Abstract
We aim to summarize the available literature on patients treated with robotic bariatric surgery (RBS) or laparoscopic bariatric surgery (LBS) and compare the clinical outcomes between RBS and LBS. A systematic literature was conducted in accordance with the PRISMA guidelines. Thirty-four observational studies met our inclusion criteria, and 27 studies of 27,997 patients were included in the meta-analysis. There were no significant differences between RBS and LBS regarding overall postoperative complications, major complications, the length of hospital stay, reoperation, conversion, and mortality. Nevertheless, RBS was burdened by longer operative times and higher hospital costs when compared with LBS. On the contrary, the incidence of anastomotic leak was lower in RBS than in LBS. Further studies with a longer follow-up are recommended.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obesity is a growing problem in the world and is associated with highly elevated risks of adverse health outcomes. The NCD Risk Factor Collaboration revealed that between 1975 and 2014, the prevalence of obesity increased from 3.2 to 10.8 % in men and from 6.4 to 14.9 % in women in their pooled analysis of 1698 population-based studies including more than 19 million participants [1]. Bariatric surgery has been approved as an effective treatment that achieves dramatic and durable weight loss in obese patients [2].
Since the first laparoscopic Roux-en-Y gastric bypass (LRYGB) was reported in 1994, laparoscopic bariatric surgery (LBS) has become widely used for the treatment of morbid obesity because of shorter hospital stay, faster convalescence, and lower postoperative complication rates compared with open bariatric procedure [3, 4]. In order to overcome the technical disadvantages of laparoscopic surgery, including lack of three-dimensional (3D) imaging and loss of some freedom of motion, robotic surgical systems were introduced in 1997. Compared with traditional laparoscopy, robotic surgical system has been considered to achieve better postoperative quality and overcome some of the limitations of laparoscopic surgery. So robotic surgery is becoming more prevalent in the fields of gynecology and urology in the last decade. More recently, it has seen more use in the field of bariatric surgery; however, the benefit between robotic BS and laparoscopic BS was still in debate.
In this review, we aimed to determine whether robotic BS is superior to laparoscopic BS in terms of postoperative complications, operative times, the length of hospital stay, and economic parameters.
Materials and Methods
Study Design
A systematic review and meta-analysis was conducted according to predefined guidelines provided by the Cochrane Collaboration (2008) [5]. All data were reported according to Meta-analysis Of Observational Studies in Epidemiology statement [6].
Search Strategy
Two authors (Author 1, Author 2) independently searched published studies indexed in the MEDLINE, EMBASE, web of science and the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library. References of all selected studies were also examined. The following main search terms were used: robotic, laparoscopic, Roux-en-Y, gastric bypass, sleeve gastrectomy, gastroplasty, biliopancreatic diversion, adjustable gastric banding, and bariatric surgery. The latest date for this search was May 2016.
Inclusion and Exclusion Criteria
Two reviewers (Author 1, Author 2) independently screened all abstracts and selected studies in the meta-analysis if they met all of the following criteria: (1) randomized, controlled trials (RCTs) or observational studies including cohort, cross-sectional, and case-control studies; (2) written in the English language; (3) conducted on human subjects; (4) compared outcomes between the laparoscopic and robotic bariatric surgery; (5) If data of ongoing studies were published as updates, results of only the longest duration periods were included. For studies without the outcomes we needed, author(s) would be contacted via e-mail for more relevant information, if necessary. Exclusion criteria were (1) reviews, comments, case reports, abstracts, animal studies, and unpublished studies.
Primary Outcomes
The main outcome was the rate of overall complications, major complications (Grade 3 and 4 complications) and minor complications (Grade 1 and 2 complications) [7]. Other primary outcomes were adverse events including anastomotic leak, stricture or stenosis, gastrointestinal (GI) or abdominal bleeding, reoperation, mortality, operative time, and length of stay (LOS).
Secondary Outcomes
The secondary outcomes included conversion rate, 30-day readmission, volume of intraoperative bleeding, ICU stay, deep-vein thrombosis (DVT), ulcer, abscess, intestinal obstruction, wound infection, trocar side hernia, pneumonia, vomiting, diarrhea, dehydration, abdominal pain, and fever.
