Abstract
We aim to summarize the existing evidence to compare the surgical outcomes of robotic Roux-en-Y gastric bypass (RRYGB) and laparoscopic RYGB (LRYGB) and to determine if these two procedures are equivalent. Literature searches were conducted by a comprehensive search in PubMed, EMBASE, and the Cochrane Library. Nineteen articles met the inclusion criteria for this review. The robotic and laparoscopic procedures had no significant differences in hospitalization time, conversion, reoperation, readmission, and postoperative complications. However, RRYGB was associated with a longer mean operative time. RRYGB was not found to be superior to LRYGB. Future studies that would report detailed meaningful postoperative outcomes, such as complications and percentage of excess weight loss, are required to determine any further differences in the efficacy between RRYGB and LRYGB.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obesity is a major health epidemic worldwide, and is associated with damages to multiple organs and poor patient quality of life [1,2,3,4]. Roux-en-Y gastric bypass (RYGB) is one of the common bariatric surgical procedures [5]. It can greatly improve weight loss and weight-related comorbidities in patients with obesity compared to lifestyle interventions and medical therapies [6, 7]. The international diabetes organizations published a statement in 2016 indicating that bariatric surgery should be considered in treating patients with type 2 diabetes and a body mass index (BMI) greater than 30 kg/m2 when hyperglycemia is inadequately controlled by optimal pharmacological treatment. However, the recommended minimum BMI for Asian patients was reduced to 27.5 kg/m2 [8].
There are currently three surgical techniques for RYGB: open, laparoscopic, and robotic. There are major advantages of laparoscopic techniques compared with open surgery, including minimal blood loss, fewer wound related complications, and shorter length of hospital stay [9]. Thus, laparoscopy has become the gold standard for bariatric surgery. In recent years, surgical scholars have attempted to combine the robotic system with surgical techniques to attain the most benefits for patients. The robotic technique has been applied to many surgical fields, including RYGB [10,11,12]. These surgical techniques have become more delicate and minimally invasive. The advantages of the robotic system include the three-dimensional view, resistance to fatigue of robotic hands, and increased mobility and range of the instrument. It could even reduce hand dominance in surgical trainees across all task domains [13]. However, only a few studies have evaluated the difference between robotic and laparoscopic techniques in terms of intraoperative and postoperative complications at present and whether the robotic is superior to laparoscopic.
Although several previous meta-analyses [14,15,16,17,18] have explored the advantages and disadvantages of robotic RYGB (RRYGB) and laparoscopic RYGB (LRYGB), there are newer, more recent trials that are not included in these studies [14, 16, 18]. Moreover, these studies also had methodological limitations [15,16,17]. Thus, the aim of our study is to summarize the existing evidence to compare the surgical outcomes between RRYGB and LRYGB and to determine if these two procedures are equivalent.
Materials and Methods
Search Strategy and Selection Criteria
The present study was conducted by a comprehensive search in PubMed, EMBASE, and the Cochrane Library to select relevant studies from inception to November 20, 2017. The search terms included “Roux-en-Y gastric bypass,” “gastric bypass,” “robotics,” “robot-assisted,” “surgery, computer-assisted,” “computer-assisted,” “telerobotics,” and “remote operations.”
The inclusion criteria were as follows: (1) English language, (2) human studies, (3) original research, including retrospective and prospective studies, (4) reporting outcomes of RYGB in obese patients, and (5) comparative studies: comparing LRYGB with RRYGB. The exclusion criteria were as follows: (1) studies involving non-bariatric procedures, (2) case reports and reviews, (3) animal studies, and (4) revision surgery.
Study Selection
After the electronic search in all the databases, two reviewers independently screened the titles and abstracts for excluding clearly irrelevant articles (articles not involving RYGB). If the abstract did not contain enough information, two investigators independently reviewed the full text. When the abstract met our inclusion criteria, the full text was reviewed. For eligible trials, the same reviewers extracted the data. If a consensus could not be reached between the two investigators, then a third investigator would resolve the disagreement.
