Abstract
Introduction
The immunosuppressive therapy for life after liver transplantation (LT) is considered as a risk factor for obesity. Seven percent of the transplanted patients develop severe or morbid obesity. The obesity induces non-alcoholic steatohepatitis (NASH), which is a major risk factor for liver cirrhosis and hepatocellular carcinoma, without forgetting the cardiovascular risk and the devastating impact of obesity on quality of life of the transplanted patients. Consequently, obesity exposes these patients to future transplant loss. Bariatric surgery has been proposed for transplant patients to reduce the obesity-related comorbidities and to improve survival. We report in this video the surgical technique of laparoscopic sleeve gastrectomy (LSG) after LT.
Methods
We have performed between 2008 and 2017 the sleeve gastrectomy (SG) after LT in nine patients. Six procedures (66%) were performed totally by laparoscopy and three by upfront laparotomy. All the patients had a standard preoperative evaluation for obesity. All the procedures were assisted by a hepatic surgeon. Postoperatively patients were transferred to the liver ICU for 24 h then to the liver unit ward.
Result
The median BMI was 41.9 kg/m2 (range 38–46.1 kg/m2). Median operative time was 120 min (range, 90–240 min). No intra-operative complications occurred. The median length of hospital stay was 7 days (range, 4–81 days). The postoperative course of the majority of the patients was uneventful except for one patient who develops a staple line leak.
Conclusion
LSG after LT is technically feasible. Larger series are needed to improve the safety of the procedure in this high-risk population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The immunosuppressive therapy for life after liver transplantation (LT) is considered as a risk factor for obesity. The prevalence of severe and morbid obesity of patients who received LT is around 7 % [1]. The obesity and some of its comorbidities, such as diabetes type 2, induce non-alcoholic steatohepatitis (NASH), which is a major risk factor for development of liver cirrhosis and hepatocellular carcinoma. In addition, obesity increases the cardiovascular risk and has a devastating impact on quality of life of the transplanted patients. Consequently, obesity exposes these patients to future transplant loss [2].
Bariatric surgery has proven to be the only effective treatment for obesity, resulting in significant weight loss and reduction in obesity-related comorbidities for long term, such as metabolic syndrome, hypertension, diabetes, sleep apnea, dyslipidemia, and NASH. For this reason, bariatric surgery (BS) has been proposed for transplant patients to improve survival [3]. However, BS after LT comes with surgical risk of severe adhesions, wound complications, and anastomotic dehiscence due to the use of steroids and/or immunosuppressive drugs [4].
The systematic review that is conducted by our team showed that sleeve gastrectomy is the most used technique in transplant patients [5]. It has been preferred over gastric banding to prevent the implantation of foreign bodies. As well as this technique was preferred on the gastric bypass, in order to preserve the endoscopic access to the bile ducts and theoretically, there is no intestinal bypass; so that, the absorption of immunosuppressive drugs is not modified [6,7,8].
We report in this video the surgical technique of a laparoscopic sleeve gastrectomy (LSG) after LT.
(MP4 104,018 kb)
Method
We have performed between 2008 and 2017 the sleeve gastrectomy (SG) after LT in nine patients. Six procedures (66%) were performed totally by laparoscopy and three by upfront laparotomy due to the required combined repair of LT incisional hernia. The median delay from LT to LSG was 44 months (range, 36.0–46.1 months). All the patients had a standard preoperative evaluation for obesity, including a comprehensive medical evaluation and counseling by a multidisciplinary board (bariatric surgeon, endocrinologist, cardiologist, pneumologist, psychologist, and dietician) for at least 6 months, according to the French guidelines for bariatric surgery [9]. Decision to proceed to BS was discussed during a multidisciplinary meeting with the participation of hepatologists and liver surgeons. All the procedures were assisted by a hepatic surgeon. Oral calcineurin inhibitor-based immunosuppression was continued throughout the perioperative period which is by itself does not involve in the genesis of staple line leakage. Thromboprophylaxis with enoxaparin for 4 weeks was started 6 h after BS. Postoperatively patients were transferred to the liver ICU for 24 h then to the liver unit ward.