Data Extraction
Two investigators (Author 1, Author 2) independently reviewed abstracts of all citations. Data verifications between the two authors were performed to ensure reliability and completeness after all abstracts were reviewed. The inclusion criteria were applied to all identified studies independently. Different decisions were resolved by consensus.
Full texts of potentially relevant articles identified through other sources were retrieved. If multiple articles from the same study were searched, only the article with the longest follow-up period was included. Data with respect to research design, type of surgery, participant characteristics, duration of study, and outcomes were independently extracted. We contacted the authors for the primary reports of the unpublished data. If the authors did not reply, the available data were used for our analyses.
Methodological Quality Assessment
We used the nine-point Newcastle-Ottawa Scale to assess the study quality for all included observational studies. This scale evaluated a quality score calculated on three fundamental methodological criteria: study participants (0–4), adjustment for confounding (0–2), or ascertainment of the exposure or outcome of interest (0–3). We arbitrarily classified quality as high (score: 7–9) versus low (score: 0–3). We excluded studies from our meta-analysis if they had poor quality. Discrepant opinions between authors were resolved to reach a consensus.
Statistical Analysis
The data were pooled using REVMAN 5.0 software (The Nordic Cochrane Centre, Copenhagen, Denmark) and STATA/SE version 13 (Stata Corp, College Station, TX, USA). For each study, we calculated ORs with 95 % confidence intervals (CIs) for dichotomous data and standardized mean differences (SMDs) with 95 % CIs for continuous data. A random-effect model (DerSimonian-Laird method) was used when significant heterogeneity was detected between studies (P < 0.10; I 2 > 50 %). Otherwise, a fixed-effect model (Mantel-Haenszel test) was used. Subgroup analyses by type of robotic procedure (robotic-assisted, totally robotic) and type of bariatric surgery (RYGB, SG, AGB) were performed. To assess the stability of the results of the meta-analysis, sensitivity analysis was performed. Publication bias was assessed by the Egger’s test and represented graphically by funnel plots. Egger’s formal statistical test was performed only when the number of included studies was adequate (10 or more) and statistical significance was defined as P < 0.1.
Results
Description of Included Studies
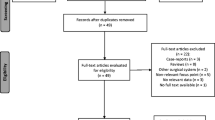
After excluding duplicate results, the initial search included 1066 articles, 1032 articles were excluded because 970 were off the topic after scanning the title and/or the abstract, 38 were not RCT or observational studies, 10 had no laparoscopic comparison group, and 14 were conference abstracts. A total of 34 articles were included in our systematic review and 27 studies of 27,997 patients were included in the meta-analysis (Fig. 1). The characteristics are outlined in Table 1.
Quality Assessment of Included Studies
NOS evaluated the quality of the included studies. Total score ranged from 4 to 8. None of the studies had low quality (total score below 3) and excluded from the meta-analysis.
Overall Complications
Twenty-three trials reported the incidence of overall complications [8–30]. Meta-analysis revealed no significant difference in the incidence of overall complications between the TRRYGB and the LRYGB (OR 0.92, 95 % CI 0.73–1.16, P = 0.49), the RARYGB and the LRYGB (OR 0.99, 95 % CI 0.77–1.27, P = 0.92), the RASG and the LSG (OR 0.79, 95 % CI 0.49–1.29, P = 0.35), and RBS and the LBS (OR 0.93, 95 % CI 0.79–1.09, P = 0.39). There was no evidence of statistical heterogeneity (I 2 = 25 %) (Fig. 2).
Forest plot describing the differences in rates of overall complications between RBS and LBS. The subgroup analysis on studies performing TRRYGB, RARYGB, and RASG is presented. There was no difference in the incidence of overall complications between RBS and LBS. RBS robotic bariatric surgery, LBS laparoscopic bariatric surgery, TRRYGB totally robotic Roux-en-Y gastric bypass, RARYGB robotic-assisted Roux-en-Y gastric bypass, RASG robotic-assisted sleeve gastrectomy
Major and Minor Complications
Major complications were reported in 9 studies [8–11, 22, 23, 30–32] and minor complications in 7 studies [8–11, 22, 23, 31]. There was no significant difference in the incidence of major complications between the TRRYGB and the LRYGB (OR 1.00, 95 % CI 0.59–1.69, P = 0.99), the RARYGB and the LRYGB (OR 1.00, 95 % CI 0.69–1.45, P = 1.00), and the RBS and the LBS (OR 1.01, 95 % CI 0.74–1.36, P = 0.97). There was a low degree of heterogeneity between studies (I 2 = 23 %) (Fig. 3).
