Abstract
Weight failure after sleeve gastrectomy (SG) is frequently observed. Consensus on the most effective treatment is lacking. The aim of this meta-analysis was to assess revisional strategies for weight regain (WR) or insufficient weight loss (IWL) following SG. The included studies reported on endoscopic gastroplasty (ESG), re-sleeve gastrectomy (re-SG), Roux-en-Y gastric bypass (RYGB), one-anastomosis gastric bypass (OAGB), single-anastomosis duodeno-ileal bypass (SADI), and duodenal switch (DS). All techniques resulted in clinically relevant weight loss. Although our data suggest that revisional OAGB was the most effective procedure, the lack of direct comparisons precludes strong conclusions. All procedures were feasible but differed regarding complication rates. Choice of procedure is depending on patient’s characteristics and surgeons’ expertise.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity has reached pandemic proportions over the last decades. This has led to a dramatic increase in the number of bariatric procedures performed annually [1]. Bariatric surgery is considered the most effective treatment for morbid obesity compared to non-surgical alternatives, providing durable weight loss and significant improvement of related comorbidities [2].
Sleeve gastrectomy (SG) is the most frequently performed bariatric procedure worldwide [1]. Insufficient weight loss (IWL) and weight regain (WR) are among possible indications for revision and incidences range from 5 to 37% in literature [3, 4]. Proposed causes of weight regain include initial large sleeve size, postoperative sleeve dilation, increased ghrelin levels, inadequate support during follow-up, and maladaptive lifestyle behaviors [4]. Weight regain is associated with recurrence of comorbidities, an impaired quality of life, and a potential negative economic impact [5,6,7]. As a result, revision rates up to 27.8% are reported after 7 years following SG with WR and IWL being the most frequent cause [3].
Among the surgical revisional procedures for WR and IWL after SG are re-sleeve gastrectomy (re-SG), Roux-en-Y gastric bypass (RYGB), one-anastomosis gastric bypass (OAGB), single-anastomosis duodeno-ileal bypass (SADI), and duodenal switch (DS) [8,9,10]. Endoscopic management by gastroplasty (ESG) has shown to be an alternative strategy by reducing the sleeve volume [11].
The aim of the present study was to define the efficacy and complication rates of revisional strategies after IWL or WR following SG. Therefore, data on the revisional procedures after SG were systematically reviewed and compared using meta-analysis.
Methods
Literature Search
A literature search was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)-statement [12]. To identify all relevant publications, we conducted systematic searches in the bibliographic databases PubMed and Embase.com from inception up to May 04, 2021, in collaboration with a medical information specialist. The following terms were used (including synonyms and closely related words) as index terms or free-text words: “Bariatric surgery,” “Sleeve gastrectomy,” “Gastric bypass,” “Weight regain”. The references of the identified studies were searched for relevant publications. Duplicate studies were excluded. All languages were accepted. The full search strategies for all databases can be found in Appendix A/Supplementary material. The study was registered in the International Prospective Register of Systematic Reviews-University of York (PROSPERO) (registry number RD42021224890).
Study Selection
Two reviewers (RF and NRS) independently screened all potentially relevant titles and abstracts for eligibility. If necessary, the full text article was checked for the eligibility criteria. Differences in judgement were resolved through a consensus procedure. All studies, both prospective and retrospective, that addressed a therapeutic intervention for WR after SG in patients > 18 years were considered for inclusion. Studies were included if they reported weight loss efficacy after intervention. Only studies with a minimum of 20 patients were included, to make a reliable assessment of pooled efficacy. Furthermore, literature reviews, case reports, and animal studies were excluded. Other exclusion criteria were as follows:
-
- A follow-up shorter than 6 months
-
- Studies addressing/concerning treatment for other indications than WR or IWL without subgroup analyses for the index subgroup
-
- Studies that did not report BMI
- The bibliographies of included studies were subsequently hand-searched for other relevant references.
