Abstract
Study aim
Retinal microvasculature changes reflect systemic small vessel damage from obesity. The impact of bariatric surgery induced weight loss on the microvasculature is relatively unknown. We hypothesized that weight loss following bariatric surgery would be associated with improved structural changes in the retinal microvasculature, reflecting an overall improvement in microvascular health.
Methods
The study included 22 obese subjects scheduled for bariatric surgery (laparoscopic Roux-en-Y gastric bypass or a sleeve gastrectomy) and 15 lean, age-matched controls. Ophthalmic examination, including fundus photography, was performed at baseline and 6-months. Retinal microvasculature caliber was analysed quantitatively using a semi-automated computer program and summarized as central retinal artery equivalent (CRAE) and venular equivalent (CRVE).
Results
Mean weight loss at 6 months was 26.1 kg ± 8 kg in the bariatric surgery group. Retinal artery caliber increased (136.0 ± 1.4 to 141.4 ± 1.4 µm, p = 0.013) and venular caliber decreased (202.9 ± 1.9 to 197.3 ± 1.9 µm, p = 0.046) in the bariatric surgery group by 6 months, with no change in arteriolar (136.6 ± 1.1 to 134.5 ± 1.2, p = 0.222) or venular (195.1 ± 2.1 to 193.3 ± 2.2, p = 0.550) caliber in the control group. The arteriolar to venular ratio increased in the bariatric surgery group, with no change in the control group at 6 months.
Conclusions
The findings suggest obesity-related microvascular changes are reversible after bariatric surgery-induced weight loss. The capacity for the retinal microvasculature to improve following bariatric surgery suggests plasticity of the human microvasculature early in the disease course.
Similar content being viewed by others
Introduction
Bariatric surgery has been shown to be an effective treatment for metabolic abnormalities and reduces the risk of cardiovascular disease (CVD) events and mortality [1, 2]. It improves glucose homeostasis, often more effectively than pharmaceutical and behavioral approaches, leading to remission of type 2 diabetes (T2D) [3, 4]. However, little is known about the impact of bariatric surgery on the microvasculature [5]. This association is particularly important as the role of the microvasculature in the pathogenesis of obesity related CVD [6, 7] and as a particularly distinguishing feature of increased CVD mortality among those with T2D, are recognized [8].
Changes in the retinal microvasculature reflect damage from obesity [9,10,11], and related conditions including hypertension [12, 13], T2D [14, 15] and other chronic disease processes [16]. The retinal arterioles are narrower in patients with hypertension [17, 18] and the venules are wider in people with obesity, diabetes or higher levels of systemic inflammatory markers [15, 19]. It is thought these retinal changes may be mediated through endothelial dysfunction [20], inflammation [21] and other mechanisms activated by increased adiposity [21]. Such a pattern of retinal microvascular changes (narrower arterioles and wider venules) have been observed early in the course of impaired glucose metabolism [22] and are closely associated with an increased future risk of diabetes [23] as well as its microvascular complications: diabetic retinopathy [24] and diabetic nephropathy [16]. Evidence of an association between retinal arteriolar narrowing and myocardial perfusion has also been shown, suggesting retinal arteriolar narrowing may serve as a marker of coronary microvascular disease [25].
To study the impact of bariatric surgery on the retinal microvasculature provides an important opportunity to further our understanding of the impact of obesity on microvascular disease, and the reversibility of these changes, essential for the development of appropriately timed treatment and intervention in high-risk populations. We hypothesized that weight loss from bariatric surgery would be associated with improved structural changes in the retinal microvasculature, specifically with a reduction in retinal arteriolar narrowing and venular widening.