Forest plot describing the differences in rates of major complications between RBS and LBS. The subgroup analysis on studies performing TRRYGB, RARYGB, and RAGB is presented. There was no difference in the incidence of major complications between RBS and LBS. RBS robotic bariatric surgery, LBS laparoscopic bariatric surgery, TRRYGB totally robotic Roux-en-Y gastric bypass, RARYGB robotic-assisted Roux-en-Y gastric bypass, RAGB robotic adjusted gastric banding
Likewise, we found no statistically significant difference in minor complications between RARYGB and the LRYGB (OR 1.00, 95 % CI 0.63–1.60, P = 1.00) and RBS and the LBS (OR 0.79, 95 % CI 0.59–1.05, P = 0.11); however, the minor complications rates were significantly lower after TRRYGB compared with LRYGB (OR 0.68, 95 % CI 0.46–0.98, P = 0.04) (Fig. 4).
Forest plot describing the differences in rates of minor complications between RBS and LBS. The subgroup analysis on studies performing TRRYGB and RARYGB is presented. There was no difference in the incidence of minor complications between RBS and LBS. But there was a significantly reduced number of minor complications associated with TRRYGB. RBS robotic bariatric surgery, LBS laparoscopic bariatric surgery, TRRYGB totally robotic Roux-en-Y gastric bypass, RARYGB robotic-assisted Roux-en-Y gastric bypass
Anastomotic Leak and Stricture, GI/Abdominal Bleeding
Anastomotic leak was reported in 19 studies [8–11, 13–19, 22–24, 27–29, 32, 33] (Fig. 5). There was no significant difference in the incidence of anastomotic leak between the RARYGB and the LRYGB (OR 1.00, 95 % CI 0.50–2.00, P = 1.00), the RASG and the LSG (OR 0.42, 95 % CI 0.12–1.53, P = 0.19). However, the anastomotic leak rates were significantly lower after RBS and TRRYGB compared with laparoscopic procedures (OR 0.5, 95 % CI 0.3–0.81, P = 0.005; OR 0.22, 95 % CI 0.09–0.55, P = 0.001). There was no significant heterogeneity (I 2 = 46 %).
Forest plot describing the differences in rates of anastomotic leak between RBS and LBS. The subgroup analysis on studies performing TRRYGB, RARYGB, and RASG are presented. There was a significantly reduction in the incidence of anastomotic leak associated with RBS. RBS robotic bariatric surgery, LBS laparoscopic bariatric surgery, TRRYGB totally robotic Roux-en-Y gastric bypass, RARYGB robotic-assisted Roux-en-Y gastric bypass, RASG robotic-assisted sleeve gastrectomy
Anastomotic stricture was available for 16 studies [8, 10, 13–15, 17–19, 22–25, 28, 32, 34, 35] (Fig. 6) and GI/abdominal bleeding in 12 studies [10, 13, 14, 17–19, 22, 23, 28, 29, 34, 35]. The meta-analysis showed no difference between studies (TRRYGB versus LRYGB, RARYGB versus LRYGB, RASG versus LSG, and RBS versus LBS) in terms of the incidence of anastomotic leak and GI/abdominal bleeding, without evidence of statistical heterogeneity (I 2 = 34 %, I 2 = 0 %, respectively).
Forest plot describing the differences in rates of anastomotic stricture between RBS and LBS. The subgroup analysis on studies performing TRRYGB, RARYGB, and RASG is presented. There was no difference in the incidence of anastomotic stricture between RBS and LBS. RBS robotic bariatric surgery, LBS laparoscopic bariatric surgery, TRRYGB totally robotic Roux-en-Y gastric bypass, RARYGB robotic-assisted Roux-en-Y gastric bypass, RASG robotic-assisted sleeve gastrectomy
Reoperation, Readmission, Mortality, and Conversion
Reoperation was reported in 10 studies [8–11, 13, 16, 24, 25, 29, 33], readmission in 7 studies [16, 17, 24, 27, 30, 33, 34], mortality in 18 studies [8, 9, 12, 13, 15, 18, 22, 24–30, 32–35], and conversion in 12 studies [8–10, 13, 14, 19, 25, 27, 29, 33–35]. There was no significant difference in the incidence of reoperation, readmission, mortality, and conversion between studies, without evidence of statistical heterogeneity (I 2 = 66 %, I 2 = 0 %, I 2 = 0 %, I 2 = 46 %, respectively).
