Abstract
Purpose
Obese patients have neurodegeneration of the optic nerve demonstrated by decreased peripapillary nerve fiber layer. Whether bariatric surgery reverses this neurodegenerative process has not been explored. We aimed to evaluate the impact of bariatric surgery in the structure of the retina and optic nerve.
Methods
Multicentric observational study. Obese patients scheduled for bariatric surgery were consecutively recruited and included in the study and evaluated before and 6–12 months after the intervention. The retinal structure was evaluated as retinal thickness in the different retinal layers in the foveal, perifoveal, and parafoveal regions using optical coherence tomography. Choroidal thickness and optic nerve retinal nerve fiber layer thickness were also evaluated.
Results
Eighty eyes from 40 participants were included. Globally, we found a significant thickening of the retina after bariatric surgery (foveal: 273.5 (21.5) μm vs 280.0 (28.8) μm, p < 0.001; parafoveal 332.4 ± 17.8 μm vs 336.6 ± 15.9 μm, p = 0.003; perifoveal: 293.4 ± 13.8 μm vs 295.7 ± 14.9 μm; p = 0.001), whereas no significant differences were found for the ganglion cell layer, choroid, or peripapillary nerve fiber layer thickness. The retinal thickening was confined to inner retinal layers and was independent of the diabetic status of the patients. After multivariate adjustment, HbA1c variation, preoperative C-peptide, preoperative hypertension, preoperative OSA, and preoperative LDL and TG levels seem to be clinical predictors of retinal thickening.
Conclusions
We found a significant thickening of the retina after bariatric surgery that was independent of the diabetic status. The thickening was confined to inner retinal layers and may represent and improve perfusion. The peripapillary nerve fiber layer remained unchanged after the surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obesity is a growing worldwide public health problem. Globally, it is estimated that more than 1.9 billion adults are overweight and, of these, 650 million are obese [1]. Obesity is associated with long-term increased mortality and morbidity due to its effect on the cardiovascular system by acting as a promoter for pathophysiological changes in vasculature and tissues [2]. Moreover, obesity has also been linked to diabetes, cancer, and other chronic diseases, representing around 4 million deaths and 120 million disability-adjusted life years worldwide [3].
It is known that the multiple adverse effects on health that obesity induces may be reversed or improved by successful weight loss [4]. As sustained weight loss is hardly achieved by conservative means, bariatric surgery emerged as a solution to face the burden that obesity and its associated co-morbidities, quality of life, and all-cause mortality represent. As more studies demonstrate that the long-term efficacy and benefits of this invasive procedure markedly overweigh the possible complications, bariatric surgery grows as a standard treatment for obesity and its related comorbidities [4,5,6].
The demonstrated efficacy of bariatric surgery in reverting the metabolic status of obese patients rendered this procedure as an option for the treatment of type 2 diabetes [7]. However, there is a debate in the literature if the benefits are also true for microvascular complications [8]. Although normalization of blood glucose levels is an important goal of diabetic retinopathy monitoring, studies 30 years ago demonstrated a paradoxical early aggravation of diabetic retinopathy during intensive insulin treatment in young type 1 diabetic patients [9,10,11]. Based on this rationale, some studies have been exploring the evolution of diabetic retinopathy after bariatric surgery, achieving controversial results [12,13,14,15,16]. As direct comparison cannot be performed from the results of these studies due to the heterogeneity of the samples used (different surgical procedures, different severity degrees of diabetic retinopathy, absent matched non-surgical control group), the debate continues. Besides the importance of documenting the evolution of diabetic retinopathy, the clinical appearance of fundus diabetic signs represents a late stage where the microcirculation is already damaged. Thus, it is essential to first document the changes that occur structurally in the retina, choroid, and optic nerve of obese patients submitted to bariatric surgery in an early non-diabetic/non-retinopathic phase so that we can better perceive the impact of bariatric surgery in these structures and, consequently, in eye diseases. Spectral-domain optical coherence tomography (OCT) uses near-infrared light to provide cross-sectional images of the retinal architecture, thus enabling the non-invasive monitoring of retinal degeneration through the quantification of the retinal layers [17]. This device enables follow-up acquisitions with demonstrated high reproducibility for the same individual, thus being an important tool for monitoring changes in the eye structures along time. By using this technology, we have previously demonstrated that obese patients have neurodegeneration of the optic nerve, as demonstrated by the decreased peripapillary retinal nerve fiber layer thickness that these patients have when compared with healthy controls [18]. Whether bariatric surgery reverses or not this neurodegenerative process has not been explored yet.