Data Extraction
For each qualified study, we recorded the following data: general information (i.e., author, publication year, journal, study location, time period, and type of study), patient demographics (i.e., number of patients, mean age, sex, preoperative BMI, and preoperative weight), primary outcomes (i.e., the percentage of excess weight loss (%EWL) at 1, 3, 6, 12, 24, and 36 months after surgery, operative time, hospitalization time, conversions, reoperation and readmission within 30 days, mortality, and postoperative complications), and secondary outcomes (i.e., cost, leak, pulmonary embolism, stricture, marginal ulcer, and wound infection).
Data on categorical outcomes were 2 × 2 tabulated, dividing patients presenting the outcome and patients free of the outcome for the laparoscopic and robotic groups. Regarding continuous outcomes, we extracted the number of patients, the mean, and the standard deviation (SD). In cases where the standard deviation was not available, it was calculated using the available data. For clinical trials that only reported the mean, range, and size, we used simple and elementary inequalities to estimate the mean and the variance [19].
Statistical Analysis
Categorical outcomes were evaluated by the odds ratio (OR) and 95% confidence interval (CI) using the random-effects model. For continuous outcomes, we used the weighted mean difference (WMD) and 95% CI to calculate the means of the random-effects model. Between-study heterogeneity was assessed using Cochran’s Q test and by estimating I2, and was quantified as low, moderate, and high, with upper limits of 25%, 50%, and 75% for I2, respectively [20]. Statistical analyses were performed using the R statistical program version 3.4.2.
Quality Assessment
The non-randomized controlled trials were evaluated done using the Newcastle-Ottawa Quality Assessment Scale (NOS) [21]. The score range of the scale varies from zero to nine stars, and studies with a score equal to or in excess of five stars were considered to have a reliable methodological quality to be included.
Publication Bias Assessment
We used the Egger’s formal statistical test which is described in the Cochrane handbook to evaluate the existence of publication bias. If the number of the included studies was more than ten, the publication bias was evaluated. For the interpretation of the results of test, statistical significance was defined as p < 0.1 [22].
Results
Study Selection
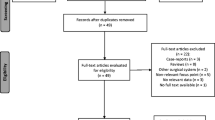
The flow diagram of the article research is shown in Fig. 1. A total of 406 potentially relevant studies have been retrieved through our search strategy, and 106 articles have been repeated. Of these studies, 210 were excluded based on title and abstract. After a full-text review of the 90 remaining articles, 71 were excluded. Finally, 19 articles [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41] met the inclusion criteria for this review and included 1 randomized controlled trial (RCT) and 18 case controlled trials (CCT).
Characteristics of the Eligible Studies
A total of 177,766 patients underwent RYGB; 172,234 treated using laparoscopic techniques and 5532 using standard robotic techniques. Fourteen studies were conducted in the USA, and 5 studies in Europe (France [25], Switzerland [26], Netherlands [29, 37], and Italy [36]).
Of these included 19 studies, 11 studies [23, 25, 27,28,29, 32,33,34,35,36, 41] were comparable in age, BMI, and F/M ratio. In Ayloo et al.’s study [24], the age of robotic group was younger, while in other two studies [26, 39], the robotic group was older. As for BMI, one study [39] had a higher BMI in robotic group, another study [37] had a lower BMI in robotic group. And the F/M ratio was not comparable in one study [37]. The age of patients was 18 to 65 years in LRYGB, 20 to 62 years in RRYGB; preoperative BMI was 23.4 to 70.3 kg/m2 in LRYGB, 33.7 to 78.2 kg/m2 in RRYGB. Seventeen articles were single-center studies, two articles [27, 40] were multi-center studies. Five articles were prospective studies, 14 articles were retrospective studies. Characteristics of eligible articles and the NOS score are reported in Table 1.