Methods
We conducted a prospective comparative study among 27 morbidly obese women who were scheduled to undergo bariatric surgery and 15 age-matched, lean controls. Full details are available elsewhere. In brief the Inclusion criteria for the bariatric surgery group were: (1) BMI > 40 kg/m2 or > 35 kg/m2 with an additional risk factor, (2) age 18–60 years or (3) conservative treatments for obesity had failed. Exclusion criteria for patient population were: (1) BMI > 60 kg/m2 or weight > 170 kg, (2) mental disorder or poor compliance, (3) eating disorder or excessive alcohol consumption, (4) active ulcus disease and (5) fasting plasma glucose > 7 mM or requiring insulin treatment [26]. Before surgery, 11 obese subjects had type 2 diabetes and 4 had impaired glucose tolerance. Anti-diabetic and antihypertensive drugs were discontinued before the study started. The study followed the tenets of the Declaration of Helsinki. The ethics committee of the Hospital District of Southwestern Finland approved the study, and all subjects gave their written informed consent before commencing the study. Four patients withdraw their consent before the study commenced and 1 during the study phase, so 22 obese subjects were studied before and 6 months after bariatric surgery. Fifteen control subjects were studied at baseline and 6 months.
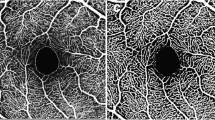
Clinical screening included physical examination, medical history, anthropometric measurements, blood pressure (BP) and laboratory tests (fasting plasma glucose, lipid profile and high sensitivity C-reactive protein). Bariatric surgery was laparoscopic Roux-en-Y gastric bypass or sleeve gastrectomy as previously described [27]. The ophthalmic study included corrected visual acuity and biomicroscopy. Pupils were dilated using tropicamide 1% and phenylephrine hyprochloride 2.5%, and digital fundus images were taken using 50-degree camera (Topcon TRC-50DX type IA, Tokyo, Japan). The images were labelled with a random number prior to sending for retinal grading. Two retinal images of each eye were taken, one centred on the optic disc and the other centred on the fovea. Images of the right eye were analysed.
The retinal microvasculature was measured using the Singapore I Vessel Assessment [SIVA], software version 3.0). SIVA automatically identifies the optic disc, places a grid with reference to the centre of optic disc, identifies vessel type and calculates retinal microvascular parameters. A trained grader was responsible for the visual evaluation of SIVA automated measurements and manually intervened if necessary, according to a standardized protocol [28]. The measured area was standardized and defined within the region between 0.5 and 2.0 disc diameters away from the disc margin and all visible vessels coursing through the specified zone were measured. The intra- and inter-grader reliability for the measurement was assessed and reported previously [28].
Based on the revised Knudtson-Parr-Hubbard formula, the retinal arteriolar and venular calibers were summarized as central retinal artery equivalent (CRAE) and central retinal vein equivalent (CRVE) [29]. Total, arteriolar and venular fractal dimensions were calculated from a skeletonized line tracing using the box-counting method, and represents a “global” measure that summarizes space-filling by the whole branching pattern of the retinal vascular tree [30]. Larger values indicated a denser, more complex branching pattern. Retinal vascular tortuosity was computed as the integral of the curvature square along the path of the vessel, normalized by the total path length; this measure is dimensionless as it represents a ratio measure [31]. The estimates were summarized as retinal arteriolar and venular tortuosity separately, representing the average tortuosity of arterioles and venules, respectively; a smaller tortuosity value indicates a straighter retinal vessel. Retinal vascular branching angle was defined as the first angle subtended between two daughter vessels at each vascular bifurcation [32]. The estimates were summarized as retinal arteriolar branching angle and retinal venular branching angle, representing the average branching angle of arterioles and venules, respectively. The retinal vascular branching angle measurement was not bounded by the standardized measured region (within 0.5 and 2.0 disc diameters).
Statistical analysis
The data analysis was performed using SPSS version 22 (SPSS Inc., USA). Descriptive information for each variable was derived and distributions assessed to determine normality. Data are presented as means ± SD. A p value of <0.050 was considered statistically significant. Student’s t tests and repeated measures anova’s were used to assess between- and within-group differences.