Quality Assessment
This meta-analysis was conducted according to the consensus statement for meta-analysis of observational studies (MOOSE statement) [13]. Two reviewers (RF and NRS) independently assessed the quality of studies using the modified Newcastle–Ottawa Scale (NOS) quality assessment tool for observational studies [14]. Possible conflicts were discussed with a third reviewer (MdB).
Data Extraction
Two reviewers (RF and JF) independently extracted study and patient characteristics, and outcome data. Disagreements were reconciled by discussion with an arbiter (MdB).
The following specifications were obtained from included studies: first author, year of publication, study design, number of patients, inclusion criteria, specification of WR/IWL criterion, gender and age of included patients, BMI at initial procedure, WR from nadir, BMI at intervention, time from initial procedure to intervention, type of intervention, location of the study, main objectives of the study, number of patients in intervention group(s), follow-up duration, follow-up rate, BMI during follow-up, TWL during follow-up, excessive body weight loss (EWL) during follow-up, mortality, type and complication rate, and main conclusion(s) of the study. Authors were contacted by email in case specific data was not specified in the article.
Statistical Analysis
Effect sizes (reduction of BMI) and standard errors were extracted or, if not reported, were calculated based on described standard deviations, pre- and after treatment scores, and the number of participants. When the standard deviation was unknown, an estimation was made based on the following formula: standard deviation = range/4 [15]. If both range and standard deviation were not given, the study was left out of pooled analysis. The pooled effect size and 95% confidence interval (95% CI) were estimated using a random-effects model with the DerSimonian and Laird approach [16]. Statistical analyses were performed with R and R Studio (R version 4.0.3) using the packages meta. P values of < 0.05 were considered statistically significant.
Results
Search Results
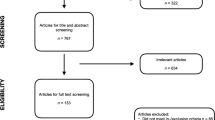
The literature search identified 5722 references: 2272 in PubMed and 3450 in Embase.com. After removing duplicates, 3592 studies were excluded by title and abstract screening. Subsequently, 44 studies were excluded by full-text evaluation, leaving 22 studies available for inclusion in this review (Fig. 1). Following data extraction, 22 studies provided data that were included for quantitative analysis [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. Main characteristics and quality assessment of all included studies are shown in Table 1.
Quantitative Analysis
Of the 22 studies including 1342 patients, 2 studies reported on ESG as a revisional procedure, 7 studies on re-SG, 6 studies on RYGB, 8 studies on OAGB, 3 studies on SADI, and 1 study on DS. Baseline characteristics are showed in Table 1. Pooled data are shown in Fig. 2.
Gastroplasty
Endoscopic sleeve gastroplasty is an incisionless, minimally invasive technique that involves remodeling the stomach via the placement of full-thickness sutures to reduce gastric capacity and delay gastric emptying [39].
Two studies described ESG and included a total of 116 patients. Endoscopic gastroplasty is performed by an endoscopic suturing device. Running sutures are placed, beginning from the level of the incisura angularis to 1–2 cm below the gastroesophageal junction to imbricate the anterior and posterior gastric wall. De Moura et al. specified WR as an increase in body weight of more than 10 kg; Maselli et al. did not specify WR (Table 1).
Pooled analyses showed a BMI at revision of 36.06 kg/m2 (95% CI, 20.83–51.29) and a reduction of 4.65 kg/m2 (95% CI, 4.02–5.29) and 5.7 kg/m2 (95% CI, 3.9–7.5) after 6 and 12 months, respectively (Fig. 2, Table 2).
Follow-up rates were 50% and 70% after 12 months. Only mild complications were reported including 4 cases of postoperative dehydration and one unintentionally narrowing of the gastroesophageal junction. Of the 67 patients who did not have GERD at the time of ESG, 6 patients (9.0%) developed GERD symptoms within the 12 months of the study (Table 3).
Re-sleeve Gastrectomy
Re-sleeve gastrectomy is a technique in which the residual gastric volume is reshaped. The indication for this procedure is WR or IWL combined with an enlarged pouch as demonstrated by CT or a barium swallow test. A bougie is used to guide transection of the left side of the gastric tube [19].