Operative Time and Length of Stay
The operative times were reported in 19 studies [8–11, 15, 17–20, 22, 24–26, 28, 29, 31–33, 36] and the length of stay in 19 studies [8–11, 13, 15, 16, 19–21, 24, 26–30, 33, 34, 36]. The meta-analysis revealed that there was an increased operative time after RBS, RARYGB, and RASG compared with laparoscopic procedures (SMD 0.61, 95 % CI 0.25–0.96, P < 0.0001; SMD 1.13, 95 % CI 0.31–1.95, P = 0.007; SMD 0.56, 95 % CI 0.29–0.83, P < 0.0001), respectively, although no significant difference was found between TRRYGB and LRYGB (SMD 0.24, 95 % CI −0.34–0.83, P = 0.42). However, the studies showed considerable statistical heterogeneity (I 2 = 96 %).
There was no significant difference between studies in terms of the length of stay (SMD −0.02, 95 % CI −0.17–0.12, P = 0.77), with significant heterogeneity (I 2 = 91 %).
Other Outcomes
All other examined outcomes were not different between the RBS and the LBS (Table 2).
Economic Outcomes
The economic outcomes were reported in 6 studies [12–15, 30, 32]. Two studies reported surgical devices [14, 32], and four reported total hospital charges [12, 15, 30, 32], but only one study analysis the overall cost, including amortization of the costs to purchase the robotic system and the additional hospital costs generated in the management of the surgical complications [13]. The five studies suggested that not only the surgical devices but also the total hospital costs of the robotic procedures are more expensive than laparoscopic surgery [12, 14, 15, 30, 32]. However, Hagens et al. found that RRYGB can be cost effective because of a reduction of costly anastomotic complications after robotic procedure [13].
Sensitive Analysis and Publication Bias
The studies of the meta-analysis of the operative time and the length of stay showed considerable statistical heterogeneity. To assess the stability of the results, sensitivity analyses were conducted by excluding 1 study at a time. None of the results was significantly altered, indicating that our results were robust. Because publication bias could affect the results of meta-analyses, we attempted to evaluate this potential publication bias by using funnel plots analysis and Egger’s test. Visual inspection of funnel plots for studies evaluating the primary outcomes suggested a symmetric distribution of studies around the effect size and the Egger’s test confirmed the lack of publication bias in the incidence of overall complications (P = 0.827), anastomotic leak (P = 0.828), stricture (P = 0.226), and mortality (P = 0.873). There is also lack of publication bias in the operative time (P = 0.224) and the length of stay (P = 0.427). However, our meta-analysis results of the incidence of major complications (P = 0.012), minor complications (P = 0.021), reoperation (P = 0.015), and conversion (P = 0.023) were influenced by publication bias, because statistically significant data are published more frequently than nonsignificant data. Publication bias was not calculated for the rest of the outcomes because less than 10 eligible studies were included in the analysis.
Discussion
It has been considered that LBS has been widely established as an effective treatment that achieves dramatic and durable weight loss in obese patients [37]. And at the same time, the application of laparoscopic techniques to morbidly obese patients adds some obstacles, such as lack of 3D imaging and increased abdominal wall torque on the ports. Therefore, robotic surgery systems had been considered to combine the advantages of minimally invasive surgery with the easier performance of open surgery since they were introduced in field of digestive surgery [38]. For the last decades, RBS has aroused interest among many general surgeons. However, compared with LBS, the advantage of RBS is not clear. In order to investigate the value and safety of RBS for morbidly obesity, we conducted this systematic review and meta-analysis.