Results
Characteristics of subjects by study group are shown in Table 1. The mean age of the bariatric surgery group was 43 years and for the control group was 45 years. There was no difference in mean systolic (mean ± SD, 130 mmHg ± 15 vs 128 mmHg ± 15, p = 0.716) or diastolic BP (84 mmHg ± 10 vs 79 mmHg ± 9, p = 0.137) between the bariatric surgery and control group at baseline. Mean weight loss 6 months post bariatric surgery was 26.1 ± 8.0 kg. Fasting plasma glucose and high sensitivity C-reactive protein decreased and HDL cholesterol increased in the bariatric surgery group, 6 months after surgery.
Retinal architecture
Ophthalmic and retinal microvascular characteristics of the study groups are shown in Table 2. Over 6 months, no change in refraction was observed in either group. Bariatric surgery increased CRAE (from 136.0 ± 1.4 µm to 141.4 ± 1.4 µm, p = 0.013) and decreased CRVE (from 202.9 ± 1.9 µm to 197.3 ± 1.9 µm, p = 0.046), 6-months after surgery, with no change in CRAE (from 136.6 ± 1.1 to 134.5 ± 1.2, p = 0.222) or CRVE (195.1 ± 2.1 to 193.3 ± 2.2, p = 0.550) in the control group (Fig. 1). The AVR increased in the bariatric surgery group (from 0.67 ± 0.01 to 0.72 ± 0.01, p = 0.002), with no change in the control group (from 0.71 ± 0.01 to 0.70 ± 0.01, p = 0.550). No change was found for branching angles or fractal dimension.
Discussion
Our study assessed the beneficial impact of bariatric surgery induced weight loss on the human microvasculature in vivo. We showed that over 6 months, bariatric surgery improved the structure of the retinal microvasculature, leading to a reduction in retinal arteriolar narrowing and venular widening (parameters previously associated with increased CVD risk), with no changes observed in the control group. These findings are consistent with the well-known association of bariatric surgery with a reduction in obesity [33] and CVD risk factors [34] and provides additional insights into the effect of weight loss on improving microvascular health.
The present study suggests that bariatric surgery has a beneficial impact on the microvasculature 6 months after surgery. While increased adiposity has been independently associated with widening of venules [9] and prospectively venule widening has been positively associated with an increased risk of becoming obese [35], implicating microvascular dysfunction in the etiology of obesity, only one previous study has assessed the impact of bariatric surgery directly on the retinal microvasculature. A study by Lammert et al. who followed a group (predominantly without diabetes) for 9 months post bariatric surgery, showed significant improvement in retinal venular widening [5]. This change was associated with improved insulin-sensitivity and reduced inflammatory markers. The present study confirms this finding and extends it, with the important addition of a control group, which showed across 6 months, that the retinal microvasculature of age-matched lean controls, remained unchanged. This suggests the changes observed in the bariatric surgery group were a result of the surgery and not time-dependent change of the microvasculature in middle-age. In a study by Johnson et al, bariatric surgery among a group with T2D was associated with an adjusted hazard ratio of 0.22, (95% CI 0.09–0.49) for microvascular events [34]. The study used a combined measure of microvascular events, which included end stage complications: blindness, laser eye or retinal surgery, post-traumatic amputation and arteriovenous access creation. The study did not include any analysis of earlier clinically significant microvascular measures and did not have information available on the duration of diabetes, which may have led to residual confounding between the groups.