%EWL and Weight Loss
Although six articles [24, 26, 34, 36, 37, 39] reported the postoperative %EWL, we did not have enough data to perform a data analysis in terms of postoperative %EWL after 1, 3, 6, 12, 24, and 36 months. After 1 month, the study by Bush et al. [26] reported that the percentage of excess BMI loss had no significant difference between the two groups. The study by Ayloo et al. [24] reported that the %EWL was not significantly different at 3 and 6 months. Among the eligible articles, three articles [26, 34, 39] reported that there were no significant differences in the %EWL between the two groups at 1 year. The study by Smeenk et al. [37] reported that the %EWL at 1 year was higher in the laparoscopic group than robotic group (P = 0.02). The study by Bush et al. [26] showed that the percentage of excess BMI loss was higher in laparoscopic group than in the robotic group at 36 months (P = 0.003); however, no significant difference was observed at 24 months.
Operative Time
Twelve articles [23,24,25,26,27, 29, 31, 34, 36, 37, 39, 41] reported the operative time in both groups. The operative time ranged from 75 min to 360 min for LRYGB and from 90 min to 405 min for RRYGB. Three articles [24, 25, 31] showed that the overall operative time was significantly shorter for RRYGB than for LRYGB; the remaining studies showed a longer operative time. In the present study, the pooled data analysis showed that RRYGB had a significantly longer operative time as shown in Fig. 2. However, the result showed a considerable statistical heterogeneity (I2 = 97%).
Hospitalization Time
Most of the eligible articles reported the hospitalization time. Six articles [28,29,30,31, 33, 38] were excluded because of lack of relevant data. Our pooled analysis showed the hospitalization time was not significantly different between the two groups [MD = − 0.01 days, 95% CI (− 0.24; 0.23); P = 0.95], with a significant heterogeneity (I2 = 86%) (Fig. 3).
Conversion
Eight articles reported the conversions [23,24,25,26, 29, 33, 34, 39]. The number of conversions ranged from 0 to 9 for the RRYGB and from 0 to 19 for the LRYGB. There was no significant difference between the two procedures, except in two studies [26, 29]. In the study by Bush et al. [26], 19 conversions were observed in LRYGB owing to several reasons, including severe adhesions, large left liver lobe, duodenal injury, stapler misfire, and other various technical problems; the number of conversions in LRYGB was higher than that in RRYGB (n = 3). In the study by Huben et al. [29], the number of conversions was 9 in RRYGB (4 owing to improper placement of the robotic ports, 2 owing to a previous open appendectomy with some adhesions, and 3 owing to substantial jejunal tears). The present pooled data analysis showed no statistical difference between RRYGB and LRYGB [OR = 1.0, 95% CI (0.16, 6.33); P = 1.0], with a significant heterogeneity (I2 = 71%) (Fig. 4).
Reoperation within 30 Days
Seven articles reported reoperations within 30 days [23, 25,26,27, 32, 39, 41]. The percentage of reoperations ranged from 0 to 2.11% for the RRYGB and from 0 to 2.28% for the LRYGB. The number of reoperations was not significantly different between the two procedures in two studies [23, 41]; in RRYGB, it was lower in two studies [26, 39] but higher in the remaining studies. The present pooled data analysis revealed that there was no significant difference between RRYGB and LRYGB [OR = 1.34, 95% CI (0.38, 4.74); P = 0.65], with a significant heterogeneity (I2 = 82%) (Fig. 5).
Readmission within 30 Days
Seven articles [24, 27, 32, 33, 39,40,41] reported the number of patients who returned to the hospital for readmission within 30 days. All of them indicated that readmission was not significantly different between robotic and laparoscopic group. The pooled data analysis also showed a similar finding [OR = 0.86, 95% CI (0.47, 1.58); P = 0.62], with a high heterogeneity (I2 = 89%).