The evaluation of structural changes in the retina, choroid, and optic nerve of obese patients submitted to bariatric surgery may bring some insights on the effect of the metabolic reversion not only in the eye but also in a systemic perspective. The aim of this study is to evaluate the impact of bariatric surgery in the structure of the retina, choroid, and optic nerve in obese non-diabetic and diabetic non-retinopathic patients.
Methods
This is a prospective study that was conducted in two Reference Centers for Obesity Treatment (Centro Hospitalar e Universitário São João, Porto, Portugal and Centro Hospitalar de Entre o Doutor e Vouga, Santa Maria da Feira, Portugal). Informed consent was obtained from all participants, and all procedures were performed in accordance with the ethical standards of the Hospital Ethics Committees and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Sampling and Preoperative Evaluation
Participants were prospectively recruited from January 2018 to December 2018. Obese patients that were candidates for bariatric surgery after multidisciplinary evaluation were consecutively invited to participate in the study. A preoperative systemic evaluation was performed including the evaluation of comorbidities, and body mass index (BMI) was determined using the formula: weight (kg)/height (m2). We only included patients with BMI > 35 kg/m2 with no previous systemic disease besides hypertension, diabetes, dyslipidemia, or obstructive sleep apnea. In this evaluation, a blood sample and urine were collected. All included subjects were also submitted to preoperative complete ophthalmological exam, including the imaging study detailed below. Individuals were excluded if they had any stage of diabetic retinopathy, a personal or familial history of glaucoma or ocular hypertension, uveitis, optic neuropathy, age-related macular degeneration, vitreoretinal, optic nerve or choroidal vascular diseases, refractive error greater than 6/− 6 diopters, recent history of decreased visual acuity, previous refractive or intraocular surgery, narrow anterior chamber angle, and secondary causes of glaucoma, such as of pigment dispersion syndrome or pseudoexfoliation syndrome.
Follow-up Evaluation
Participants were submitted to bariatric surgery (gastric bypass) and both systemic and ophthalmological follow-up systemic evaluations were scheduled between 6 and 12 months after the surgery. The parameters evaluated included those from the preoperative evaluation detailed below.
Clinical and Serum Analyses
Anthropometric data (weight, height) were converted to the body mass index (BMI) for analysis, according to the formula: BMI = weight (kg)/height (m2). Data on comorbidities (hypertension, diabetes, dyslipidemia, and existing obstructive sleep apnea (OSA)) were also collected.
The fasting serum profile included serum hemoglobin (Hb, g/dL), total protein content (g/dL), total cholesterol (mg/dL), high-density lipoprotein cholesterol (HDL, mg/dL), low-density lipoprotein cholesterol (LDL, mg/dL), triglycerides (TG, mg/dL), glucose (Glu, mg/dL), glycosylated hemoglobin (HbA1c, %), creatinine (Cr, mg/dL), C-peptide (μg/mL), and insulin (μU/mL). Urine microalbumin (mg/24 h) was also quantified. Glomerular filtration rate (GFR, mL/min/1.73 m2) was estimated using the CKD-EPI equation [19].
Image Acquisition and Analysis
The imaging protocol was explained to the participants before any acquisition. All images were obtained by the same trained ophthalmic professional and in the same environment conditions. Acquisitions were repeated if necessary to obtain high-quality images. Both eyes from the same participant were inputted for analysis.