There have been three earlier meta-analyses related to RRYGB [39–41]. Markar et al. reported a reduction in the incidence of anastomotic stricture in RRYGB compared with LRYGB, but this did not perform economic analysis and assess risk of bias [41]. A meta-analysis comparing RRYGB with LRYGB performed by Bailey et al. involved only 10 studies and did not perform subgroup analysis [39]. According to a recent meta-analysis, the result suggested comparable clinical outcomes between RRYGB and LRYGB, but a lack of the data of SG and AGB makes the result less reliable [40]. The present meta-analysis including 27 studies of 27,997 patients mainly compared the clinical outcomes between RBS and LBS, and refined subgroup analysis may produce reliable results.
Our result revealed that compared to conventional laparoscopy, although similar results were found regarding overall postoperative complications and hospital stay, RBS was burdened by longer operative times and greater hospital costs. However, RBS provided a real advantage of a lower incidence of anastomotic leak over LBS. In additions, robotic-assisted RYGB and SG generally took longer than the standard laparoscopic procedures, and TRRYGB decreased the incidences of minor complications and anastomotic leak than LRYGB.
Some recent studies have shown the difference between the learning curve for RBS and that of LBS. This has been reported to be 10 cases for RRYGB and 20 cases for RSG versus 70–100 cases for LRYGB or LSG [42–45]. In other words, it takes only a short period of time for the surgeon to learn how to use the robotic surgical system. So some experts considered that this shorter learning curve might result in shorter operative times [20, 31]. However, our result showed that LBS could be performed with shorter operative times than RBS, especially robotic-assisted RYGB and SG. Although the learning curve for RBS appears to be shorter than that for LBS, the addition of docking time and instrument exchange in robotic surgical procedure might have account for the longer operative times [46].
Concerning to economic outcomes, six studies compared costs between RBS and LBS [12–15, 30, 32], which came from different countries and used different costing techniques. Therefore, we could not combine cost estimates in our meta-analysis. Five of the six studies suggested higher hospital costs associated with the robotic versus laparoscopic approach [12, 14, 15, 30, 32]. The factors contributing to the increased hospital costs associated with robotic approach may include semi-disposable robotic instruments, increased length of operating room time, postoperative ICU stay, and hospitalization. Additionally, because of its initial purchase price and yearly maintenance fees, the robotic surgical procedure has been considered as expensive consumables [47]. The overall hospital costs per case should include the amortization costs of the robotic system [13, 48]. In addition, the postoperative complications, reoperation, and conversion may result in additional hospital costs. Understandably, high rates of postoperative complications, reoperation, and conversion have a negative impact on the overall costs. Hagen et al. reported a lower rate of anastomotic leak in RRYGB than LRYGB (0 versus. 4 %), leading to a cost reduction in the robotic cases [13]. Our meta-analysis found that the robotic procedure could decrease the incidence of costly anastomotic leak, which might result in some financial advantages. With regarding to the incidence of reoperation and conversion, although there is little difference between RBS and LBS, our result may be influenced by published bias that influenced by the fact that statistically significant data are published more frequently. If there is a lower incidence of postoperative complications in robotic procedure, the hospital cost per case can be minimized through increased robotic utilization, and additional hospital costs generated in the management of the surgical complications can be decreased significantly.
Except for surgical devices, use of the operating room, postoperative ICU stay, and hospitalization, the overall hospital costs should also include amortization costs of the robotic system (e.g., its initial purchase price and yearly maintenance fees) and the additional hospital costs generated in the management of the surgical complications, but most of studies included in our review did not mention this part. Whether RBS can be cost effective depending on balancing greater robotic overhead costs with the savings associated with avoiding stapling devices use and costly postoperative complications [13]. Well-designed and well-conducted RCT studies investigating postoperative complications and the overall cost are needed before adoption of a robotic approach to bariatric surgery.
The heterogeneity between the studies for operative time is statistically significant, which might be resulted from different definitions adopted for operative time by each surgical team and the operative surgeon’s level of robotic and laparoscopic surgical experience. Additionally, patients with history of abdominal surgery might suffer from longer operative time.