Mortality
Sixteen articles reported the mortality [23,24,25,26,27,28,29,30, 32, 33, 35,36,37, 39,40,41], and 11 studies showed that the number of patients who died was zero in both LRYGB and RRYGB [23, 24, 28,29,30, 32, 33, 35, 37, 39, 41]. The pooled data analysis showed that the number of patients who died was significantly lower in RRYGB than in LRYGB [OR = 2.37, 95% CI (1.21, 4.67); P = 0.01]. Adding the study by Celio et al. [27] had a significant impact on the result when we performed sensitivity analyses to assess the robustness of our outcome. After omitting their study, the mortality in the two groups had no significant difference [OR = 1.1, 95% CI (0.25, 4.86), I2 = 0, P = 0.9].
Postoperative Complications
Fourteen articles reported the total postoperative complications [24,25,26,27,28, 31, 33,34,35,36,37,38,39, 41]. The pooled data analysis demonstrated no significant differences in the total surgical complications between the two groups. Regarding the major complications after surgeries, our meta-analysis also showed that there were no significant differences in terms of leak, stricture, pulmonary embolism, wound infection, and marginal ulcer between RRYGB and LRYGB (Table 2).
Cost
Five articles [28,29,30, 36, 40] reported the cost in the two groups, and all of them showed that the cost in RRYGB was higher than that in LRYGB.
Sensitivity Analyses
There was a considerable statistical heterogeneity in the operative time, hospitalization time, conversion, leaks, and reoperation and readmission within 30 days in the present pooled meta-analysis (Table 2). We performed sensitivity analyses to assess the strength of our results and investigate the potential source of the high heterogeneity. Almost all results were not altered, except for mortality, when one large sample study was excluded by conducting sensitivity analyses [OR = 1.1, 95% CI (0.25, 4.86), I2 = 0, P = 0.9)]. After omitting each of the included studies for each outcome, we found that the study by Villamere et al. [40] might be the source of heterogeneity for readmission. The heterogeneity of the pooled data analysis markedly decreased after their study was excluded [OR = 1.18, 95% CI (0.88, 1.58), I2 = 11%, P = 0.26]. Similarly, the study by Bush et al. [26] contributed to the high heterogeneity for postoperative leak, and the heterogeneity became low after their study was excluded [OR = 1.4, 95% CI (0.71, 2.78), I2 = 34%, P = 0.33). Adding the studies by Bush et al. [26] and Huben et al. [29] probably resulted in the considerable heterogeneity for conversion, and the heterogeneity has become low after they were excluded [exclusion of the study by Bush et al.: OR = 1.96, 95% CI (0.33, 11.73), I2 = 46%, P = 0.46; exclusion of the study by Huben et al.: OR = 0.47, 95% CI (0.11, 1.93), I2 = 45%, P = 0.29].
Publication Bias
We used the Egger’s regression test to explore the publication bias of our meta-analysis. The Egger’s test showed no evidence of publication bias in operative time (P = 1), hospitalization time (P = 0.81), total postoperative complications (P = 0.39), and leaks (P = 0.49). Publication bias was not calculated for the remaining outcomes because less than 10 eligible studies were included in the analysis.
Discussion
Our meta-analysis evaluated the safety and efficiency of RRYGB by comparing its surgical outcomes to those of another alternative technique. Overall, 18 CCTs and one RCT were included (172,234 LRYGB and 5532 RRYGB). The present pooled analysis demonstrated that the robotic and laparoscopic procedures were comparable, although the former was associated with longer mean operative time and higher cost.