Re-sleeve was evaluated by five studies who met inclusion criteria for pooled analyses including 224 patients. The study by Mehmet et al. and Al Sabah et al. was excluded from the pooled analysis since the standard deviation was missing. In all studies but for Frieder et al., residual gastric volume was increased and measured by CT-volumetry or barium swallow test. WR or IWL was defined in four studies as EWL less than 50%. In one study, WR or IWL was not defined (Table 1).
Pooled BMI at revision was 39.17 kg/m2 (95% CI, 36.57–41.77), and reduction was 9.82 kg/m2 (95% CI, 4.28–15.37), 11.98 kg/m2 (95% CI, − 35.03–58.99), 8.04 kg/m2 (95% CI, − 5.72–10.36), and 10.8 kg/m2 (95% CI, 11.17–17.01) after 12, 24, 36, and > 48 months, respectively (Fig. 2; Table 2).
Follow-up rates were 100% in two studies and 95% in one study, and Rebibo et al. reported a follow-up of 100% after 1 year and 24% after 4 years.
In all studies, major complications were seen in 15 cases (6.7%), and staple-line leakage was predominant with 8 cases (3.6%). Specific location of leakage was not reported. One mortality case was reported; the patient died after re-SG due to pulmonary embolism at postoperative day 30 after a postoperative gastric leak (Table 3).
Roux-en-Y Gastric Bypass
During a RYGB, a gastric pouch is created, and subsequently a gastrojejunal anastomosis and jejuno-jejunostomy is made leaving a bilio-pancreatic limb of variable lengths and an alimentary limb of 150 cm.
Six studies reported on RYGB with a total of 309 patients. Two studies did not specify their operative technique [27, 36]. All other studies mentioned an alimentary limb of 150 cm. Bilio-pancreatic limb ranged from 20 to 30 cm in one study [35] to 70–100 cm in three others [19, 24, 25]. Indication for intervention was EWL < 50% in two studies and < 50% EWL or > 50% WR from nadir in two studies; and in one study, indication for re-intervention was 50% EWL and eventually regaining weight (Table 1).
Pooled BMI at revision was 39.93 kg/m2 (95% CI, 37–42.87) and reduction was 7.59 kg/m2 (95% CI, 3.51–11.67), 3.0 kg/m2 (95% CI, 0.74–5.26), 6.67 kg/m2 (95% CI, 5.59–7.74), and 7.47 kg/m2 (95% CI, − 20.17–35.11) after 12, 24, 36, and > 48 months, respectively (Fig. 2, Table 2).
Follow-up rates were not reported in one study and differed among other studies from 64 to 100% after 1 year.
Major complications were seen in 25 cases (8.1%) of which anastomotic stenosis was most common (2.6%). Among minor complications, nutritional deficiency was predominant with an incidence of 13.6% (Table 3).
One-Anastomosis Gastric Bypass
A revisional OAGB is performed by creating a gastric pouch [40]. A gastrojejunal anastomosis is created with a relatively long biliary limb.
A total of eight studies described OAGB after SG, including 484 patients. The length of the biliary limb ranged from 170 to 220 cm. In six studies, IWL was not defined. Two studies defined IWL as < 50% EWL. WR was not defined in five studies. One study defined this as regain of 10 kg or > 25% EWL, one study as 50% regain of the weight lost post SG, and another study as any weight regain with a BMI > 35 kg/m2 (Table 1).
Pooled BMI at revision was 41.78 kg/m2 (95% CI, 40.53–43.02) and reduction was 11.48 kg/m2 (95% CI, 8.03–14.92), 14.43 kg/m2 (95% CI, − 33.17–62.02), 12.74 kg/m2 (95% CI, − 0.94–26.42), and 17.80 kg/m2 (95% CI, 16.33–19.27) after 12, 24, 36, and > 48 months, respectively (Fig. 2, Table 2).
FU ranged from 48 to 100% after 12 months (n = 2 studies) and from 86 to 96% after 24 months (n = 2 studies). FU rates were not reported in two cases.
Major complication occurred in 22 cases (4.5%). GI-perforation occurred in 6 cases (1.2%). Staple-line leakage was reported in one case, anastomotic leakage in four cases, and iatrogenic bowel perforation in one case. GI-bleeding and GI-ulceration were reported in 5 cases (1%). Minor complications occurred in 11 cases (2.3%) (Table 3).