Heidelberg Spectralis spectral-domain optical coherence tomography (SD-OCT Heidelberg Engineering, Heidelberg, Germany) was employed to all patients to evaluate macular ultrastructure by acquiring a horizontal raster cube scan centered at the fovea with 61 lines (120 μm apart) covering the 30° × 25° posterior pole area. All scans were averaged nine times using the TruTrack technology from Heidelberg. Images were manually reviewed, and low-quality scans were excluded. Retinal layers were segmented using an automated proprietary software algorithm (Version 1.10.2.0, Spectralis; Heidelberg Engineering, Heidelberg, Germany): the global retinal (all layers), the retinal nerve fiber layer, the ganglion cell layer, the inner retinal layers, the outer retinal layers. Each image was reviewed in a masked fashion. To enhance accuracy, if necessary, segmentation lines were adjusted, and non-centered or poor-quality images were discarded. For the retinal layers, three concentric regions were defined based on the Early Treatment Diabetic Retinopathy Study (ETDRS) macula grid centered on the umbo: the fovea of 1 mm diameter, the parafoveal ring 1–3 mm from the umbo, and the perifoveal ring 3–6 mm from the umbo. The automated quantification of retinal thickness included in the device was used. The choroid was manually segmented in the acquired images. Choroidal thickness was measured using the calipers provided in the Spectralis Heidelberg software in the subfoveal area and at 500-μm intervals from the fovea to 1500 μm in the nasal and temporal regions. The “follow-up” function from the device was used to prospectively acquire scans in the same position in follow-up evaluations.
Heidelberg Spectralis SD-OCT (Heidelberg Engineering, Heidelberg, Germany) was also employed to assess optic nerve retinal nerve fiber layer thickness. A 256 A-scan image with 3.5-mm diameter circular rings, centered on the optic nerve, was acquired, and the mean value was expressed as retinal nerve fiber layer thickness. Each image was reviewed in a masked fashion. To enhance accuracy, if necessary, segmentation lines were adjusted, and non-centered or poor-quality images were discarded. Retinal nerve fiber layer thickness (μm) was recorded as global and six regional (temporal, temporal superior, nasal superior, nasal, nasal inferior, and temporal inferior) sectors. The follow-up function from the device was used to prospectively acquire scans in the same position in follow-up evaluations.
Fundus photography was also performed in each evaluation to access diabetic retinopathy signs or other relevant fundus abnormalities.
Statistics
The sample size was estimated to detect an effect size of 0.5, a power of 90%, and an alpha value of 0.05. Using these criteria, at least 36 individuals should be used as reference for sampling. Descriptive statistics for continuous variables are presented as mean and standard deviation (SD) or as median (interquartile range or range), according to the skewness of the distribution that was evaluated using Kolmogorov-Smirnov test. Categorical variables are presented as number (n) and percentage (%). For continuous variables, intragroup comparisons were performed using paired t test or Wilcoxon test depending on the skewness of the distributions. A linear regression model was used for adjustments to possible clinical predictors. Univariate analysis was primarily performed for clinically relevant predictors selected according to literature, and a final multivariate model using the forward approach was applied to significant predictors found in univariate analysis. All statistical analyses were performed using IBM SPSS Statistics v. 25 (SPSS Inc., Chicago, IL, USA). Significance was set at 0.05.
Results
Eighty eyes from 40 patients were included in this study (19 from Centro Hospitalar de Entre o Douro e Vouga and 21 from Centro Hospitalar e Universitário São João). The main baseline preoperative characteristics of our sample are described in Table 1.
No patients were excluded from the study as none of the patients had diabetic retinopathy or any other condition that was an exclusion criterion.
Postoperative Evolution
The BMI curve after bariatric surgery is demonstrated in Fig. 1. No relevant systemic intercurrences were registered along follow-up evaluations.