There were five previous meta-analyses that compared the safety and efficiency between RRYGB and LRYGB among patients with obesity; however, their results were inconsistent. Markar et al. [18] published a meta-analysis in 2011 that included seven trials, which exhibited lower incidences of anastomotic stricture in RRYGB; however, their study only compared limited parameters, which did not include conversion, reoperation, and readmission. Fourman et al. [16] did not perform a sensitivity analysis nor assess the risk of bias in their systematic review. Although Bailey et al. [14] reported the overall complications, leak, stricture, bleeding, and reoperation, they did not find significant differences between the two procedures. However, they only involved 10 trials and did not contain currently available evidence. One study that included 25 trials, 11 of which were non-comparative trials, showed that RRYGB was associated with significantly less frequent anastomotic strictures, reoperations, and decreased length of hospital stay than LRYGB [15]. According to a recent meta-analysis [17], RRYGB and LRYGB were comparable; however, some of the articles in that study were reported from the same medical center and the study time period overlapped.
Conversely, 3 studies showed that the operative time was significantly longer in LRYGB; however, the pooled data analysis demonstrated that it was higher in RRYGB, which is consistent with the analysis results by Li et al. [17]. In our eligible studies, only four studies [24, 25, 37, 41] have specifically defined the operative time; however, the definitions varied. And most articles did not clearly state the surgeon have finished the learning curve of robot or laparoscopy. Previous studies [24, 25, 35, 37] showed the learning curve for RRYGB varies from 10 to 30 procedures. With the completion of the learning curve and the increase of the operator’s experience, the operative time gradually decreased and became stable. There was a learning curve of 35 patients before RRYGB had been performed; thus, the study by Huben et al. [29] revealed that the operative time, including the robot setup time, was not significantly different between LRYGB and RRYGB when performed by the same surgeon. Thus, we suspect the increased time in RRYGB greatly might be the docking and setup time of robot. The unclear definition of operative time and surgeon experience might be the sources of heterogeneity. We also attempted to perform a subgroup study according to the definition of operative time; unfortunately, we failed because we lacked sufficient data. Three previous meta- analyses [14, 15, 18] reported that the overall operative time was not significantly different between RRYGB and LRYGB. A possible reason for the differing conclusions of previous meta-analyses and the present meta-analysis is that there have been more recently published trials associated with RRYGB in the interim.
Conversion is an important parameter to assess the feasibility of these minimally invasive techniques. According to our pooled data analysis, there were no differences in conversion, reoperation, and readmission within 30 days between the two techniques, which are in accordance with the findings of previous meta-analyses [14, 15, 17]. This indicates that RRYGB could be regarded as a safe and feasible procedure.
As major parameters for the evaluation of the safety of these surgical techniques, the anastomotic leak rate was 1.15%, and the pulmonary embolism rate was 1.17%, as determined by a systematic review that included 71 studies and 107,874 patients who underwent bariatric surgery [42]. Although the robotic system provided surgeons with enhanced visual control and dexterity, it did not reduce the overall complications of RYGB [14, 15], including leak, pulmonary embolism, stricture, wound infection, and marginal ulcer. Similar results were found in other surgeries [12, 43, 44], including sleeve gastrectomy [12], which confirms that our results were accurate.
We found that adding one study [27] that greatly influenced the result when we compared the mortality under the two approaches. In their study, there was a significant reduction in the number of patients who had an American Society of Anesthesiologists (ASA) classification > 2 in the RRYGB group, and 135,040 underwent laparoscopic RYGB which account for 78.4% of totally LRYGB. Thus, the risk of death was greater in laparoscopic group, and this study should be excluded to avoid serious bias. After omitting the study, the mortality in two groups had no significant difference. Therefore, as indicated by our study and previous data [14, 15, 17], the robotic technique might not affect the safety or mortality rate during the intraoperative or postoperative period.
Postoperative weight loss is an important outcome to evaluate the effectiveness of bariatric surgery. However, we did not perform a meta-analysis in terms of postoperative %EWL because the data cannot be extracted. Currently, the eligible articles have showed the weight loss from both techniques was not significantly different at 1, 3, and 6 months. A few studies [26, 29] reported that the weight loss in LRYGB was greater than that in RRYGB after 12 months. However, there is insufficient evidence on long-term outcomes indicating that LRYGB is superior to RRYGB. Therefore, to assess how RRYGB and LRYGB perform over time accurately, more high-quality studies are needed to detail the weight loss and follow-up on the majority of the study group to minimize bias.