Single-Anastomosis Duodeno-Ileal Bypass
During a SADI, the first part of the duodenum is divided about 2–4 cm distal to the pyloric ring with a linear stapler. The ileocecal junction is identified, and 250–300-cm ileum is measured and brought up to the proximally divided duodenum [20]. Then, a duodeno-ileostomy is created.
Three studies reported SADI in 150 patients as revisional procedure. Their technique was as described above. WR or IWL was not defined in these studies (Table 1).
Pooled BMI at revision was 44.20 kg/m2 (95% CI, 40.58–47.82), and reduction was 11.19 kg/m2 (95% CI, − 11.03–33.42) and 13.5 kg/m2 (95% CI, 11.49–15.51) after 12 and 36 months, respectively (Fig. 2, Table 2).
Two studies reported a follow-up rate after 24 months of 88% and 89%, respectively. One study reported a FU rate of 55% after 36 months. Follow-up rates were not reported in one study.
Major complications occurred in 9 cases (6%). One patient died following a small bowel perforation caused by an incarcerated hernia 9 months after the revision. Among other indications for reoperations were staple-line leak (n = 1), abscess (n = 2), anastomosis leak (n = 1), small bowel perforation (n = 1), incisional hernia (n = 1), internal hernia (n = 1), and cholecystolithiasis (n = 1). In 54 cases (36%) minor complications occurred. Nutritional deficiency was reported in 48 cases (32%) (Table 3).
Duodenal Switch
For the conversion of SG to DS, the ileum is divided 250–300 cm proximal to the ileocecal valve, with the proximal loop becoming the biliopancreatic limb and the distal loop becoming the alimentary limb. The duodenum is also divided 2 cm distal to the pylorus, and its continuity is established by an anastomosis to the distal end of the divided ileum (alimentary limb) to provide continuity for food passage. The cut end of the divided ileum that becomes the biliopancreatic limb (proximal end) and the alimentary limb are connected by anastomosis at the 60–100-cm point from the cecum to form the common channel where digestion and absorption occur.
Shimon et al. reported on DS after SG for 21 patients. The revisional procedure was performed when WR resulted in a BMI > 35 kg/m2 (Table 1).
BMI at intervention was 46 kg/m2 (95% CI, 42.95–49.05). During follow-up, BMI reduction was 13.55 kg/m2 (95% CI, 10.85–16.25) after 12 months for 21 patients and 14.09 kg/m2 (95% CI, 11.17–17.01) for 18 patients after > 48 months (Fig. 2, Table 2).
Three patients (14%) in the DS group had nutritional complications: one had vitamin A deficiency that was treated successfully with oral supplementation, and two had severe malnutrition that required laparoscopic common channel lengthening (Table 3).
Discussion
This systematic review and meta-analysis summarizes and compares efficacy and complications of revisional procedures for WR and IWL after SG. All revisional procedures were found to result in clinically relevant weight loss during follow-up (Fig. 3, Table 2). The results suggest that OAGB is the best revisional procedure in terms of efficacy and complication rate.
OAGB led to a BMI reduction of 11.48 kg/m2 after 12 months and 14.43 kg/m2 after 24 months. One study showed a BMI decrease of even 17.80 kg/m2 after 60 months. OAGB was a relatively safe procedure with an incidence of major complications of 4.5% including 1.2% anastomotic leakage and minor complications in 2.3%. We propose that the introduction of the malabsorptive component of this procedure is a possible explanation for additional weight loss following this procedure. The low rate of minor side effects and nutritional deficiencies in OAGB compared to RYGB suggests that methods of reporting complications varied between the studies.