Retinal, Choroid, and Optic Nerve Structure
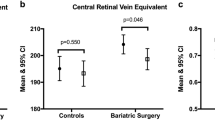
Table 2 represents the retinal layers, optic nerve, and choroid thickness before bariatric surgery and at the final follow-up. Globally, we verified a significant increase in the retinal thickness after bariatric surgery that was due to increased inner layer thickness. No significant differences were found for choroidal and optic nerve retinal nerve fiber layer thickness.
Clinical Predictors for Retinal Thickening
We investigated possible clinical predictors for global retinal thickening after bariatric surgery in the foveal, parafoveal, and perifoveal regions. The results from the univariate analysis are presented in supplementary table 1. The final predictors for global retinal thickness included in multivariate adjustment are presented in Table 3. Briefly, increased HbA1c drop after surgery was associated with retinal thickening after surgery in the foveal region. In the parafoveal region, the same was verified for increased preoperative C-peptide. In the perifoveal region, both preoperative hypertension and OSA predicted an increase in retinal thickness after surgery. Serum LDL and TG also remained associated with postoperative retinal thickening in this region.
Diabetic Retinopathy
No diabetic patient developed fundoscopic or tomographic signs of diabetic retinopathy or maculopathy in the follow-up evaluations.
Discussion
In this study, we explore the impact of bariatric surgery on the structure of the retina, choroid, and optic nerve. Globally, we found a significant thickening of the retina after bariatric surgery, whereas no significant differences were found for the choroid and optic nerve thickness. The retinal thickening was confined to inner retinal layers and was independent of the diabetic status of the patients. After multivariate adjustment, HbA1c variation, preoperative C-peptide, preoperative hypertension, preoperative OSA, and preoperative LDL and TG levels seem to be clinical predictors of retinal thickening after bariatric surgery.
As bariatric surgery grows worldwide as a standard procedure for obesity and its comorbidity treatment, it is essential to recognize the multiorgan impact of the procedure so that we can further understand the possible long-term complications and need of monitoring these patients. Regarding the eye, few studies have documented the impact of bariatric surgery, and the interest has been focused on documenting the evolution of clinically evident signs of diabetic retinopathy after the surgery. Diabetic retinopathy represents a late phase of the microvascular disease process. Thus, our aim with this study was to evaluate the impact of bariatric surgery on the structure of the retina, optic nerve, and choroid in an earlier stage in non-diabetic or diabetic non-retinopathic patients, so that we can perceive the impact of the procedure in the absence of clinically evident pathological conditions. In our sample, no patients were excluded due to the presence of diabetic retinopathy, and no additional case of diabetic retinopathy was noted after the surgery. Thus, in our study, bariatric surgery did not trigger the development of diabetic retinopathy, which corroborates the findings of previous studies [20,21,22].
To our knowledge, only three human-based studies have explored the association between bariatric surgery and eye structures. Dogan et al. [23] documented the increased macular thickness in obese patients submitted to sleeve gastrectomy, whereas topographic optic nerve parameters did not show significant differences. Posarelli et al. [24] studied a sample of obese patients submitted to gastric bypass and also documented an increase in foveal thickness. Brynskov et al. [25] restricted their study to type 2 diabetic patients submitted to bariatric surgery and also documented a thickened macula that correlated with the fall in HbA1c value. Our study corroborates the findings of the previous studies as, globally, we found a significant thickening of the retina after bariatric surgery. However, we further explored the structural changes and concluded that the inner retina layers were thickened, not the outer ones, and this occurred independently of diabetic status of the patients. In addition, we found no significant differences for the choroid and neuronal structures after the surgery, including the retina and optic nerve fiber layer thickness and ganglion cell layer thickness. Thus, our study brings some new important insights to the previous literature. Firstly, as mentioned, we documented a retinal thickening after bariatric surgery that was due to increased inner retinal layers. As the inner retina is densely vascularized [26], it is possible that this thickening of the retina represents an increased and improved perfusion of the retina that is triggered by bariatric surgery. It is known that obesity is associated with microvascular dysfunction that culminates in reduced vasodilator reactivity to flow [27]. By improving the metabolic profile, bariatric surgery may reverse this stage. In fact, recent studies have demonstrated that bariatric surgery may improve peripheral endothelial function and coronary microvascular dilator function [28] as well as improve vascular reactivity in the brain [29]. Future studies documenting the changes in retinal microvasculature using OCT angiography may further clarify our findings and explore the pathophysiology behind this process.
