adsorption property of Acid Red G from aqueous solution. Synth Met 191:66–73. https://doi.org/10.1016/j.synthmet.2014.02.013
Gil A, Assis F, Albeniz S, Korili S (2011) Removal of dyes from wastewaters by adsorption on pillared clays. Chem Eng J 168:1032–1040. https://doi.org/10.1016/j.cej.2011.01.078
Jangid NK, Chauhan NPS, Punjabi PB (2014) Novel dye-substituted polyanilines: conducting and antimicrobial properties. Polym Bull 71:2611–2630. https://doi.org/10.1007/s00289-014-1210-6
Jangid NK, Chauhan NPS, Punjabi PB (2015) Preparation and characterization of polyanilines bearing rhodamine 6-G and Azure B as pendant groups. J Macromol Sci A 52:95–104. https://doi.org/10.1080/10601325.2015.980714
Jumasiah A, Chuah TG, Gimbon J, Choong TSY, Azni I (2005) Adsorption of basic dye onto palm kernel shell activated carbon: sorption equilibrium and kinetics studies. Desalination 186:57–64. https://doi.org/10.1016/j.desal.2005.05.015
Kamboh MA, Solangi IB, Sherazi S, Memon S (2011) A highly efficient calix-[4]-arene based resin for the removal of azo dyes. Desalination 268:83–89. https://doi.org/10.1016/j.desal.2010.10.001
Khan AR, Tahir H, Uddin F, Hameed U (2005) Adsorption of methylene blue from aqueous solution on the surface of wool fiber and cotton fiber. J Appl Sci Environ Manage 9:29–35. https://doi.org/10.4314/jasem.v9i2.17287
Khare SK, Panday KK, Srivastava RM, Singh VN (1987) Removal of victoria blue from aqueous solution by fly ash. J Chem Technol Biotechnol 38:99–104. https://doi.org/10.1002/jctb.280380206
Kopecká J, Kopecký D, Vrňata M, Fitl P, Stejskal J, Trchová M, Bober P, Morávková Z, Prokeš J, Sapurina I (2014) Polypyrrole nanotubes: mechanism of formation. RSC Adv 4:1551–1558. https://doi.org/10.1039/c3ra45841e
Kumar R, Ansari MO, Parveen N, Barakat MA, Cho MH (2015) Simple route for the generation of differently functionalized PVC@ graphene–polyaniline fiber bundles for the removal of Congo red from wastewater. RSC Adv 5:61486–61494. https://doi.org/10.1039/c5ra10378a
Langmuir I (1916) The constitution and fundemental properties of solids and liquids. Part I. Solids. J Am Chem Soc 38:2221–2295. https://doi.org/10.1021/ja02268a002
Lay M, Méndez JA, Pèlach MÀ, Bun KN, Vilaseca F (2016) Combined effect of carbon nanotubes and polypyrrole on the electrical properties of cellulose-nanopaper. Cellulose 23:3925–3937. https://doi.org/10.1007/s10570-016-1060-5
Li J, Feng J, Yan W (2013) Excellent adsorption and desorption characteristics of polypyrrole/TiO2 composite for methylene blue. Appl Surf Sci 279:400–408. https://doi.org/10.1016/j.apsusc.2013.04.127
Liu K, Li H, Wang Y, Gou X, Duan Y (2015a) Adsorption and removal of rhodamine B from aqueous solution by tannic acid functionalized graphene. Colloids Surf A Physicochem Eng Asp 477:35–41. https://doi.org/10.1016/j.colsurfa.2015.03.048
Liu W, Li X, Li M, Li Y, Ge M (2015b) Preparation of polyaniline/filter-paper composite for removal of Coomassie Brilliant Blue. Polym Polym Compos 23:191–198 (WOS:000353862600010)
Maráková N, Humpolíček P, Kašpárková V, Capáková Z, Martinková L, Bober P, Trchová M, Stejskal J (2017) Antimicrobial activity and cytotoxicity of cotton fabric coated with conducting polymers, polyaniline or polypyrrole, and with deposited silver nanoparticles. Appl Surf Sci 396:169–176. https://doi.org/10.1016/j.apsusc.2016.11.024
Matheickal J, Yu Q (1997) Biosorption of lead (II) from aqueous solutions by Phellinus badius. Miner Eng 10:947–957. https://doi.org/10.1016/S0892-6875(97)00076-9