Five articles included in our analysis revealed that the cost of RRYGB was higher than that of LRYGB. However, we did not perform the pooled analysis due to 4 studies only showed the mean of cost. But the result was in accordance with other studies of robotic cost [12, 45]. A study with a large sample size demonstrated that the cost positively correlated with the length of hospital stay, which could double after a week, and that robotic-assisted surgeries have the highest impact on costs [46]. The increased expense for robotic surgery can be attributed to the maintenance, instrument, and equipment costs, and the unnecessary financial burden might be the reason why many medical centers and insurances had difficulties in adopting the robotic approach. However, some studies have found that the robotic approach has lower costs because it reduces complications and has shorter hospital and ICU stays, which indicates that the robotic method is cost-effective [45, 47].
The majority of the eligible articles in our meta-analysis were retrospective trials and only one article was an RCT. Therefore, the inherent pitfall of the present meta-analysis is important because low quality articles and limited data might pose a certain bias [48, 49]. Finally, most articles did not report a follow-up period, and only a few studies reported the %EWL after surgery; thus, we were unable to assess the efficacy of RRYGB fully because this datum was unavailable.
Conclusion
This meta-analysis of studies comparing RRYGB and LRYGB indicated that their clinical outcomes were comparable. RRYGB had similar clinical outcomes and was not found to be superior to LRYGB. However, this result should be confirmed by additional high-quality studies. Future studies that would report detailed meaningful postoperative outcomes, such as complications and %EWL, are required to demonstrate any further differences in the efficacy between RRYGB and LRYGB.
References
Martin KA, Mani MV, Mani A. New targets to treat obesity and the metabolic syndrome. Eur J Pharmacol. 2015;763(Pt A):64–74.
Abbasi A, Juszczyk D, van Jaarsveld CHM, et al. Body mass index and incident type 1 and type 2 diabetes in children and young adults: a retrospective cohort study. J Endocr Soc. 2017;1(5):524–37.
Lu JL, Molnar MZ, Naseer A, et al. Association of age and BMI with kidney function and mortality: a cohort study. Lancet Diabetes Endo. 2015;3(9):704–14.
Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer-related mortality. Physiol Rev. 2015;95(3):727–48.
Kang JH, Le QA. Effectiveness of bariatric surgical procedures: a systematic review and network meta-analysis of randomized controlled trials. Medicine (Baltimore). 2017;96(46):e8632.
Colquitt JL, Pickett K, Loveman E, et al. Surgery for weight loss in adults. Cochrane Database of Syst Rev. 2014.
Sheng B, Truong K, Spitler H, et al. The long-term effects of bariatric surgery on type 2 diabetes remission, microvascular and macrovascular complications, and mortality: a systematic review and Meta-analysis. Obes Surg. 2017;27(10):2724–32.
Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39(6):861–77.
Rausa E, Bonavina L, Asti E, et al. Rate of death and complications in laparoscopic and open Roux-en-Y gastric bypass. A meta-analysis and meta-regression analysis on 69,494 patients. Obes Surg. 2016;26(8):1956–63.
Jung MK, Hagen ME, Buchs NC, et al. Robotic bariatric surgery: a general review of the current status. Int J Med Robot. 2017;13(4).
Madhuri TK, Butler-Manuel S. Robotic-assisted vs traditional laparoscopic surgery for endometrial cancer: a randomized controlled trial. Am J Obstet Gynecol. 2017;216(6):619.
Magouliotis DE, Tasiopoulou VS, Sioka E, et al. Robotic versus laparoscopic sleeve gastrectomy for morbid obesity: a systematic review and meta-analysis. Obes Surg. 2017;27(1):245–53.