Besides the potential vascular improvement, there were no specific changes in the peripapillary nerve fiber layer. Our group has previously demonstrated that obese patients have optic nerve degeneration when compared with matched controls [18]. As no significant changes were noticed in neuronal components after bariatric surgery, the neurodegenerative component of obesity may be irreversible despite the improved metabolic condition; this is in accord with what occurs in other retinal neurodegenerative diseases [30].
In our study, we also explored the association between clinical factors and the retinal thickening after bariatric surgery. Interestingly, although the clinical diagnosis of diabetes did not impact the retina changes, we found both increased HbA1c drop after the surgery and increased preoperative serum C-peptide to be predictors of retinal changes after the intervention, regardless of the diabetic status. Our findings support the recent evidence that the hyperglycemia and insulin resistance in obesity may have a role in microvascular tissue damage even before the clinical diagnosis of diabetes [31]. In fact, Carlsson et al. [32] have recently reported the results from a prospective, non-randomized, matched cohort study of 4047 patients that supports our findings. Carlsson and colleagues compared microvascular disease outcomes after obesity surgery versus usual care, with analyses stratified by baseline glycemic status (normal, prediabetes, screen-detected diabetes, and established diabetes). The researchers showed that obesity surgery was associated with reduced risk of incident microvascular disease in all subgroups of patients, but that the greatest reduction in relative risk was in patients with prediabetes at baseline. The association between hyperglycemia, insulin resistance, and microvascular impairment in non-diabetic obese individuals is a topic that warrants further research.
We acknowledge that our study has several limitations. Firstly, we are aware that preoperative obese patients’ metabolic status is heterogeneous and may vary according to several non-controllable factors: the duration of the exposition to high-fat diet, the severity and duration of the comorbidities (and the treatment that was applied), the genomic and familiar environment, and the individual predisposition to a pro-inflammatory response, among others. Secondly, a longer follow-up would determine if these changes are correlated or not with possible clinically relevant findings in the future. Besides all the potential limitations, our study has as an important strength the fact that it explores the structural remodeling that occurs after bariatric surgery in the eye, thus bringing new insights into the possible subjacent physiological processes. OCT is a robust technology with high repeatability that has been previous demonstrated [33]. This technology has been widely used in ophthalmology as a strong correlation has been demonstrated between OCT images and retina histology [34]. Although these changes are not clinically meaningful in the sense that no changes in vision occurred and no clinical attitude (treatment, intervention) is expected to be performed because of the retinal thickness increase, they are meaningful in a research perspective as retinal thickness is not expected to increase with time [35].
Conclusion
With bariatric surgery implementation as a first-line treatment continuing to grow worldwide, it is essential to monitor its impact at a multisystemic level. Bariatric surgery induces a retinal thickening that was independent of the diabetic status of the patient. The retinal thickening was confined to inner retinal layers and may represent and improve perfusion after the surgery. Neuronal structures, including the ganglion cell layer and macular and optic nerve layer thickness, remained unchanged after the surgery; and thus, the neurodegeneration component in obesity may not be reversible with bariatric surgery. Further studies exploring retinal microvasculature changes using OCT-angiography may explore and reinforce these findings.
References
WHO. Obesity and overweight - key facts. 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
Ayer J, Charakida M, Deanfield JE, et al. Lifetime risk: childhood obesity and cardiovascular risk. Eur Heart J. 2015;36:1371–6.
Afshin A, Forouzanfar MH, Reitsma MB, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27.
Bray GA, Frühbeck G, Ryan DH, et al. Management of obesity. Lancet. 2016;387:1947–56.