Technique of LSG
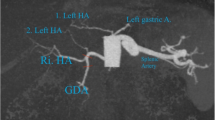
The patient is placed in a supine position. Open pneumoperitoneum is performed at the left paramedian line, 10 cm down to the xiphoid. This incision will be used for the optical trochar. After exploration of the peritoneal cavity, a 5-mm trochar is added in the left hypochondrium (in general, it is an area with fewer adhesions as it is beyond the dissection area of the liver transplantation). We start the dissection of the adhesions by a single trochar; once we have the place, a 12-mm trochar is added at the right paramedian line and at the same horizontal line as the optical trochar. We proceed by a sharp and blunt dissection of hepato-epiploic adhesions of the left hepatic lobe until identification of the diaphragm, the esophageal hiatus, and the angle of Hiss. A special attention to avoid the decapsulation of the grafted liver can be also fragile for handling. In most of the cases, there is no need to add a trochar for liver retractor because the liver is stuck spontaneously to the abdominal wall. Distal gastric dissection is performed until the identification of the pylorus. Then, we proceed by dissecting the greater curvature 5–6 cm from the pylorus until the left diaphragmatic crus—dissection of the posterior adhesions. After calibration by a 36-French tube, the SG is realized using a standard stapler as for the general population. The integrity of the staple line is checked by a blue test. Finally, the hemostasis of the staple line is achieved by bipolar cauterization and addition of biological glue.
Result
The median BMI was 41.9 kg/m2 (range, 38–46.1 kg/m2). Median operative time was 120 min (range, 90–240 min). No intra-operative complications occurred. The median length of hospital stay was 7 days (range, 4–81 days). The postoperative course of the majority of the patients was uneventful except for one patient who develops a staple line leak, needing multiple surgical and endoscopic reoperations. Once the leak is controlled, the patient developed a gastroparesis with severe dysphagia to solid. Neither invasive nor none invasive test ruled out any abnormality. Manometry confirmed total aperistalsis of the stomach remnant. After several weeks of parenteral nutrition, and failure of medical treatment, the SG was converted by laparotomy into a Roux-en-Y gastric bypass (RYGB). The patient developed a leak from the gastric pouch, leading progressively to multiorgan failure and death 19 months after SG and 11 months after conversion to RYGB.
References
Annual Report of the US Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1994–2009. Department of Health and Human Services, Health Resources and Services Administration, Healthca. Available from: https://srtr.transplant.hrsa.gov/annualreports/2012/pdf/2012_SRTR_ADR.pdf
Blackburn GL, Mun EC. Effects of weight loss surgeries on liver disease. Semin Liver Dis. 2004;24(4):371–9.
Hafeez S, Ahmed MH. Bariatric surgery as potential treatment for nonalcoholic fatty liver disease: a future treatment by choice or by chance. J Obes. 2013;2013:839275.
D’Amico G, Tulla K, Tzvetanov I. Bariatric Surgery and Transplantation. Glob Bariatric Surg. 2018;471–478
Lazzati A, Iannelli A, Schneck A-S, et al. Bariatric surgery and liver transplantation: a systematic review a new frontier for bariatric surgery. Obes Surg. 2015;25(1):134–42.
Elli EF, Gonzalez-Heredia R, Sanchez-Johnsen L, et al. Sleeve gastrectomy surgery in obese patients post-organ transplantation. Surg Obes Relat Dis. 2015;
Butte JM, Devaud N, Jarufe NP, et al. Sleeve gastrectomy as treatment for severe obesity after orthotopic liver transplantation. Obes Surg. 2007;17(11):1517–9.
Heimbach JK, Watt KDS, Poterucha JJ, et al. Combined liver transplantation and gastric sleeve resection for patients with medically complicated obesity and end-stage liver disease. Am J Transplant. 2013;13(2):363–8.
LavilleM RM, Chavrier G, et al. Recommendations regarding obesity surgery. Obes Surg. 2005;15(10):1476–80.
Lin MYC, Tavakol MM, Sarin A, et al. Safety and feasibility of sleeve gastrectomy in morbidly obese patients following liver transplantation. Surg Endosc. 2013;27(1):81–5.
Khoraki J, Katz MG, Funk LM, et al. Feasibility and outcomes of laparoscopic sleeve gastrectomy after solid organ transplantation. Surg Obes Relat Dis. 2015;12(1):75–83.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
The procedure was performed in accordance with the ethical standards of the institutional and the national research committee.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Informed Consent
Informed consent was obtained from the individual participant included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Bou Nassif, G., Salloum, C., Paolino, L. et al. Laparoscopic Sleeve Gastrectomy After Orthotopic Liver Transplantation, Video Reported. OBES SURG 29, 1436–1438 (2019). https://doi.org/10.1007/s11695-019-03751-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-019-03751-5