Badalato GM, Shapiro E, Rothberg MB, et al. The da vinci robot system eliminates multispecialty surgical trainees' hand dominance in open and robotic surgical settings. JSLS. 2014;18(3).
Bailey JG, Hayden JA, Davis PJ, et al. Robotic versus laparoscopic Roux-en-Y gastric bypass (RYGB) in obese adults ages 18 to 65 years: a systematic review and economic analysis. Surg Endosc. 2014;28(2):414–26.
Economopoulos KP, Theocharidis V, McKenzie TJ, et al. Robotic vs. laparoscopic Roux-En-Y gastric bypass: a systematic review and meta-analysis. Obes Surg. 2015;25(11):2180–9.
Fourman MM, Saber AA. Robotic bariatric surgery: a systematic review. Surg Obes Relat Dis. 2012;8(4):483–8.
Li K, Zou J, Tang J, et al. Robotic versus laparoscopic bariatric surgery: a systematic review and meta-analysis. Obes Surg. 2016;26(12):3031–44.
Markar SR, Karthikesalingam AP, Venkat-Ramen V, et al. Robotic vs. laparoscopic Roux-en-Y gastric bypass in morbidly obese patients: systematic review and pooled analysis. Int J Med Robot. 2011;7(4):393–400.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13.
Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Wells GA BS, O'Connell D, Peterson J, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. (Available from: URL: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [cited 2009 Oct 19]).
Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Ahmad A, Carleton JD, Ahmad ZF, et al. Laparoscopic versus robotic-assisted Roux-en-Y gastric bypass: a retrospective, single-center study of early perioperative outcomes at a community hospital. Surg Endosc. 2016;30(9):3792–6.
Ayloo SM, Addeo P, Buchs NC, et al. Robot-assisted versus laparoscopic Roux-en-Y gastric bypass: is there a difference in outcomes? World J Surg. 2011;35(3):637–42.
Benizri E, Renaud M, Reibel N, et al. Perioperative outcomes after totally robotic gastric bypass: a prospective nonrandomized controlled study. Am J Surg. 2013;206(2):145–51.
Buchs NC, Morel P, Azagury DE, et al. Laparoscopic versus robotic Roux-En-Y gastric bypass: lessons and long-term follow-up learned from a large prospective monocentric study. Obes Surg. 2014;24(12):2031–9.
Celio AC, Kasten KR, Schwoerer A, et al. Perioperative safety of laparoscopic versus robotic gastric bypass: a propensity matched analysis of early experience. Surg Obes Relat Dis. 2017;13(11):1847–52.
Curet MJ, Curet M, Solomon H, et al. Comparison of hospital charges between robotic, laparoscopic stapled, and laparoscopic handsewn Roux-en-Y gastric bypass. J Robot Surg. 2009;3(2):75–8.
Hubens G, Balliu L, Ruppert M, et al. Roux-en-Y gastric bypass procedure performed with the da Vinci robot system: is it worth it? Surg Endosc. 2008;22(7):1690–6.
Lyn-Sue JR, Winder JS, Kotch S, et al. Laparoscopic gastric bypass to robotic gastric bypass: time and cost commitment involved in training and transitioning an academic surgical practice. J Robot Surg. 2016;10(2):111–5.
Mohr CJ, Nadzam GS, Curet MJ, et al. Totally robotic Roux-en-Y gastric bypass. Arch Surg. 2005;140(8):779–86.
Moon RC, Gutierrez JC, Royall NA, et al. Robotic Roux-en-Y gastric bypass, is it safer than laparoscopic bypass? Obes Surg. 2016;26(5):1016–20.
Myers SR, McGuirl J, Wang J. Robot-assisted versus laparoscopic gastric bypass: comparison of short-term outcomes. Obes Surg. 2013;23(4):467–73.