Re-sleeve gastrectomy was indicated as a revisional procedure only in case of an enlarged sleeve pouch. In all but one study, an increased residual gastric volume of more than 250 mL was measured preoperatively. Re-sleeve was effective, with a decrease in BMI of 10 kg/m2 after 12 and > 48 months. Overall complication rate was considered low. A relatively high rate of anastomotic leakage of 3.6% was reported, possibly due to the addition of stapler lines on the greater curvature which has been stapled previously. Sleeve anatomy has been commonly proposed as possible cause for WR or IWL [4]. An initial large sleeve volume with either an incomplete excised fundus is considered as primary dilatation. As this could be an obvious mechanism for IWL, WR could be explained by an increasingly extending fundus with release of larger amounts of grehlin [41]. Progressive dilatation of the gastric tube is described as secondary dilatation [42, 43].
RYGB resulted in a BMI reduction of 7.7 kg/m2 after 12 months and 7.47 kg/m2 after > 48 months. There was a relatively high complication rate (8.1% major complications). Minor complications occurred in 13.9% of cases most frequently due to nutritional deficiencies. Adequate high follow-up rates are mandatory.
SADI is a relatively difficult technical procedure, as reflected by the major complication rate of 6%. Thus, the role as revisional procedure after SG is not yet established. Interestingly, one study showed a decrease of 13.50 kg/m2 after 36 months. The high rate of deficiencies in 32% of the patients necessitates adequate patient follow-up.
Only one study reported on DS after failed SG in patients with a very high BMI of 46 kg/m2 at revision [38]. Weight loss after 12 months was 13.6 kg/m2 and 14.1 kg/m2 after > 48 months. DS results in the best weight loss after failed SG, at the expense of high complication rate and a difficult surgical technique [44, 45]. A high number of 29% of the patients was missing from the follow-up in this study. Missing follow-up visits after DS could potentially be life threatening in case of severe nutritional deficiencies and or electrolyte disturbances.
Endoscopic gastroplasty was considered the least effective procedure and led to a decrease in BMI of only 5.7 kg/m2 at last follow-up after 12 months. It was associated with only minor complications except for one case with a stenosis. Although it is a relatively safe procedure, follow-up was short and efficacy seems low. Interventions were performed by multiple centers, potentially introducing heterogeneity. This is an evolving technology which should not be done outside the setting of a study. Longer follow-up is warranted to provide data on efficacy.
The comparisons of the results after various surgical revisional procedures for WR and IWL are new and meaningful. Previous reviews reported efficacy for revisional procedures after SG including complication-related indications [46,47,48]. In our review, selection bias was minimized by excluding studies with complication-related indications. Small studies (< 20 patients) were excluded with the intent to limit the potential negative effect of the learning curve (i.e., complication rate overestimation).
The results from this meta-analysis should be interpreted with caution. Our review and meta-analysis does have limitations. The conclusions are based on retrospective studies including incomplete data. Heterogeneity bias is potentially introduced by the diversity of indications and surgical techniques [49], patient selection, surgeons’ experience, preoperative gastric volumetry, outcomes reporting, definition of postoperative complications and preoperative comorbidities. Percentage TWL is considered most appropriate for expression of weight loss; however, these data were not consistently provided by the studies [50]. There is a reasonable possibility of publication bias due to the retrospective nature of the included studies. Furthermore, we included studies with a minimum follow-up of 1 year which is a relatively short period concerning weight changes in bariatric surgery. Follow-up rates were considerably poor. It should be noted that SG is a relatively new procedure, and long-term follow-up data are scarce or non-existent. The inclusion of observational studies is appropriate and internally consistent with an evidence-based approach [51].
Randomized controlled trials with follow-up of several years are needed. Indication in these studies should be explicit with standard preoperative imaging of residual gastric volume. A uniform definition of WR and IWL might reduce the risk of selection bias.
Conclusion
This is the first systematic review and meta-analysis comparing the efficacy and safety of different treatments for WR or IWL following SG. All treatment modalities are effective for weight loss. Data in this review showed a good balance for OAGB based on weight loss and complication rate. ESG as an evolving technique is the least effective procedure. SADI and DS are associated with high complication rates. Heterogeneity and poor follow-up rates preclude strong conclusions and clinical decision making. Controlled prospective trials with longer follow-up are needed in order to choose the best revisional treatment for long-term success.