It is well established in the literature that arterioles and venules are differentially associated with cardiometabolic risk factors. Narrower arterioles in general are associated with higher BP [12], while wider venules with inflammation and obesity [9]. The AVR provides a ratio of the two measures. We have previously reported that each SD reduction in the AVR was associated with a 30% increased risk of hypertension, independent of baseline blood pressure and other risk factors over 10 years [36]. The present study and that of Lammert et al. both support the differential association between arterioles and venules. The change in CRAE and the AVR were possibly a consequence of the lowering of systolic BP (by 3 mmHg and diastolic BP by 5 mmHg) that occurred with weight loss, though this requires confirmation in a larger study, powered to assess covariates. In general both studies of children and adults have observed narrowing of CRAE and a reduction in the AVR with increased BP [36, 37]. While few prospective studies have assessed the impact of blood pressure lowering on the retinal microvasculature [6, 38, 39], a study of untreated hypertensive patients by Hughes et al showed that arterioles widened with anti-hypertension treatment over 12 months [38]. The blood pressure drop was large (31 mmHg in the amlodipine arm and 25 mmHg in the lisinopril arm) compared to the current study as the population had untreated hypertension. Additional changes in arteriolar branching angles and arteriolar density were observed. In the present study, no change was observed for tortuosity, branching angles or fractal dimension, while BP was reduced in the present study after surgery, the drop may not have been sufficient to observe changes in these measures and as the population mean BP was within the normal range, a large drop was not expected.
The primary limitation of this study is its small sample size, along with only recruiting women. Further investigation is warranted in a well-documented, large prospective study of patients undergoing bariatric surgery, where the findings of this study can be confirmed with covariate adjustment. Future directions for this work could include an assessment of weight loss maintenance and weight regain with changes in the retinal microvasculature over time.
Conclusions
The findings suggest obesity-related microvascular changes (retinal arteriolar narrowing and venular widening) are reversible after bariatric surgery-induced weight loss. The capacity for the retinal microvasculature to improve following bariatric surgery suggests plasticity of the human microvasculature early in the disease course.
References
Sjostrom L, Peltonen M, Jacobson P, Sjostrom CD, Karason K, Wedel H, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65.
Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KG, Zimmet PZ, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by International Diabetes Organizations. Diabetes Care. 2016;39:861–77.
Arterburn D, Bogart A, Coleman KJ, Haneuse S, Selby JV, Sherwood NE, et al. Comparative effectiveness of bariatric surgery vs. nonsurgical treatment of type 2 diabetes among severely obese adults. Obes Res Clin Pract. 2013;7:e258–68.
Schauer PR, Mingrone G, Ikramuddin S, Wolfe B. Clinical Outcomes of Metabolic Surgery: Efficacy of Glycemic Control, Weight Loss, and Remission of Diabetes. Diabetes Care. 2016;39:902–11.
Lammert A, Hasenberg T, Kraupner C, Schnulle P, Hammes HP. Improved arteriole-to-venule ratio of retinal vessels resulting from bariatric surgery. Obesity. 2012;20:2262–7.
Antonio PR, Marta PS, Luis DD, Antonio DP, Manuel ST, Rafael MS, et al. Factors associated with changes in retinal microcirculation after antihypertensive treatment. J Hum Hypertens. 2014;28:310–5.
Ting DSW, Tan GSW, Agrawal R, Yanagi Y, Sie NM, Wong CW, et al. Optical Coherence Tomographic Angiography in Type 2 Diabetes and Diabetic Retinopathy. JAMA Ophthalmol. 2017;135:306–12.
Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, et al. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126:1858–68.
Tapp RJ, Ness A, Williams C, Howe LD, Tilling K, Witt N, et al. Mc GTSA and Hughes AD. Differential effects of adiposity and childhood growth trajectories on retinal microvascular architecture. Microcirculation. 2013;20:609–16.
Wang JJ, Taylor B, Wong TY, Chua B, Rochtchina E, Klein R, et al. Retinal vessel diameters and obesity: a population-based study in older persons. Obesity. 2006;14:206–14.
Boillot A, Zoungas S, Mitchell P, Klein R, Klein B, Ikram MK, et al. Obesity and the microvasculature: a systematic review and meta-analysis. PLoS ONE. 2013;8:e52708.
Tapp RJ, Hussain SM, Battista J, Hutri-Kahonen N, Lehtimaki T, Hughes AD, et al. Impact of blood pressure on retinal microvasculature architecture across the lifespan: the Young Finns Study. Microcirculation. 2015;22:146–55.
Wang JJ, Mitchell P, Leung H, Rochtchina E, Wong TY, Klein R. Hypertensive retinal vessel wall signs in a general older population: the Blue Mountains Eye Study. Hypertension. 2003;42:534–41.