McKay G (1979) Waste color removal from textile effluents. Am Dyestuff Rep 68:29–36 (WOS:A1979GT25600002)
Namasivayam C, Sumithra S (2005) Removal of Direct Red 12B and methylene blue from water by adsorption onto Fe(III)/Cr(III) hydroxide, an industrial solid waste. J Environ Manage 74:207–215. https://doi.org/10.1016/j.jenvman.2004.08.016
Nassar MY, Moustafa MM, Taha MM (2016) Hydrothermal tuning of the morphology and particle size of hydrozincite nanoparticles using different counterions to produce nanosized ZnO as an efficient adsorbent for textile dye removal. RSC Adv 6:42180–42195. https://doi.org/10.1039/c6ra04855b
Nassar MY, Ayman AA, Amin AS (2017a) A facile Pechini sol–gel synthesis of TiO2/Zn2TiO2/ZnO/C nanocomposite: an efficient catalyst for the photocatalytic degradation of Orange G textile dye. RSC Adv 7:30411–30421. https://doi.org/10.1039/c7ra90074k
Nassar MY, Mohamed TY, Ahmed IS, Samir I (2017b) MgO nanostructure via a sol–gel combustion synthesis method using different fuels: an efficient nano-adsorbent for the removal of some anionic textile dyes. J Mol Liq 225:730–740. https://doi.org/10.1016/j.molliq.2016.10.135
Nassar MY, Alia EI, Zakaria ES (2017c) Tunable auto-combustion preparation of TiO2 nanostructures as efficient adsorbents for the removal of an anionic textile dye. RSC Adv 7:8034–8050. https://doi.org/10.1039/c6ra27924d
Nassar MY, Mohamed TY, Ahmed IS, Ibrahim SA, Mohamed NM, Khatab M (2017d) Hydrothermally synthesized Co3O4, α-Fe2O3, and CoFe2O4 nanostructures: efficient nano-adsorbents for the removal of Orange G textile dye from aqueous media. J Inorg Organomet Polym 27:1526–1537. https://doi.org/10.1007/s10904-017-0613-x
Ofomaja AE, Ho YS (2007) Equilibrium sorption of anionic dye from aqueous solution by palm kernel fibre as sorbent. Dyes Pigm 74:60–66. https://doi.org/10.1016/j.dyepig.2006.01.014
Ovando Medina VM, Díaz Flores PE, Martínez Gutiérrez H, Moreno Ruiz LA, Antonio Carmona ID, Hernández Ordoñez M (2014) Composite of cellulosic agricultural waste coated with semiconducting polypyrrole as potential dye remover. Polym Compos 35:186–193. https://doi.org/10.1002/pc.22649
Palanisamy P, Agalya A, Sivakumar P (2013) Polypyrrole composite—a potential material for the removal of acid dyes. Asian J Chem 25:5891–5896 (WOS:000325089600002)
Riaz U, Ashraf S, Aqib M (2014) Microwave-assisted degradation of acid orange using a conjugated polymer, polyaniline, as catalyst. Arab J Chem 7:79–86. https://doi.org/10.1016/j.arabjc.2013.07.001
Rußler A, Sakakibara K, Rosenau T (2011) Cellulose as matrix component of conducting films. Cellulose 18:937–944. https://doi.org/10.1007/s10570-011-9555-6
Salavati H, Kohestani T (2013) Preparation, characterization and photochemical degradation of dyes under UV light irradiation by inorganic–organic nanocomposite. Mater Sci Semicond Process 16:1904–1911. https://doi.org/10.1016/j.mssp.2013.07.014
Sasso C, Bruyant N, Benevenri D, Faure-Vincent J, Zeno E, Petit-Conil M, Chaussy D, Belgacem MN (2011) Polypyrrole (PPy) chemical synthesis with xylan in aqueous medium and production of highly conducting PPy/nanofibrillated cellulose films and coatings. Cellulose 18:1455–1467. https://doi.org/10.1007/s10570-011-9583-2
Sharma YC (2009) Optimization of parameters for adsorption of methylene blue on a low-cost activated carbon. J Chem Eng Data 55:435–439. https://doi.org/10.1021/je900408s