References
Angrisani L, Santonicola A, Iovino P, Vitiello A, Zundel N, Buchwald H, et al. Bariatric Surgery and Endoluminal Procedures: IFSO Worldwide Survey 2014. Obes Surg. 2017;27.
Puzziferri N, Roshek TB, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long-term Follow-up After Bariatric Surgery. JAMA. 2014;312.
Clapp B, Wynn M, Martyn C, Foster C, O’Dell M, Tyroch A. Long term (7 or more years) outcomes of the sleeve gastrectomy: a meta-analysis. Surg Obes Relat Dis. 2018;14.
5. Lauti M, Kularatna M, Hill AG, MacCormick AD. Weight Regain Following Sleeve Gastrectomy—a Systematic Review. Obes Surg. 2016;26.
Karlsson J, Taft C, Rydén A, Sjöström L, Sullivan M. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. Int J Obes. 2007;31.
Sheppard CE, Lester ELW, Chuck AW, Birch DW, Karmali S, de Gara CJ. The Economic Impact of Weight Regain. Gastroenterol Res Pract. 2013;2013.
Brethauer SA, Aminian A, Romero-Talamás H, Batayyah E, Mackey J, Kennedy L, et al. Can Diabetes Be Surgically Cured? Long-Term Metabolic Effects of Bariatric Surgery in Obese Patients with Type 2 Diabetes Mellitus. Ann Surg. 2013;258.
Frantzides CT, Alexander B, Frantzides AT. Laparoscopic Revision of Failed Bariatric Procedures. JSLS. 2019;23.
Linke K, Schneider R, Gebhart M, Ngo T, Slawik M, Peters T, et al. Outcome of revisional bariatric surgery for insufficient weight loss after laparoscopic Roux-en-Y gastric bypass: an observational study. Surg Obes Relat Dis. 2020;16.
Poublon N, Chidi I, Bethlehem M, Kuipers E, Gadiot R, Emous M, et al. One anastomosis gastric bypass vs. Roux-en-Y gastric bypass, remedy for insufficient weight loss and weight regain after failed restrictive bariatric surgery. Obes Surg. 2020;30.
Eid G. Sleeve gastrectomy revision by endoluminal sleeve plication gastroplasty: a small pilot case series. Surg Endosc. 2017;31.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLOS Med. 2021;18.
Stroup DF. Meta-analysis of Observational Studies in Epidemiology: A Proposal for Reporting; JAMA. 2000;283.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5.
DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: An update. Contemp Clin Trials. 2007;28.
AlSabah S, Alsharqawi N, Almulla A, Akrof S, Alenezi K, Buhaimed W, et al. Approach to Poor Weight Loss After Laparoscopic Sleeve Gastrectomy: Re-sleeve Vs. Gastric Bypass. Obes Surg. 2016;26.
AlSabah S, al Haddad E, Al-Subaie S, Ekrouf S, Alenezi K, Almulla A, et al. Short-Term Results of Revisional Single-Anastomosis Gastric Bypass After Sleeve Gastrectomy for Weight Regain. Obes Surg. 2018;28.
Antonopulos C, Rebibo L, Calabrese D, Ribeiro-Parenti L, Arapis K, Dhahri A, et al. Comparison of Repeat Sleeve Gastrectomy and Roux-en-Y Gastric Bypass in Case of Weight Loss Failure After Sleeve Gastrectomy. Obes Surg. 2019;29.
Bashah M, Aleter A, Baazaoui J, El-Menyar A, Torres A, Salama A. Single Anastomosis Duodeno-ileostomy (SADI-S) Versus One Anastomosis Gastric Bypass (OAGB-MGB) as Revisional Procedures for Patients with Weight Recidivism After Sleeve Gastrectomy: a Comparative Analysis of Efficacy and Outcomes. Obes Surg. 2020;30.
de la Cruz M, Büsing M, Dukovska R, Torres AJ, Reiser M. Short- to medium-term results of single-anastomosis duodeno-ileal bypass compared with one-anastomosis gastric bypass for weight recidivism after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2020;16.
de Moura DTH, Barrichello Jr S, de Moura EGH, de Souza TF, dos Passos Galvão Neto M, Grecco E, et al. Endoscopic sleeve gastroplasty in the management of weight regain after sleeve gastrectomy. Endoscopy. 2020;52.