Cheung CY, Ikram MK, Klein R, Wong TY. The clinical implications of recent studies on the structure and function of the retinal microvasculature in diabetes. Diabetologia. 2015;58:871–85.
Sabanayagam C, Lye WK, Klein R, Klein BE, Cotch MF, Wang JJ, et al. Retinal microvascular calibre and risk of diabetes mellitus: a systematic review and participant-level meta-analysis. Diabetologia. 2015;58:2476–85.
Sabanayagam C, Shankar A, Koh D, Chia KS, Saw SM, Lim SC, et al. Retinal microvascular caliber and chronic kidney disease in an Asian population. Am J Epidemiol. 2009;169:625–32.
Wong TY, Klein R, Klein BE, Meuer SM, Hubbard LD. Retinal vessel diameters and their associations with age and blood pressure. Invest Ophthalmol Vis Sci. 2003;44:4644–50.
Ding J, Wai KL, McGeechan K, Ikram MK, Kawasaki R, Xie J, et al. and Meta-Eye Study G. Retinal vascular caliber and the development of hypertension: a meta-analysis of individual participant data. J Hypertens. 2014;32:207–15.
Wong TY, Islam FM, Klein R, Klein BE, Cotch MF, Castro C, et al. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the multi-ethnic study of atherosclerosis (MESA). Invest Ophthalmol Vis Sci. 2006;47:2341–50.
Bruyndonckx L, Hoymans VY, Lemmens K, Ramet J, Vrints CJ. Childhood obesity-related endothelial dysfunction: an update on pathophysiological mechanisms and diagnostic advancements. Pediatr Res. 2016;79:831–7.
Gishti O, Jaddoe VW, Hofman A, Wong TY, Ikram MK, Gaillard R. Body fat distribution, metabolic and inflammatory markers and retinal microvasculature in school-age children. The Generation R Study. Int J Obes. 2015;39:1482–7.
Sorensen BM, Houben AJ, Berendschot TT, Schouten JS, Kroon AA, van der Kallen CJ, et al. Type 2 diabetes are associated with generalized microvascular dysfunction: the Maastricht Study. Circulation. 2016. Nov 1;134(18):1339–1352
Nguyen TT, Wang JJ, Islam FM, Mitchell P, Tapp RJ, Zimmet PZ, et al. Retinal arteriolar narrowing predicts incidence of diabetes: the Australian Diabetes, Obesity and Lifestyle (AusDiab) Study. Diabetes. 2008;57:536–9.
Cheung CY, Lamoureux E, Ikram MK, Sasongko MB, Ding J, Zheng Y, et al. Retinal vascular geometry in Asian persons with diabetes and retinopathy. J Diabetes Sci Technol. 2012;6:595–605.
Wang L, Wong TY, Sharrett AR, Klein R, Folsom AR, Jerosch-Herold M. Relationship between retinal arteriolar narrowing and myocardial perfusion: multi-ethnic study of atherosclerosis. Hypertension. 2008;51:119–26.
Honka H, Koffert J, Hannukainen JC, Tuulari JJ, Karlsson HK, Immonen H, et al. The effects of bariatric surgery on pancreatic lipid metabolism and blood flow. J Clin Endocrinol Metab. 2015;100:2015–23.
Helmio M, Victorzon M, Ovaska J, Leivonen M, Juuti A, Jaser N, et al. SLEEVEPASS: a randomized prospective multicenter study comparing laparoscopic sleeve gastrectomy and gastric bypass in the treatment of morbid obesity: preliminary results. Surg Endosc. 2012;26:2521–6.
Cheung CY, Tay WT, Mitchell P, Wang JJ, Hsu W, Lee ML, et al. Quantitative and qualitative retinal microvascular characteristics and blood pressure. J Hypertens. 2011;29:1380–91.