With any new technology, it is crucial that surgeons and operating room teams who adopt robotics are appropriately trained in its safe execution. There are a number of established curriculums for training and there are several avenues for surgeons and other team members to obtain this training in the form of skills labs to introduce the robotic platform, surgical simulators, case observations, mini-fellowships, and wet labs. The first cases performed by novice robotic surgeons should be proctored by an experienced surgeon, and it is imperative that surgeons continue to use robotics regularly in their practice. This enables them and the surgical team to improve their skills and efficiency. In today’s climate of efficiency, hybrid approaches or staged introduction can be a way to introduce robotics without significantly increasing operative times during the learning curve.
Despite a low utilization of robotic-assisted laparoscopic elective general and bariatric surgical procedures [30], the current as well as future proposed robotic platforms offer potential in the advancement of decreased incision, single incision, and incision-less (endoscopic and natural orifice) surgery. The continued integration of radiologic imaging and other adjuncts for augmented visualization to allow for creation of a real time operative map may be especially useful in complex revisional surgeries. The robotic platform provides superior visualization, increased degrees of movement, and ergonomic advantages, and with future innovations and research, we are likely to see even more widespread adoption of this tool in bariatric surgical procedures. In addition, the robotic approach not only improves the surgeon’s performance but also makes the surgeon feel safer and more comfortable. Unfortunately, the surgeon’s comfort and/or physical exhaustion is not addressed or even mentioned in most of the comparison studies between laparoscopy and robotic surgery, so further studies exploring these parameters are needed.
Our review has some strengths and limitations. Strengths included the comprehensive search method, data extraction, and study quality assessment made by two independent reviewers. There are also some limitations in our study. First, although comprehensive search strategies focused on laparoscopic bariatric surgery and robotic bariatric surgery was implemented, this review is subject to publication bias inevitably. Second, most of the included studies are observational reports and not blinded, which are of suboptimal quality and subject to selection bias. Finally, most of studies included in our study had follow-up periods of only 1–12 months. A study conducted by Scozzari et al. suggested a higher incidence of complications with longer follow-up [32]. Therefore, long-term outcomes such as anastomotic stricture may be underestimated for RBS and LBS.
This systematic review and meta-analysis demonstrates the need for additional studies comparing the RBS with the LBS. Large, randomized prospective studies with a longer follow-up are needed before we can definitively make conclusions regarding other important postoperative clinical outcomes (e.g., weight loss, the remission rate of T2DM, OSA, hypertension, dyslipidemia, etc.) and on the overall hospital costs.
Conclusions
Although similar results were found regarding overall postoperative complications and hospital stay, RBS was burdened by longer operative times and greater hospital costs. However, if the rate of postoperative complication had been considered as a primary and reasonable parameter for surgeons to evaluate when determining the efficacy of a new surgical technique designed to improve a preexisting procedure, RBS did lead to a technical improvement over LBS. The cost saving might occur in the robotic platform because of the decreased costly anastomotic leak, and further studies with a longer follow-up should be performed to explore postoperative complications and overall hospital costs.
References
Collaboration NCDRF. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19· 2 million participants. Lancet. 2016;387:1377–96.
Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA. 2002;288:2793–6.
Weller WE, Rosati C. Comparing outcomes of laparoscopic versus open bariatric surgery. Ann Surg. 2008;248:10–5.
Nguyen NT, Goldman C, Rosenquist CJ, et al. Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality of life, and costs. Ann Surg. 2001;234:279–91.
Higgins J, Altman DG. Assessing risk of bias in included studies. Cochrane handbook for systematic reviews of interventions: Cochrane book series. 2008. 187–241.
Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–12.
Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Ahmad A, Carleton JD, Ahmad ZF, et al. Laparoscopic versus robotic-assisted Roux-en-Y gastric bypass: a retrospective, single-center study of early perioperative outcomes at a community hospital. Surgical endoscopy. 2015.
Ayloo S, Roh Y, Choudhury N. Laparoscopic, hybrid, and totally robotic Roux-en-Y gastric bypass. J Robot Surg. 2016;10:41–7.
Benizri EI, Renaud M, Reibel N, et al. Perioperative outcomes after totally robotic gastric bypass: a prospective nonrandomized controlled study. Am J Surg. 2013;206:145–51.
Buchs NC, Morel P, Azagury DE, et al. Laparoscopic versus robotic Roux-en-Y gastric bypass: lessons and long-term follow-up learned from a large prospective monocentric study. Obes Surg. 2014;24:2031–9.