Debs T, Petrucciani N, Kassir R, Juglard G, Gugenheim J, Iannelli A, et al. Laparoscopic Conversion of Sleeve Gastrectomy to One Anastomosis Gastric Bypass for Weight Loss Failure: Mid-Term Results. Obes Surg. 2020;30.
Dijkhorst PJ, Boerboom AB, Janssen IMC, Swank DJ, Wiezer RMJ, Hazebroek EJ, et al. Failed Sleeve Gastrectomy: Single Anastomosis Duodenoileal Bypass or Roux-en-Y Gastric Bypass? A Multicenter Cohort Study. Obes Surg. 2018;28.
D’Urso A, Vix M, Perretta S, Ignat M, Scheer L, Mutter D. Indications and Long-Term Outcomes of Conversion of Sleeve Gastrectomy to Roux-en-Y Gastric Bypass. Obes Surg. 2021;31.
Filip S, Hutopila I, Copaescu C. Re-sleeve Gastrectomy - An Efficient Revisional Bariatric Procedure - 3 Years Results. Chirurgia (Bucur). 2019;114.
Frieder JS, Aleman R, Gomez CO, Ferri F, Okida LF, Funes DR, et al. Outcomes of reoperative surgery in severely obese patients after sleeve gastrectomy: a single-institution experience. Surg Obes Relat Dis. 2020;16.
Gerges WB, Omran H, Makram F. Conversion of laparoscopic sleeve gastrectomy after weight loss failure into laparoscopic one anastomosis gastric bypass: short-term safety and efficacy and effect of indications on outcome. Surg Endosc. 2021. https://doi.org/10.1007/s00464-021-08374-5.
Jamal MH, Elabd R, AlMutairi R, Albraheem A, Alhaj A, Alkhayat H, et al. The Safety and Efficacy of One Anastomosis Gastric Bypass as a Revision for Sleeve Gastrectomy. Obes Surg. 2020;30.
Maselli DB, Alqahtani AR, Abu Dayyeh BK, Elahmedi M, Storm AC, Matar R, et al. Revisional endoscopic sleeve gastroplasty of laparoscopic sleeve gastrectomy: an international, multicenter study. Gastrointest Endosc. 2021;93.
Mehmet B, Yasemin A. Re-Sleeve Gastrectomy for Failed Primary Laparoscopic Sleeve Gastrectomy. J Coll Phys Surg Pak. 2019;29.
Nedelcu M, Noel P, Iannelli A, Gagner M. Revised sleeve gastrectomy (re-sleeve). Surg Obes Relat Dis. 2015;11.
Omarov T, Samadov E, Bayramov N, Unlu A, Coskun AK. The Effectiveness and Feasibility of Laparoscopic Re-sleeve Gastrectomy. Obes Surg. 2020;30.
Pizza F, D’Antonio D, Carbonell Asíns JA, Lucido FS, Tolone S, Docimo L, et al. One Anastomosis Gastric Bypass after Sleeve Gastrectomy Failure: Does a Single Procedure Fit for all? Obes Surg. 2021;31.
Quezada N, Hernández J, Pérez G, Gabrielli M, Raddatz A, Crovari F. Laparoscopic sleeve gastrectomy conversion to Roux-en-Y gastric bypass: experience in 50 patients after 1 to 3 years of follow-up. Surg Obes Relat Dis. 2016;12.
Rayman S, Assaf D, Azran C, Sroka G, Assalia A, Beglaibter N, et al. Sleeve Gastrectomy Failure—Revision to Laparoscopic One-Anastomosis Gastric Bypass or Roux-n-Y Gastric Bypass: a Multicenter Study. Obes Surg. 2021;31.
Rebibo L, Dhahri A, Robert B, Regimbeau J-M. Repeat sleeve gastrectomy: optimization of outcomes by modifying the indications and technique. Surg Obes Relat Dis. 2018;14.