Cheung CY, Hsu W, Lee ML, Wang JJ, Mitchell P, Lau QP, et al. A new method to measure peripheral retinal vascular caliber over an extended area. Microcirculation. 2010;17:495–503.
Liew G, Wang JJ, Cheung N, Zhang YP, Hsu W, Lee ML, et al. The retinal vasculature as a fractal: methodology, reliability, and relationship to blood pressure. Ophthalmology. 2008;115:1951–6.
Hart WE, Goldbaum M, Cote B, Kube P, Nelson MR. Measurement and classification of retinal vascular tortuosity. Int J Med Inform. 1999;53:239–52.
Zamir M, Medeiros JA, Cunningham TK. Arterial bifurcations in the human retina. J Gen Physiol. 1979;74:537–48.
Bray GA, Fruhbeck G, Ryan DH, Wilding JP. Management of obesity. Lancet. 2016;387:1947–56.
Johnson BL, Blackhurst DW, Latham BB, Cull DL, Bour ES, Oliver TL, et al. Bariatric surgery is associated with a reduction in major macrovascular and microvascular complications in moderately to severely obese patients with type 2 diabetes mellitus. J Am Coll Surg. 2013;216:545–56. discussion556-8
Shankar A, Sabanayagam C, Klein BE, Klein R. Retinal microvascular changes and the risk of developing obesity: population-based cohort study. Microcirculation. 2011;18:655–62.
Wong TY, Shankar A, Klein R, Klein BE, Hubbard LD. Prospective cohort study of retinal vessel diameters and risk of hypertension. BMJ. 2004;329:79.
Gishti O, Jaddoe VW, Felix JF, Klaver CC, Hofman A, Wong TY, et al. Retinal microvasculature and cardiovascular health in childhood. Pediatrics. 2015;135:678–85.
Hughes AD, Stanton AV, Jabbar AS, Chapman N, Martinez-Perez ME. and Mc GTSA. Effect of antihypertensive treatment on retinal microvascular changes in hypertension. J Hypertens. 2008;26:1703–7.
Dahlof B, Stenkula S, Hansson L. Hypertensive retinal vascular changes: relationship to left ventricular hypertrophy and arteriolar changes before and after treatment. Blood Press. 1992;1:35–44.
Acknowledgements
This study was conducted within the Finnish Centre of Excellence in Cardiovascular and Metabolic Diseases supported by the Academy of Finland, the University of Turku, the Turku University Hospital, the Åbo Academy University, and the Finnish Eye Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no conflicts of interest. This study was conducted within the Finnish Centre of Excellence in Cardiovascular and Metabolic Diseases supported by the Academy of Finland, the University of Turku, the Turku University Hospital, the Åbo Academy University, and the Finnish Eye Foundation. This study was registered at ClinicalTrials.gov under registration number NCT01373892.
Additional information
Synopsis
Bariatric surgery led to a reduction in retinal arteriolar narrowing and venular widening, with no changes observed in the control group. These findings are consistent with the observed cardiovascular risk reduction post bariatric surgery.
Rights and permissions
About this article
Cite this article
Viljanen, A., Soinio, M., Cheung, C.Yl. et al. Effects of bariatric surgery on retinal microvascular architecture in obese patients. Int J Obes 43, 1675–1680 (2019). https://doi.org/10.1038/s41366-018-0242-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0242-7
- Springer Nature Limited
This article is cited by
-
How Does Weight Loss After Bariatric Surgery Impact the Ocular Parameters? A Review
Obesity Surgery (2023)
-
Bariatric surgery—what the ophthalmologist needs to know
Eye (2022)
-
Retinal microvascular associations with cardiometabolic risk factors differ by diabetes status: results from the UK Biobank
Diabetologia (2022)
-
Longitudinal Effect of Bariatric Surgery on Retinal Microcirculation and Target Organ Damage: the BASTOD Study
Obesity Surgery (2022)
-
Bariatric Surgery Induces Retinal Thickening Without Affecting the Retinal Nerve Fiber Layer Independent of Diabetic Status
Obesity Surgery (2020)