Curet MJ, Curet M, Solomon H, et al. Comparison of hospital charges between robotic, laparoscopic stapled, and laparoscopic handsewn Roux-en-Y gastric bypass. J Robot Surg. 2009;3:75–8.
Hagen ME, Pugin F, Chassot G, et al. Reducing cost of surgery by avoiding complications: the model of robotic Roux-en-Y gastric bypass. Obes Surg. 2012;22:52–61.
Hubens G, Balliu L, Ruppert M, et al. Roux-en-Y gastric bypass procedure performed with the da Vinci robot system: is it worth it? Surg Endosc. 2008;22:1690–6.
Lyn-Sue JR, Winder JS, Kotch S, et al. Laparoscopic gastric bypass to robotic gastric bypass: time and cost commitment involved in training and transitioning an academic surgical practice. J Robot Surg. 2016.
Moon RC, Gutierrez JC, Royall NA, et al. Robotic Roux-en-Y gastric bypass, is it safer than laparoscopic bypass? Obes Surg. 2016;26:1016–20.
Myers SR, McGuirl J, Wang J. Robot-assisted versus laparoscopic gastric bypass: comparison of short-term outcomes. Obes Surg. 2013;23:467–73.
Parini U, Fabozzi M, Contul RB, et al. Laparoscopic gastric bypass performed with the Da Vinci intuitive robotic system: preliminary experience. Surgical Endoscopy And Other Interventional Techniques. 2006;20:1851–7.
Park CW, Lam ECF, Walsh TM, et al. Robotic-assisted Roux-en-Y gastric bypass performed in a community hospital setting: the future of bariatric surgery? Surg Endosc. 2011;25:3312–21.
Sanchez BR, Mohr CJ, Morton JM, et al. Comparison of totally robotic laparoscopic Roux-en-Y gastric bypass and traditional laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2005;1:549–54.
Scozzari G, Zanini M, Cravero F, et al. High incidence of trocar site hernia after laparoscopic or robotic Roux-en-Y gastric bypass. Surg Endosc. 2014;28:2890–8.
Smeenk RM, van’t Hof G, Elsten E, et al. The results of 100 robotic versus 100 laparoscopic gastric bypass procedures: a single high volume centre experience. Obes Surg. 2016;26:1266–73.
Snyder BE, Wilson T, Leong BY, et al. Robotic-assisted Roux-en-Y gastric bypass: minimizing morbidity and mortality. Obes Surg. 2010;20:265–70.
Wood MH, Kroll JJ, Garretson B. A comparison of outcomes between the traditional laparoscopic and totally robotic Roux-en-Y gastric bypass procedures. J Robot Surg. 2014;8:29–34.
Ayloo S, Buchs NC, Addeo P, et al. Robot-assisted sleeve gastrectomy for super-morbidly obese patients. Journal of laparoendoscopic & advanced surgical techniques Part A. 2011;21:295–9.
Elli E, Gonzalez-Heredia R, Sarvepalli S, et al. Laparoscopic and robotic sleeve gastrectomy: short- and long-term results. Obes Surg. 2015;25:967–74.
Kannan U, Ecker BL, Choudhury R, et al. Laparoscopic hand-assisted versus robotic-assisted laparoscopic sleeve gastrectomy: experience of 103 consecutive cases. Surg Obes Relat Dis. 2016;12:94–9.
Romero RJ, Kosanovic R, Rabaza JR, et al. Robotic sleeve gastrectomy: experience of 134 cases and comparison with a systematic review of the laparoscopic approach. Obes Surg. 2013;23:1743–52.
Vilallonga R, Manuel Fort J, Caubet E, et al. Robotic sleeve gastrectomy versus laparoscopic sleeve gastrectomy: a comparative study with 200 patients. Obes Surg. 2013;23:1501–7.
Villamere J, Gebhart A, Vu S, et al. Utilization and outcome of laparoscopic versus robotic general and bariatric surgical procedures at Academic Medical Centers. Surg Endosc. 2015;29:1729–36.
Mohr CJ, Nadzam GS, Curet MJ. Totally robotic Roux-en-Y gastric bypass. Arch Surg. 2005;140:779–86.