Stejskal J, Trchová M, Bober P, Morávková Z, Kopecký D, Vrňata M, Prokeš J, Varga M, Watzlová E (2016) Polypyrrole salts and bases: superior conductivity of nanotubes and their stability towards the loss of conductivity by deprotonation. RSC Adv 6:88382–99391. https://doi.org/10.1039/c6ra19461c
Tan I, Ahmad A, Hameed B (2008) Adsorption of basic dye using activated carbon prepared from oil palm shell: batch and fixed bed studies. Desalination 225:13–28. https://doi.org/10.1016/j.desal.2007.07.005
Tavoli F, Alizadeh N (2014) In situ UV–vis spectroelectrochemical study of dye doped nanostructure polypyrrole as electrochromic film. J Electroanal Chem 720:128–133. https://doi.org/10.1016/j.jelechem.2014.03.022
Temkin M, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim URSS 12(3):217–222
Vadivelan V, Kumar KV (2005) Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J Colloid Interface Sci 286:90–100. https://doi.org/10.1016/j.jcis.2005.01.007
Walker G, Hansen L, Hanna J-A, Allen S (2003) Kinetics of a reactive dye adsorption onto dolomitic sorbents. Water Res 37:2081–2089. https://doi.org/10.1016/S0043-1354(02)00540-7
Wang S, Lu S, Li X, Zhang X, He S, He T (2013) Study of H2SO4 concentration on properties of H2SO4 doped polyaniline counter electrodes for dye-sensitized solar cells. J Power Sources 242:438–446. https://doi.org/10.1016/j.jpowsour.2013.05.060
Wang T et al (2014) Adsorption removal of organic dyes on covalent triazine framework (CTF). Microporous Mesoporous Mater 187:63–70. https://doi.org/10.1016/j.micromeso.2013.12.016
Wang N, Li J, Lv W, Feng J, Yan W (2015) Synthesis of polyaniline/TiO2 composite with excellent adsorption performance on Acid Red G. RSC Adv 5:21132–21141. https://doi.org/10.1039/c4ra16910g
Xiong J, Jiao C, Li C, Zhang D, Lin H, Chen Y (2014) A versatile amphiprotic cotton fiber for the removal of dyes and metal ions. Cellulose 21:3073–3087. https://doi.org/10.1007/s10570-014-0318-z
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Colloid Interface Sci 209:172–184. https://doi.org/10.1016/j.cis.2014.04.002
Yang CX, Lei L, Zhou PX, Zhang Z, Lei ZQ (2015) Preparation and characterization of poly(AA co PVP)/PGS composite and its application for methylene blue adsorption. J Colloid Interface Sci 443:97–104. https://doi.org/10.1016/j.jcis.2014.11.040
Zhang S, Zhao L, Zeng M, Li J, Xu J, Wang X (2014) Hierarchical nanocomposites of polyaniline nanorods arrays on graphitic carbon nitride sheets with synergistic effect for photocatalysis. Catal Today 224:114–121. https://doi.org/10.1016/j.cattod.2013.12.008
Zhang Y, Yu F, Cheng W, Wang J, Ma J (2017) Adsorption equilibrium and kinetics of the removal of ammoniacal nitrogen by zeolite X/activated carbon composite synthesized from elutrilithe. J Chem 2017:1–9. https://doi.org/10.1155/2017/1936829
Zhu WJ, Lin L, Liao Q, Chen X, Qian ZQ, Shen JY, Liang JL, Yao JM (2016) Functionalization of cellulose with hyperbranched polyethylenimine for selective dye adsorption and separation. Cellulose 23:3785–3797. https://doi.org/10.1007/s10570-016-1045-4
Acknowledgements
The authors would like to thank Tanta University and the Technology Agency of the Czech Republic (TE01020022) for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ayad, M.M., Amer, W.A., Zaghlol, S. et al. Polypyrrole-coated cotton textile as adsorbent of methylene blue dye. Chem. Pap. 72, 1605–1618 (2018). https://doi.org/10.1007/s11696-018-0442-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-018-0442-6