Fried M, Ribaric G, Buchwald JN, et al. Metabolic surgery for the treatment of type 2 diabetes in patients with BMI. Obes Surg. 2010;20:776–90. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/s11695-010-0113-3
Wolfe BM, Kvach E, Eckel RH. Treatment of obesity: weight loss and bariatric surgery. Circ Res. 2016;118:1844–55. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27230645http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC4888907
Sheng B, Truong K, Spitler H, et al. The long-term effects of bariatric surgery on type 2 diabetes remission, microvascular and macrovascular complications, and mortality: a systematic review and meta-analysis. Obes Surg. 2017;27:2724–32.
Feldman-Billard S, Larger É, Massin P. Early worsening of diabetic retinopathy after rapid improvement of blood glucose control in patients with diabetes. Diabetes Metab Elsevier Masson SAS. 2017;44:1–11. https://doi.org/10.1016/j.diabet.2017.10.014.
Dahl-Jørgensen K, Brinchmann-Hansen O, Hanssen KF, et al. Rapid tightening of blood glucose control leads to transient deterioration of retinopathy in insulin dependent diabetes mellitus: the Oslo study. Br Med J (Clin Res Ed). 1985;290:811–5.
Ballegooie EV, Hooymans JMM, Timmerman Z, et al. Rapid deterioration of diabetic retinopathy during treatment with continuous subcutaneous insulin infusion. Diabetes Care. 1984;7:236–42. Available from: http://www.bmj.com/cgi/doi/10.1136/bmj.290.6471.811
Lauritzen T, Larsen H-W, Frost-Larsen K, et al. Effect of 1 year of near-normal blood glucose levels on retinopathy in insulin-dependent diabetics. Lancet. 1983;321:200–4. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673683925850
Brynskov T, Laugesen CS, Svenningsen AL, Floyd AK, Sørensen TL. Monitoring of diabetic retinopathy in relation to bariatric surgery: a prospective observational study. Obes Surg [Internet]. Obes Surg; 2016;26:1279–1286. https://doi.org/10.1007/s11695-015-1936-8
Miras AD, Chuah LL, Khalil N, et al. Type 2 diabetes mellitus and microvascular complications 1 year after Roux-en-Y gastric bypass: a case–control study. Diabetologia. 2015;58:1443–7.
Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA - J Am Med Assoc. 2014;311:2297–304.
Viljanen A, Soinio M, Cheung CY lui, Hannukainen JC, Karlsson HK, Wong TY, et al. Effects of bariatric surgery on retinal microvascular architecture in obese patients. Int J Obes. Springer US; 2018; Available from: https://doi.org/10.1038/s41366-018-0242-7
Abbatini F, Capoccia D, Casella G, et al. Long-term remission of type 2 diabetes in morbidly obese patients after sleeve gastrectomy. Surg Obes Relat Dis. 2013;9:498–502.
Demir S, Özer S, Alim S, Güneş A, Ortak H, Yılmaz R. Retinal nerve fiber layer and ganglion cell-inner plexiform layer thickness in children with obesity. Int J Ophthalmol. 2016 [cited 2018 Aug 4];9:434–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27158616
Laiginhas R, Guimarães M, Cardoso P, et al. Retinal nerve fiber layer thickness decrease in obesity as a marker of neurodegeneration. Obes Surg. 2019;
Raman M, Middleton RJ, Kalra PA, et al. Estimating renal function in old people: an in-depth review. Int Urol Nephrol Springer Netherlands. 2017;49:1979–88.
Miras AD, Chuah LL, Lascaratos G, et al. Bariatric surgery does not exacerbate and may be beneficial for the microvascular complications of type 2 diabetes. Diabetes Care. 2012;35:2012.
Morén A, Sundbom M, Ottosson J, et al. Gastric bypass surgery does not increase the risk for sight-threatening diabetic retinopathy. Acta Ophthalmol. 2017:2015–8.