Park CW, Lam EC, Walsh TM, et al. Robotic-assisted Roux-en-Y gastric bypass performed in a community hospital setting: the future of bariatric surgery? Surg Endosc. 2011;25(10):3312–21.
Sanchez BR, Mohr CJ, Morton JM, et al. Comparison of totally robotic laparoscopic Roux-en-Y gastric bypass and traditional laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2005;1(6):549–54.
Scozzari G, Rebecchi F, Millo P, et al. Robot-assisted gastrojejunal anastomosis does not improve the results of the laparoscopic Roux-en-Y gastric bypass. Surg Endosc. 2011;25(2):597–603.
Smeenk RM, Van't Hof G, Elsten E, et al. The results of 100 robotic versus 100 laparoscopic gastric bypass procedures: a single high volume centre experience. Obes Surg. 2016;26(6):1266–73.
Snyder BE, Wilson T, Leong BY, et al. Robotic-assisted roux-en-Y gastric bypass: minimizing morbidity and mortality. Obes Surg. 2010;20(3):265–70.
Stefanidis D, Bailey SB, Kuwada T, et al. Robotic gastric bypass may lead to fewer complications compared with laparoscopy. Surg Endosc. 2018;32(2):610–6.
Villamere J, Gebhart A, Vu S, et al. Utilization and outcome of laparoscopic versus robotic general and bariatric surgical procedures at academic medical centers. Surg Endosc. 2015;29(7):1729–36.
Wood MH, Kroll JJ, Garretson B. A comparison of outcomes between the traditional laparoscopic and totally robotic Roux-en-Y gastric bypass procedures. J Robot Surg. 2014;8(1):29–34.
Chang SH, Freeman NLB, Lee JA, et al. Early major complications after bariatric surgery in the USA, 2003-2014: a systematic review and meta-analysis. Obes Rev 2017.
Froghi S, Ahmed K, Khan M, et al. Evaluation of robotic and laparoscopic partial nephrectomy for small renal tumours (T1a). BJU Int. 2013;112:E322–33.
Xiong J, Nunes Q, Tan C, et al. Comparison of short-term clinical outcomes between robotic and laparoscopic gastrectomy for gastric cancer: a meta-analysis of 2495 patients. J Laparoendosc Adv Surg Tech A. 2013;23(12):965–76.
Hagen ME, Pugin F, Chassot G, et al. Reducing cost of surgery by avoiding complications: the model of robotic Roux-en-Y gastric bypass. Obes Surg. 2012;22(1):52–61.
Khorgami Z, Aminian A, Shoar S, et al. Cost of bariatric surgery and factors associated with increased cost: an analysis of national inpatient sample. Surg Obes Relat Dis. 2017;13(8):1284–9.
Salman M, Bell T, Martin J, et al. Use, cost, complications, and mortality of robotic versus nonrobotic general surgery procedures based on a nationwide database. Am Surg. 2013;79(6):553–60.
Tian J, Zhang J, Ge L, et al. The methodological and reporting quality of systematic reviews from China and the USA are similar. J Clin Epidemiol. 2017;85:50–8.
Yao L, Sun R, Chen Y, et al. The quality of evidence in Chinese meta-analyses needs to be improved. J Clin Epidemiol. 2016;74:73–9.
Funding
Supported by Foundation of Lanzhou Science and Technology Bureau (Grant no. 2017-ZD-38); Supported by Natural Science Foundation of Gansu Province (Grant no. 18JR3RA052).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval
This study does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
This study does not include informed consent.
Non-Blinded COI Statement
Every author has nothing to disclose.
Rights and permissions
About this article
Cite this article
Wang, L., Yao, L., Yan, P. et al. Robotic Versus Laparoscopic Roux-en-Y Gastric Bypass for Morbid Obesity: a Systematic Review and Meta-Analysis. OBES SURG 28, 3691–3700 (2018). https://doi.org/10.1007/s11695-018-3458-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-3458-7