Scozzari G, Rebecchi F, Millo P, et al. Robot-assisted gastrojejunal anastomosis does not improve the results of the laparoscopic Roux-en-Y gastric bypass. Surg Endosc. 2011;25:597–603.
Edelson PK, Dumon KR, Sonnad SS, et al. Robotic vs. conventional laparoscopic gastric banding: a comparison of 407 cases. Surg Endosc. 2011;25:1402–8.
Ayloo SM, Addeo P, Buchs NC, et al. Robot-assisted versus laparoscopic Roux-en-Y gastric bypass: is there a difference in outcomes? World J Surg. 2011;35:637–42.
Buchs NC, Azagury DE, Pugin F, et al. Roux-en-Y gastric bypass for super obese patients: what approach? Int J Med Robot Comput Assist Surg: MRCAS. 2015.
Schraibman V, Macedo AL, Epstein MG, et al. Comparison of the morbidity, weight loss, and relative costs between robotic and laparoscopic sleeve gastrectomy for the treatment of obesity in Brazil. Obes Surg. 2014;24:1420–4.
Elder KA, Wolfe BM. Bariatric surgery: a review of procedures and outcomes. Gastroenterology. 2007;132:2253–71.
Maeso S, Reza M, Mayol JA, et al. Efficacy of the Da Vinci surgical system in abdominal surgery compared with that of laparoscopy: a systematic review and meta-analysis. Ann Surg. 2010;252:254–62.
Bailey JG, Hayden JA, Davis PJ, et al. Robotic versus laparoscopic Roux-en-Y gastric bypass (RYGB) in obese adults ages 18 to 65 years: a systematic review and economic analysis. Surg Endosc. 2014;28:414–26.
Economopoulos KP, Theocharidis V, McKenzie TJ, et al. Robotic vs. laparoscopic Roux-en-Y gastric bypass: a systematic review and meta-analysis. Obes Surg. 2015;25:2180–9.
Markar SR, Karthikesalingam AP, Venkat-Ramen V, et al. Robotic vs. laparoscopic Roux-en-Y gastric bypass in morbidly obese patients: systematic review and pooled analysis. The International Journal of Medical Robotics and Computer Assisted Surgery. 2011;7:393–400.
Schauer P, Ikramuddin S, Hamad G, et al. The learning curve for laparoscopic Roux-en-Y gastric bypass is 100 cases. Surgical Endoscopy and Other Interventional Techniques. 2003;17:212–5.
Zacharoulis D, Sioka E, Papamargaritis D, et al. Influence of the learning curve on safety and efficiency of laparoscopic sleeve gastrectomy. Obes Surg. 2012;22:411–5.
Vilallonga R, Fort JM, Gonzalez O, et al. The initial learning curve for robot-assisted sleeve gastrectomy: a surgeon’s experience while introducing the robotic technology in a bariatric surgery department. Minim Invasive Surg. 2012. 2012.
Baptista V, Wassef W. Bariatric procedures: an update on techniques, outcomes and complications. Curr Opin Gastroenterol. 2013;29:684–93.
Sgarbura O, Vasilescu C. The decisive role of the patient-side surgeon in robotic surgery. Surg Endosc. 2010;24:3149–55.
Turchetti G, Palla I, Pierotti F, et al. Economic evaluation of da Vinci-assisted robotic surgery: a systematic review. Surg Endosc. 2012;26:598–606.
Barbash GI, Glied SA. New technology and health care costs—the case of robot-assisted surgery. N Engl J Med. 2010;363:701–4.
Acknowledgments
My deepest gratitude goes to Professor Pin Zhang for her constant encouragement and guidance. Without his consistent and illuminating instruction, this study could not have reached its present form. This work did not receive any specific grant from any funding agency in the public, commercial, or not for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent Statement
Informed consent was obtained from all individual participants included in the study.
Additional information
Kun Li and Jianan Zou contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, K., Zou, J., Tang, J. et al. Robotic Versus Laparoscopic Bariatric Surgery: a Systematic Review and Meta-Analysis. OBES SURG 26, 3031–3044 (2016). https://doi.org/10.1007/s11695-016-2408-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-016-2408-5