Shimon O, Keidar A, Orgad R, Yemini R, Carmeli I. Long-Term Effectiveness of Laparoscopic Conversion of Sleeve Gastrectomy to a Biliopancreatic Diversion with a Duodenal Switch or a Roux-en-Y Gastric Bypass due to Weight Loss Failure. Obes Surg. 2018;28.
Moura DTH de, Moura EGH de, Thompson CC. Endoscopic sleeve gastroplasty: From whence we came and where we are going. World J Gastrointest Endosc. 2019;11.
Chakhtoura G, Zinzindohoué F, Ghanem Y, Ruseykin I, Dutranoy J-C, Chevallier J-M. Primary Results of Laparoscopic Mini-Gastric Bypass in a French Obesity-Surgery Specialized University Hospital. Obes Surg. 2008;18.
Yehoshua RT, et al. Laparoscopic sleeve gastrectomy—volume and pressure assessment. Obes Surg. 2008;18:1083.
Noel P, et al. Revised sleeve gastrectomy: another option for weight loss failure after sleeve gastrectomy. Surg Endosc. 2014;28:1096–102.
Nedelcu M, Noel P, Iannelli A, et al. Revised sleeve gastrectomy (re-sleeve). Surg Obes Relat Dis. 2015;11:1282–8.
Homan J, Betzel B, Aarts EO, van Laarhoven KJHM, Janssen IMC, Berends FJ. Secondary surgery after sleeve gastrectomy: Roux-en-Y gastric bypass or biliopancreatic diversion with duodenal switch. Surg Obes Relat Dis. 2015;11.
Casillas RA, Um SS, Zelada Getty JL, Sachs S, Kim BB. Revision of primary sleeve gastrectomy to Roux-en-Y gastric bypass: indications and outcomes from a high-volume center. Surg Obes Relat Dis. 2016;12.
Guan B, Chong TH, Peng J, Chen Y, Wang C, Yang J. Mid-long-term Revisional Surgery After Sleeve Gastrectomy: a Systematic Review and Meta-analysis. Obes Surg. 2019;29.
Matar R, Monzer N, Jaruvongvanich V, Abusaleh R, Vargas EJ, Maselli DB, et al. Indications and Outcomes of Conversion of Sleeve Gastrectomy to Roux-en-Y Gastric Bypass: a Systematic Review and a Meta-analysis. Obes Surg. 2021;31.
Aiolfi A, Micheletto G, Marin J, Bonitta G, Lesti G, Bona D. Resleeve for failed laparoscopic sleeve gastrectomy: systematic review and meta-analysis. Surg Obes Relat Dis. 2020;16.
van de Laar AW, van Rijswijk AS, Kakar H, Bruin SC. Sensitivity and Specificity of 50% Excess Weight Loss (50%EWL) and Twelve Other Bariatric Criteria for Weight Loss Success. Obes Surg. 2018;28.
van Rijswijk A-S, van Olst N, Schats W, van der Peet DL, van de Laar AW. What Is Weight Loss After Bariatric Surgery Expressed in Percentage Total Weight Loss (%TWL)? A Systematic Review. Obes Surg. 2021;31.
Shrier I, Boivin J-F, Steele RJ, Platt RW, Furlan A, Kakuma R, et al. Should Meta-Analyses of Interventions Include Observational Studies in Addition to Randomized Controlled Trials? A Critical Examination of Underlying Principles. Am J Epidemiol. 2007;166.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent does not apply.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• All revisional procedures for weight failure after sleeve gastrectomy result in weight loss
• Results suggest that one-anastomosis gastric bypass has a good balance between efficacy and safety
• Duodenal switch and single-anastomosis duodeno-ileal bypass were associated with high complication rates
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Franken, R.J., Sluiter, N.R., Franken, J. et al. Treatment Options for Weight Regain or Insufficient Weight Loss After Sleeve Gastrectomy: a Systematic Review and Meta-analysis. OBES SURG 32, 2035–2046 (2022). https://doi.org/10.1007/s11695-022-06020-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-022-06020-0