Coleman KJ, Haneuse S, Johnson E, et al. Long-term microvascular disease outcomes in patients with type 2 diabetes after bariatric surgery: evidence for the legacy effect of surgery. Diabetes Care. 2016;39:1400–7.
Dogan B, Dogan U, Erol MK, Habibi M, Bulbuller N. Optical coherence tomography parameters in morbidly obese patients who underwent laparoscopic sleeve gastrectomy. J Ophthalmol. Hindawi Limited; 2016 [cited 2018 May 20];2016:5302368. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27413543
Posarelli C, Salvetti G, Piaggi P, et al. Ophthalmologic evaluation of severely obese patients undergoing bariatric surgery: a pilot, monocentric, prospective, open-label study. PLoS One. 2019;14:1–11.
Brynskov T, Laugesen CS, Floyd AK, et al. Thickening of inner retinal layers in the parafovea after bariatric surgery in patients with type 2 diabetes. Acta Ophthalmol. 2016;94:668–74.
Daruich A, Matet A, Moulin A, Kowalczuk L, Nicolas M, Sellam A, et al. Mechanisms of macular edema: beyond the surface. Prog. Retin. Eye Res. Elsevier Ltd; 2018. Available from: https://doi.org/10.1016/j.preteyeres.2017.10.006
Grizelj I, Cavka A, Bian JT, et al. Reduced flow-and acetylcholine-induced dilations in visceral compared to subcutaneous adipose arterioles in human morbid obesity. Microcirculation. 2015;22:44–53.
Tarzia P, Lanza GA, Sestito A, et al. Long-term effects of bariatric surgery on peripheral endothelial function and coronary microvascular function. Obes Res Clin Pract. 2017;11:114–7.
Tucker WJ, Thomas BP, Puzziferri N, et al. Impact of bariatric surgery on cerebral vascular reactivity and cognitive function: a non-randomized pilot study. Pilot Feasibility Stud. 2020;6:1–13.
Kadłubowska J, Malaguarnera L, Wąż P, et al. Neurodegeneration and neuroinflammation in diabetic retinopathy: potential approaches to delay neuronal loss. Curr Neuropharmacol. 2016;14:831–9.
Dimitriadis GK, Randeva HS, Miras AD. Microvascular complications after metabolic surgery. Lancet Diabetes Endocrinol . Elsevier Ltd; 2017;5:240–1. Available from: https://doi.org/10.1016/S2213-8587(17)30042-6
Carlsson LM, Sjöholm K, Karlsson C, et al. Long-term incidence of microvascular disease after bariatric surgery or usual care in patients with obesity stratified by baseline glucose status. Lancet Diabetes Endocrinol. 2017;5:271–9.
Pierro L, Giatsidis SM, Mantovani E, Gagliardi M. Macular thickness interoperator and intraoperator reproducibility in healthy eyes using 7 optical coherence tomography instruments. Am J Ophthalmol. Elsevier Inc.; 2010;150:199–204.e1. Available from: https://doi.org/10.1016/j.ajo.2010.03.015
Vajzovic L, Hendrickson AE, O’Connell RV, et al. Maturation of the human fovea: correlation of spectral-domain optical coherence tomography findings with histology. Am J Ophthalmol. 2012;154:779–789.e2. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3624763/pdf/nihms412728.pdf
Nieves-Moreno M, Martínez-de-la-Casa JM, Morales-Fernández L, et al. Impacts of age and sex on retinal layer thicknesses measured by spectral domain optical coherence tomography with Spectralis. PLoS One. 2018;13:1–9.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This manuscript is not submitted or published elsewhere.
Electronic Supplementary Material
ESM 1
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Laiginhas, R., Guimarães, M., Cardoso, P. et al. Bariatric Surgery Induces Retinal Thickening Without Affecting the Retinal Nerve Fiber Layer Independent of Diabetic Status. OBES SURG 30, 4877–4884 (2020). https://doi.org/10.1007/s11695-020-04904-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-020-04904-7